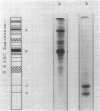
Abstract
Exposure of isolated cell envelopes from purified infectious elementary (EB) of Chlamydia psittaci to sodium carbonate-bicarbonate buffer at pH 10 plus ethylenediaminetetraacetate (EDTA) results in partial solubilization of the total protein. The released materials represent 20% of the dry weight, 16% of the total protein, 40% of the total carbohydrate, and 9% of the total lipid of the cell envelopes. Sucrose density gradient centrifugation, and Sephadex G-200, Sepharose 4B, or diethylaminoethyl-cellulose column chromatography, reveal a protein-carbohydrate-lipid complex of several hundred thousand molecular weight that contains 50% protein. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the isolated EB cell envelopes reveals two major protein bands, A and B, with estimated molecular masses of approximately 85,000 and 53,000, respectively, both of which also stain for the presence of carbohydrate and lipid. Gel electrophoresis of the protein-carbohydrate-lipid complex reveals two protein bands, C and D, with estimated molecular weights of approximately 17,000 and 13,000, respectively, which contain lipid and a small amount of carbohydrate; bands A and B are not present in the complex. Gel electrophoresis of the cell envelope residues after extraction of the complex with alkali and EDTA shows a single main band, corresponding to the position of band B, which contains protein, carbohydrate, and lipid; band A is completely missing. B and A is believed to be a component of the complex, which is split into two subunits on alkali solubilization.
Full text
PDF

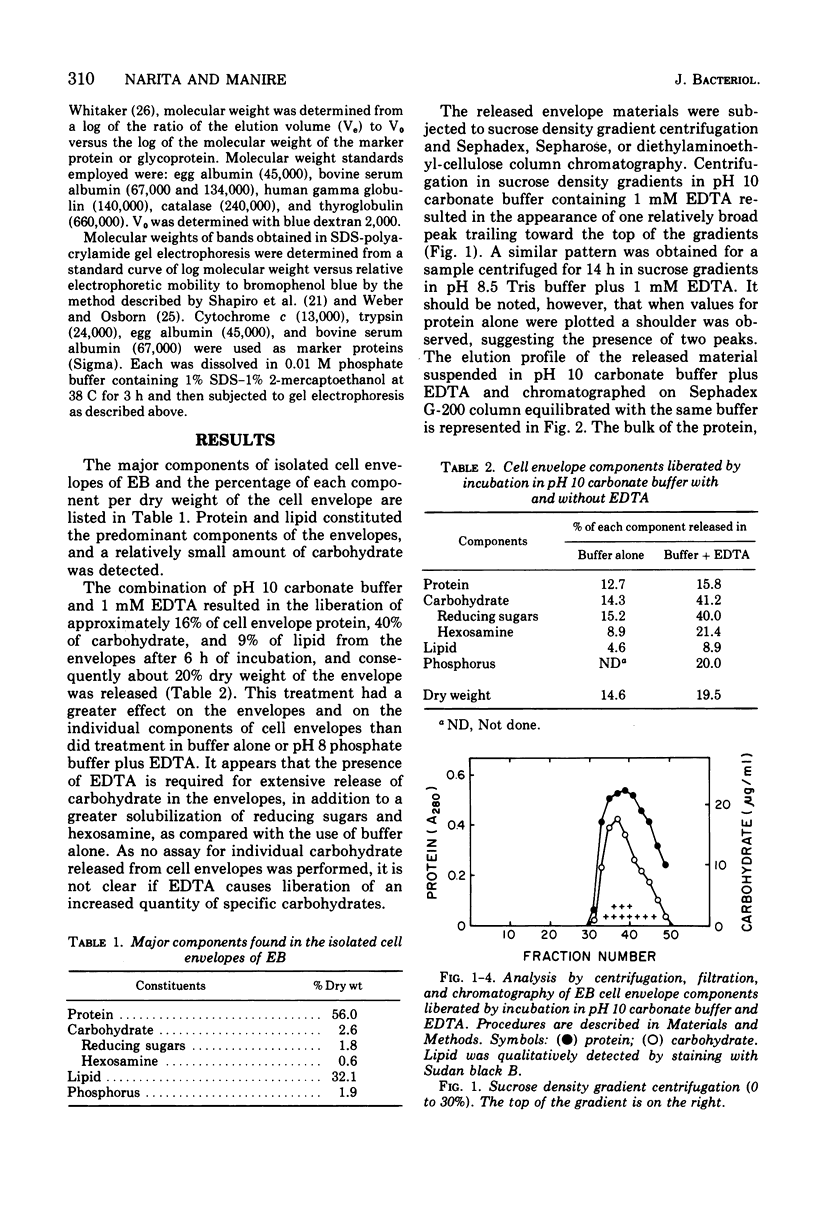
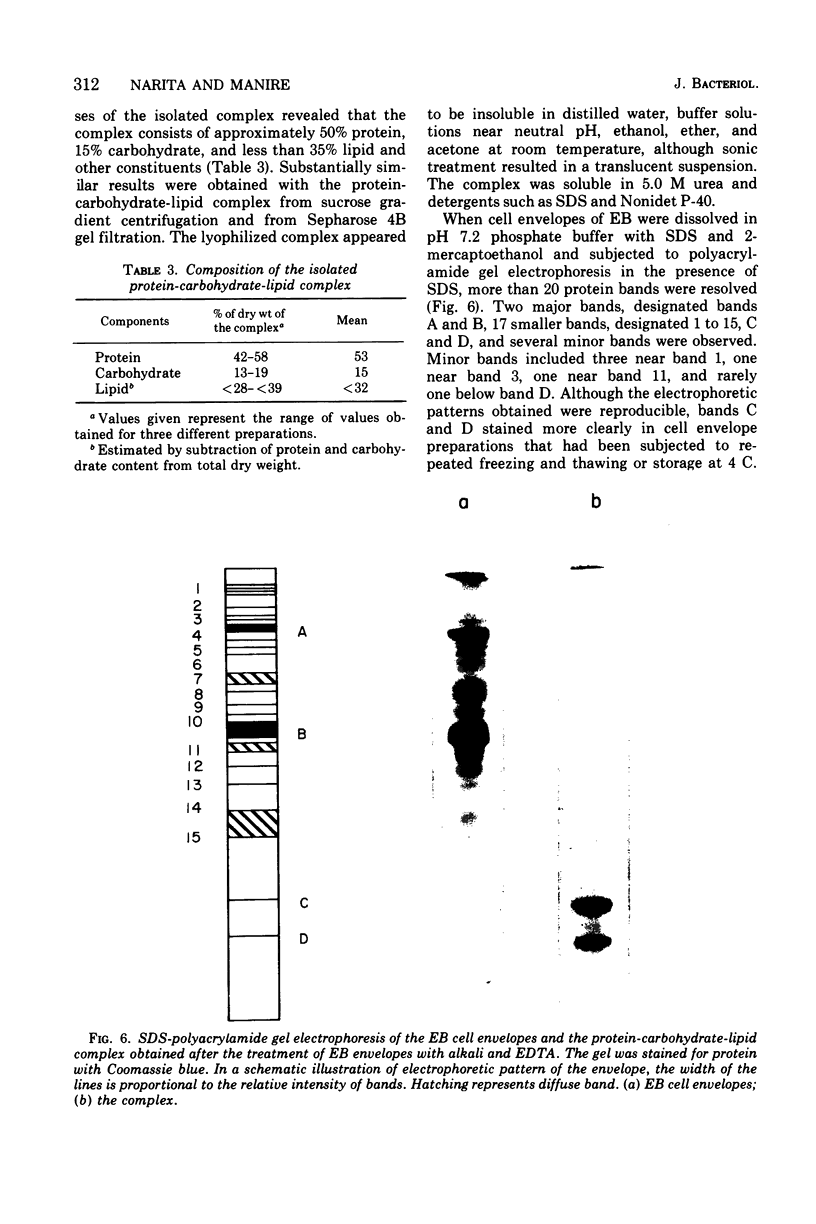
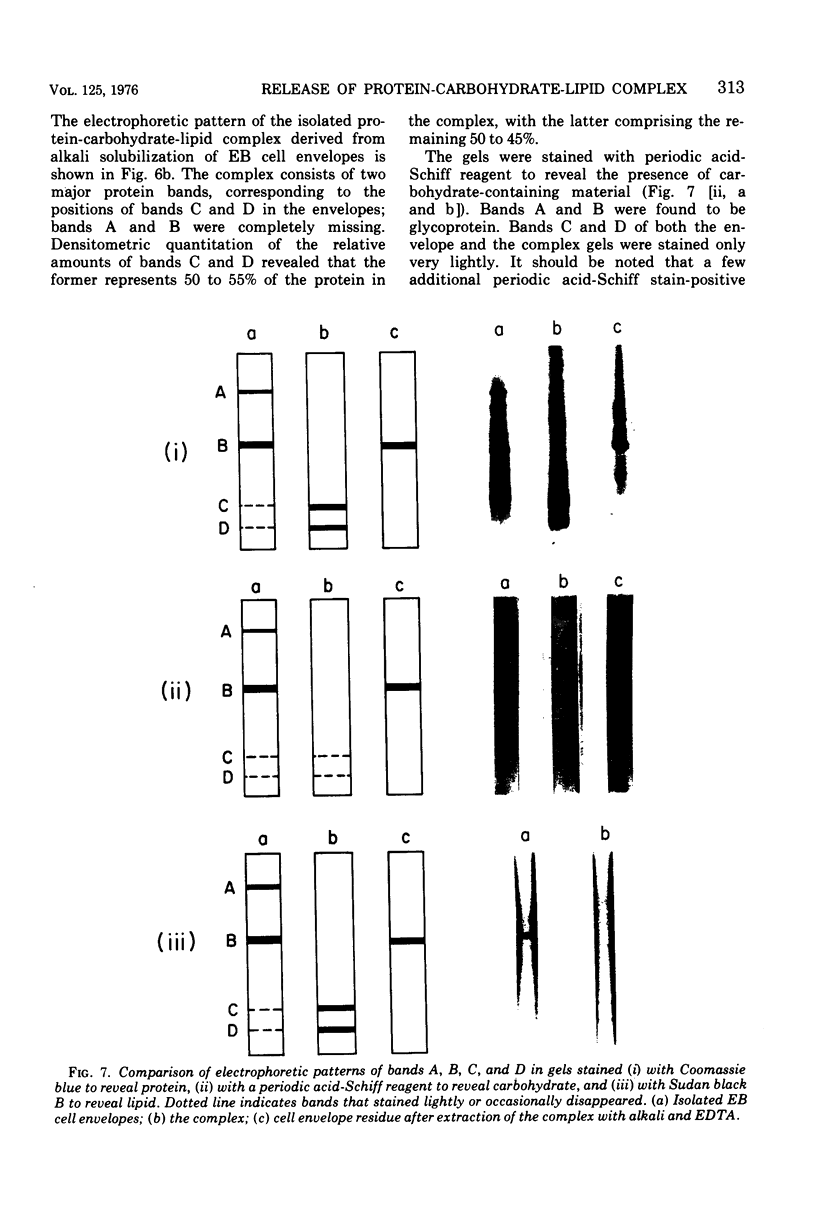
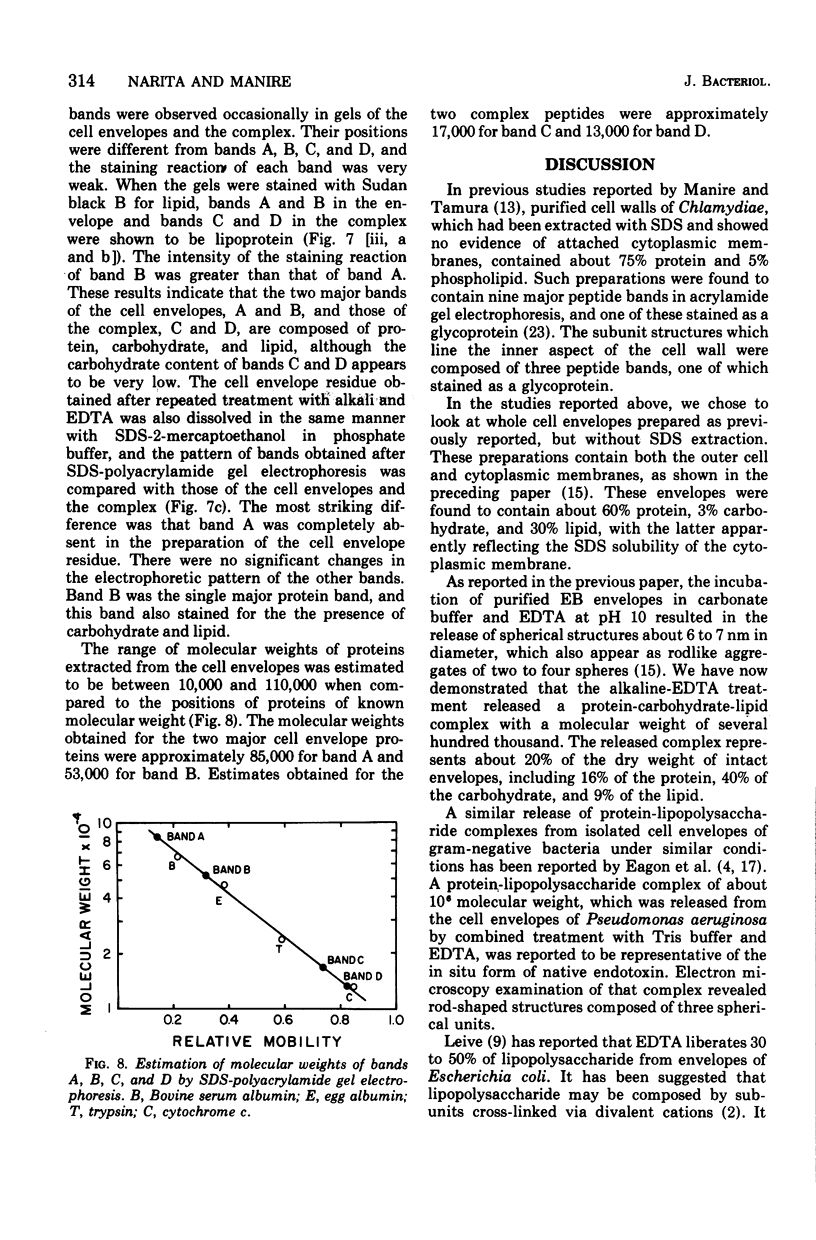


Images in this article
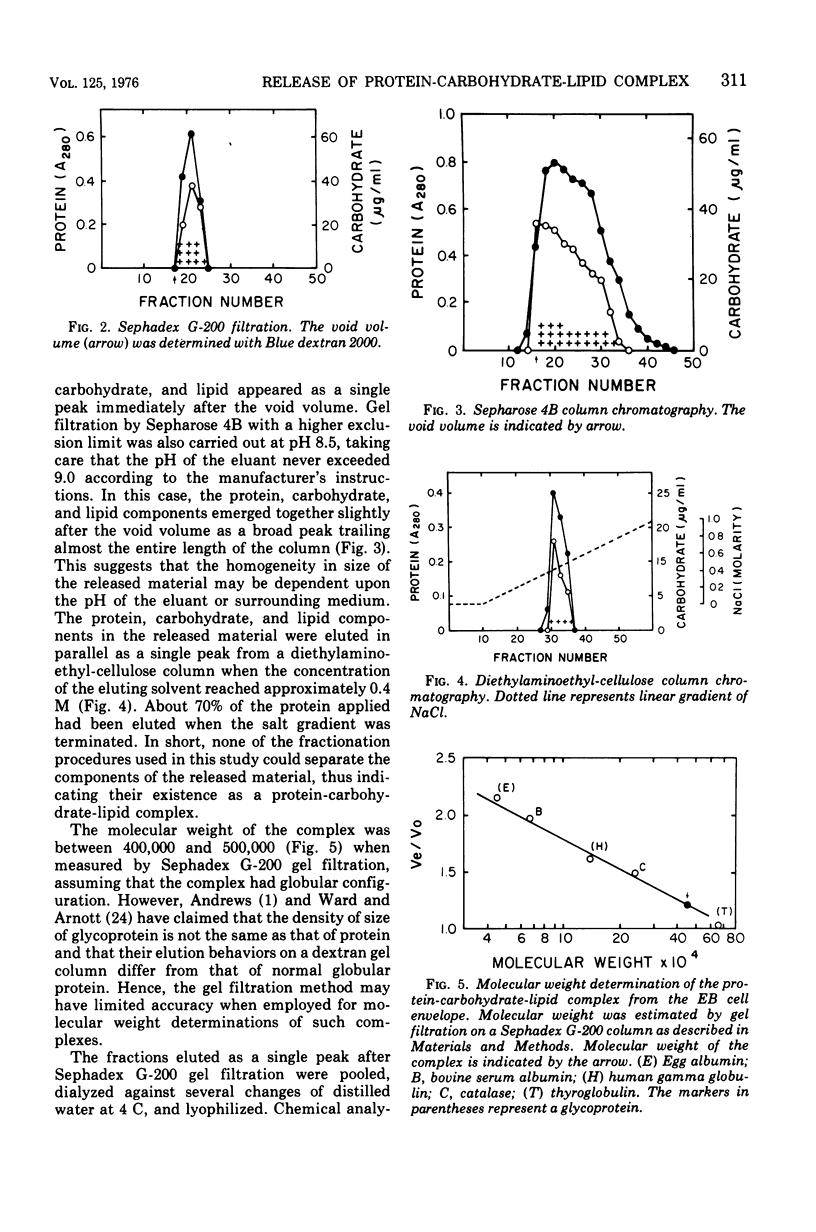
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbell M. A., Eagon R. G. Role of Multivalent Cations in the Organization, Structure, and Assembly of the Cell Wall of Pseudomonas aeruginosa. J Bacteriol. 1966 Aug;92(2):380–387. doi: 10.1128/jb.92.2.380-387.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Cox S. T., Jr, Eagon R. G. Action of ethylenediaminetetraacetic acid, tris(hydroxymethyl)-aminomethane, and lysozyme on cell walls of Pseudomonas aeruginosa. Can J Microbiol. 1968 Aug;14(8):913–922. doi: 10.1139/m68-153. [DOI] [PubMed] [Google Scholar]
- Inouye M., Yee M. L. Homogeneity of envelope proteins of Escherichia coli separated by gel electrophoresis in sodium dodecyl sulfate. J Bacteriol. 1973 Jan;113(1):304–312. doi: 10.1128/jb.113.1.304-312.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobylka D., Khettry A., Shin B. C., Carraway K. L. Proteins and glycoproteins of the erythrocyte membrane. Arch Biochem Biophys. 1972 Feb;148(2):475–487. doi: 10.1016/0003-9861(72)90166-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Manire G. P., Tamura A. Preparation and chemical composition of the cell walls of mature infectious dense forms of meningopneumonitis organisms. J Bacteriol. 1967 Oct;94(4):1178–1183. doi: 10.1128/jb.94.4.1178-1183.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Manire G. P. Electron Microscopic Observations on the Fine Structure of Cell Walls of Chlamydia psittaci. J Bacteriol. 1970 Dec;104(3):1332–1337. doi: 10.1128/jb.104.3.1332-1337.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita T., Wyrick P. B., Manire G. P. Effect of alkali on the structure of cell envelopes of Chlamydia psittaci elementary bodies. J Bacteriol. 1976 Jan;125(1):300–307. doi: 10.1128/jb.125.1.300-307.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Rogers S. W., Gilleland H. E., Jr, Eagon R. G. Characterization of a protein-lipopolysaccharide complex released from cell walls of Pseudomonas aeruginosa by ethylenediaminetetraacetic acid. Can J Microbiol. 1969 Jul;15(7):743–748. doi: 10.1139/m69-130. [DOI] [PubMed] [Google Scholar]
- Rottem S., Razin S. Electrophoretic patterns of membrane proteins of Mycoplasma. J Bacteriol. 1967 Aug;94(2):359–364. doi: 10.1128/jb.94.2.359-364.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Examination of the protein composition of the cell envelope of Escherichia coli by polyacrylamide gel electrophoresis. J Bacteriol. 1970 Nov;104(2):882–889. doi: 10.1128/jb.104.2.882-889.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Stinnett J. D., Gilleland H. E., Jr, Eagon R. G. Proteins released from cell envelopes of Pseudomonas aeruginosa on exposure to ethylenediaminetetraacetate: comparison with dimethylformamide-extractable proteins. J Bacteriol. 1973 Apr;114(1):399–407. doi: 10.1128/jb.114.1.399-407.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Tanaka A., Manire G. P. Separation of the polypeptides of Chlamydia and its cell walls by polyacrylamide gel electrophoresis. J Bacteriol. 1974 Apr;118(1):139–143. doi: 10.1128/jb.118.1.139-143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD D. N., ARNOTT M. S. GEL FILTRATION OF PROTEINS, WITH PARTICULAR REFERENCE TO THE GLYCOPROTEIN, LUTEINIZING HORMONE. Anal Biochem. 1965 Aug;12:296–302. doi: 10.1016/0003-2697(65)90094-1. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]