Abstract
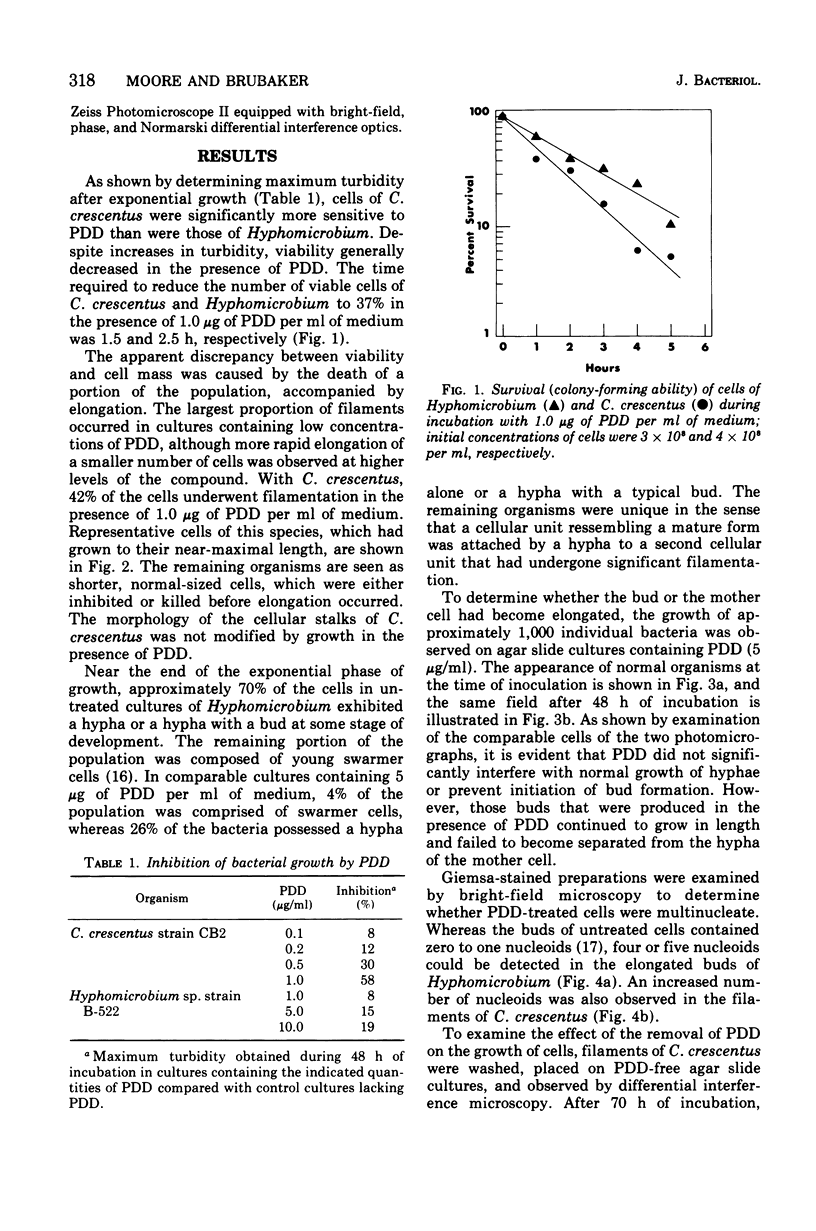
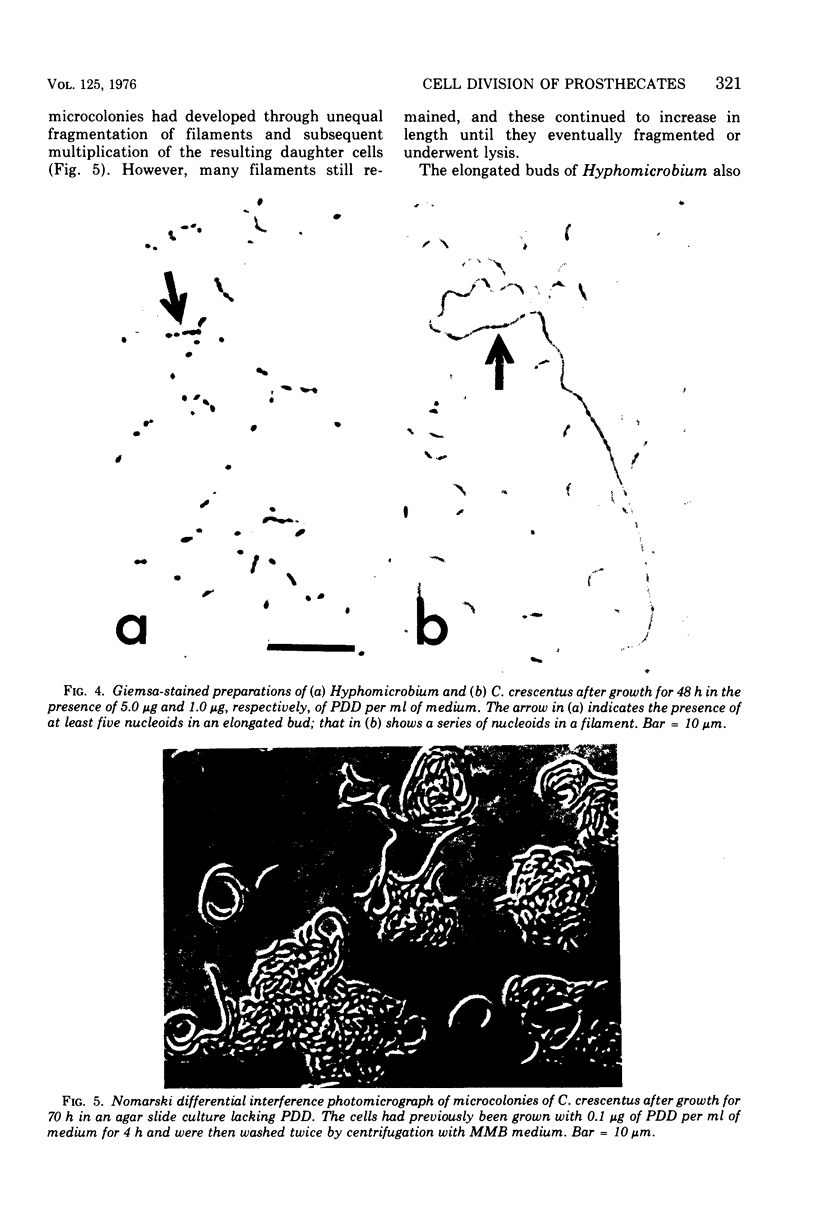
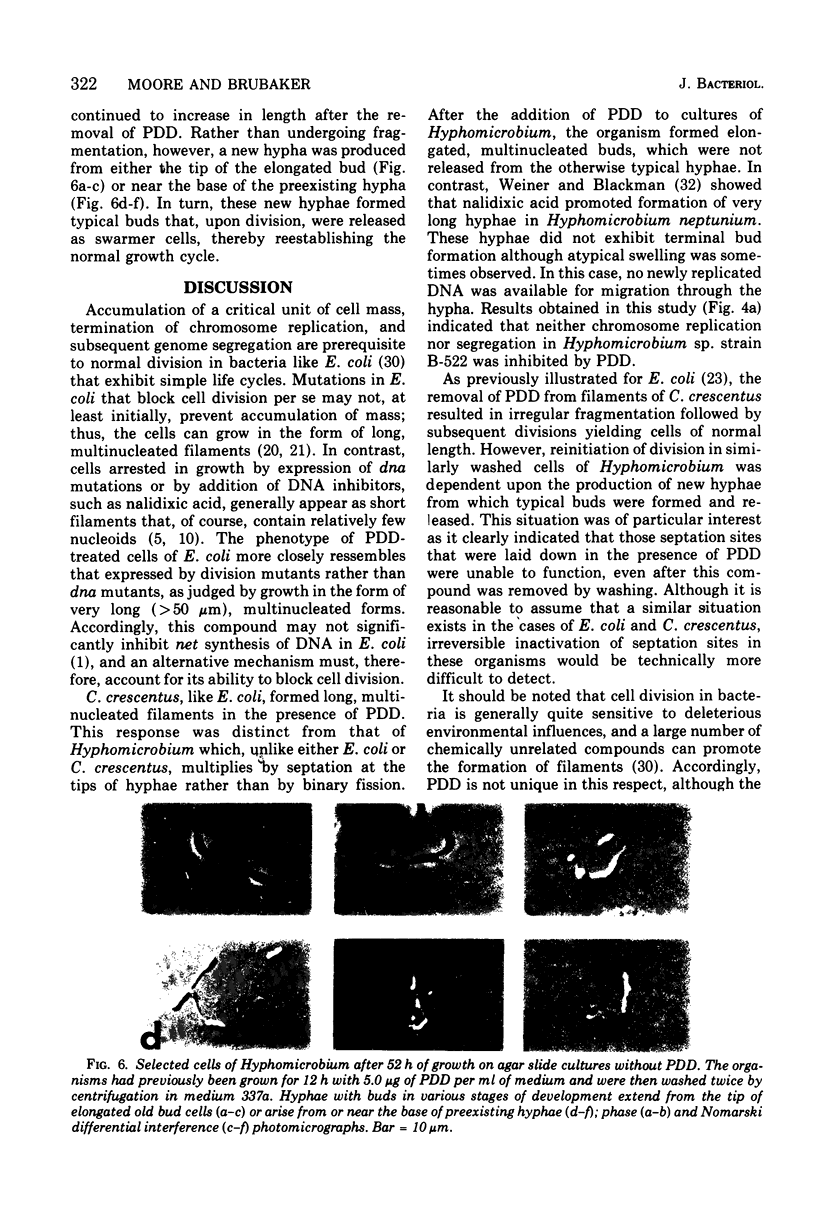
Low concentrations of the radiomimetic agent cis-platinum(II)diamminodichloride (PDD) inhibited cell division in Caulobacter crescentus (0.1 mug/ml) and Hyphomicrobium sp. strain B-522 (1.0 mug/ml) without altering the length of prosthecae. After exposure, cells of C. crescentus appeared as long filaments, whereas only the bud portion of Hyphomicrobium underwent elongation. PDD-treated cells of both species were multinucleated. After the removal of PDD by washing, filaments of C. crescentus fragmented unequally and then normal growth resumed. In Hyphomicrobium (where division involves release of swarmer cells that arise as buds on the distal ends of hyphae), potential septation sites formed in the presence of PDD remained inactive after washing. Reinitiation of cell division in this species was dependent upon the synthesis of new hyphae that could arise from either end of the elongated bud. This finding suggests that the PDD-induced lesion at a given septation site is irreversible and, upon removal of this compound, alternate sites must be synthesized for the subsequent occurrence of cell division.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck D. J., Brubaker R. R. Effect of cis-platinum(II)diamminodichloride on wild type and deoxyribonucleic acid repair deficient mutants of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1247–1252. doi: 10.1128/jb.116.3.1247-1252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck D. J., Brubaker R. R. Mutagenic properties of cis-plantinum(II)diammino-dichloride in Escherichia coli. Mutat Res. 1975 Feb;27(2):181–189. doi: 10.1016/0027-5107(75)90077-9. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. [Prophage induction and cell division in E. coli. II. Linked (recA, zab) and unlinked (lex) suppressors of tif-1-mediated induction and filamentation]. Mol Gen Genet. 1972;119(2):153–174. doi: 10.1007/BF00269134. [DOI] [PubMed] [Google Scholar]
- Clark D. J. Regulation of deoxyribonucleic acid replication and cell division in Escherichia coli B-r. J Bacteriol. 1968 Oct;96(4):1214–1224. doi: 10.1128/jb.96.4.1214-1224.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S. Repair of DNA containing interstrand crosslinks in Escherichia coli: sequential excision and recombination. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1064–1068. doi: 10.1073/pnas.70.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnen S. T., Newton A. Chromosome replication during development in Caulobacter crescentus. J Mol Biol. 1972 Mar 14;64(3):671–680. doi: 10.1016/0022-2836(72)90090-3. [DOI] [PubMed] [Google Scholar]
- Harder H. C., Rosenberg B. Inhibitory effects of anti-tumor platinum compounds on DNA, RNA and protein syntheses in mammalian cells in virtro. Int J Cancer. 1970 Sep 15;6(2):207–216. doi: 10.1002/ijc.2910060207. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Horácek P., Drobník J. Interaction of cis-dichlorodiammineplatinum (II) with DNA. Biochim Biophys Acta. 1971 Dec 16;254(2):341–347. doi: 10.1016/0005-2787(71)90842-2. [DOI] [PubMed] [Google Scholar]
- Howle J. A., Gale G. R. Cis-dichlorodiammineplatinum (II). Persistent and selective inhibition of deoxyribonucleic acid synthesis in vivo. Biochem Pharmacol. 1970 Oct;19(10):2757–2762. doi: 10.1016/0006-2952(70)90102-4. [DOI] [PubMed] [Google Scholar]
- Howle J. A., Thompson H. S., Stone A. E., Gale G. R. Cis-dichlorodiammineplatinum [II]: inhibition of nucleic acid synthesis in lymphocytes stimulated with phytohemagglutinin. Proc Soc Exp Biol Med. 1971 Jul;137(3):820–825. doi: 10.3181/00379727-137-35675. [DOI] [PubMed] [Google Scholar]
- Moore R. L., Hirsch P. Deoxyribonucleic acid base sequence homologies of some budding and prosthecate bacteria. J Bacteriol. 1972 Apr;110(1):256–261. doi: 10.1128/jb.110.1.256-261.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. L., Hirsch P. First generation synchrony of isolated Hyphomicrobium swarmer populations. J Bacteriol. 1973 Oct;116(1):418–423. doi: 10.1128/jb.116.1.418-423.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. L., Hirsch P. Nuclear apparatus of Hyphomicrobium. J Bacteriol. 1973 Dec;116(3):1447–1455. doi: 10.1128/jb.116.3.1447-1455.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mound D. W., Walker A. C., Kosel C. Suppression of lex mutations affecting deoxyribonucleic acid repair in Escherichia coli K-12 by closely linked thermosensitive mutations. J Bacteriol. 1973 Nov;116(2):950–956. doi: 10.1128/jb.116.2.950-956.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBERG B., VANCAMP L., KRIGAS T. INHIBITION OF CELL DIVISION IN ESCHERICHIA COLI BY ELECTROLYSIS PRODUCTS FROM A PLATINUM ELECTRODE. Nature. 1965 Feb 13;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Clark D. J. Cell division of Escherichia coli BUG-6: effect of varying the length of growth at the nonpermissive temperature. J Bacteriol. 1972 Apr;110(1):117–121. doi: 10.1128/jb.110.1.117-121.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. J., Pascoe J. M. Cross-linking of complementary strands of DNA in mammalian cells by antitumour platinum compounds. Nature. 1972 Feb 4;235(5336):282–284. doi: 10.1038/235282a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg B., Renshaw E., Vancamp L., Hartwick J., Drobnik J. Platinum-induced filamentous growth in Escherichia coli. J Bacteriol. 1967 Feb;93(2):716–721. doi: 10.1128/jb.93.2.716-721.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg B., Van Camp L., Grimley E. B., Thomson A. J. The inhibition of growth or cell division in Escherichia coli by different ionic species of platinum(IV) complexes. J Biol Chem. 1967 Mar 25;242(6):1347–1352. [PubMed] [Google Scholar]
- Rosenberg B., VanCamp L. The successful regression of large solid sarcoma 180 tumors by platinum compounds. Cancer Res. 1970 Jun;30(6):1799–1802. [PubMed] [Google Scholar]
- Rosenberg B., VanCamp L., Trosko J. E., Mansour V. H. Platinum compounds: a new class of potent antitumour agents. Nature. 1969 Apr 26;222(5191):385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- Schmidt J. M. Prosthecate bacteria. Annu Rev Microbiol. 1971;25:93–110. doi: 10.1146/annurev.mi.25.100171.000521. [DOI] [PubMed] [Google Scholar]
- Schmidt J. M., Stanier R. Y. The development of cellular stalks in bacteria. J Cell Biol. 1966 Mar;28(3):423–436. doi: 10.1083/jcb.28.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M., Schaechter M. Control of cell division in bacteria. Bacteriol Rev. 1974 Jun;38(2):199–221. doi: 10.1128/br.38.2.199-221.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner R. M., Blackman M. A. Inhibition of deoxyribonucleic acid synthesis and bud formation by nalidixic acid in Hyphomicrobium neptunium. J Bacteriol. 1973 Dec;116(3):1398–1404. doi: 10.1128/jb.116.3.1398-1404.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]