Abstract
A mammalian recombinant strategy was established to dissect rules of basement membrane laminin assembly and secretion. The α-, β-, and γ-chain subunits of laminin-1 were expressed in all combinations, transiently and/or stably, in a near-null background. In the absence of its normal partners, the α chain was secreted as intact protein and protein that had been cleaved in the coiled-coil domain. In contrast, the β and γ chains, expressed separately or together, remained intracellular with formation of ββ or βγ, but not γγ, disulfide-linked dimers. Secretion of the β and γ chains required simultaneous expression of all three chains and their assembly into αβγ heterotrimers. Epitope-tagged recombinant α subunit and recombinant laminin were affinity-purified from the conditioned medium of αγ and αβγ clones. Rotary-shadow electron microscopy revealed that the free α subunit is a linear structure containing N-terminal and included globules with a foreshortened long arm, while the trimeric species has the typical four-arm morphology of native laminin. We conclude that the α chain can be delivered to the extracellular environment as a single subunit, whereas the β and γ chains cannot, and that the α chain drives the secretion of the trimeric molecule. Such an α-chain-dependent mechanism could allow for the regulation of laminin export into a nascent basement membrane, and might serve an important role in controlling basement membrane formation.
Keywords: basement membrane, recombinant protein, coiled-coil
Basement membranes are cell-associated heteropolymers that are essential for tissue development and maintenance. The functions of these extracellular matrices are both architectural and informational, with basement membranes acting as substratum, filter, and solid-phase agonist (1–3). Major components of these matrices are the different members of the laminin glycoprotein family, each a heterotrimer assembled from α-, β-, and γ-chain subunits joined through a long arm coiled-coil domain (4), each with shared and unique functions, and each with its own spatial and temporal expression pattern.
An understanding of subunit chain assembly and secretion is important for an understanding of the regulation of isoform induction and chain switching during development and repair. While there is information on the physical chemical interactions that contribute to coiled-coil formation (5–10) in vitro, little is known about the rules of chain assembly and secretion in vivo. In this study, we developed and applied a strategy to dissect laminin assembly and secretion that allowed us to selectively express all three subunits in a near-null background. We studied laminin 1 (α1β1γ1), the most extensively characterized isoform, whose activities are dependent on correct post-translational modifications, subunit association, and domain folding (5, 11–14). We report the surprising finding that the α-chain subunit can be secreted without its subunit partners. In contrast to previous models, we find that two-chain laminins cannot be secreted even though, in certain combinations, they can assemble. We also find that α-chain expression and assembly are essential for secretion of the other subunits. We therefore propose that the α chain drives the secretion of its β- and γ-chain partners, a mechanism that might be used to regulate basement membrane formation.
MATERIALS AND METHODS
DNA Constructions and Analysis.
(i) A 5.7-kb DNA fragment containing the full-length mouse laminin β1 cDNA (15) was isolated from a pUC19-β1 plasmid, and XhoI linkers were added to both ends. The fragment was then inserted into the pCIS vector at the XhoI site. A 7.6-kb DNA fragment containing the full-length mouse laminin γ1 cDNA (16) in pUC-19 was excised with KpnI, blunted, and ligated into a baculovirus expression vector pVL1393 that had been previously digested with EcoRI and blunted (pVL1393-B2). A fragment bearing the complete ORF was then isolated from pVL1393-B2 following digestion with SalI and inserted into the mammalian pCIS vector after linearization with XhoI. pCIS containing a full-length mouse laminin α-chain cDNA (17) was prepared as described (13). To facilitate recombinant protein purification, the α-chain cDNA was modified to contain a nucleotide sequence encoding the FLAG epitope, an octapeptide with the amino acid sequence DYKDDDDK. Laminin α cDNA with the FLAG sequence at the C terminus was prepared from α1-pCIS by replacing a DNA fragment starting from the last XmaI site in the coding region of the α1 cDNA to its end, with a synthetic double-stranded DNA fragment containing the 3′ end of the α1cDNA and FLAG sequence immediately preceding the natural stop codon (5′-CCGGGCCTGAGCCTGACTACAAGGACGACGATGACAAGTAATTAAT-3′). A construct of a full-length mouse laminin α cDNA with the FLAG sequence at the N terminus was also prepared by using a plasmid vector derived from pRc/CMV (InVitrogen) containing a BM40 (SPARC) signal peptide and FLAG sequences (Billy Hudson, Kansas University Medical Center, Kansas City). (ii) Total RNA isolation and reverse transcriptase (RT)-PCR were performed according to standard procedures (18).
Transfection and Cloning of Human Cells.
(i) Transfection of human embryonic kidney 293 cells (adenovirus transformed, ATCC CRL 1573), maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, was carried out by calcium phosphate precipitation as previously described (13). Some studies were conducted after transient transfection with one, or simultaneously with two or three, laminin cDNAs. Secreted protein, screened with anti-E8 antibody, was precipitated from serum-free conditioned medium, and intracellular soluble protein was obtained from cell lysates prepared by freeze–thawing of cells. (ii) To obtain clones of cells stably expressing a laminin chain, cells were cotransfected with laminin cDNA and an antibiotic-resistance vector (puromycin, pSV-2pac; G418, pSV-2neo; hygromycin, JD214). To obtain cells stably expressing two or three laminin chains, cells were either cotransfected with two laminin cDNAs under a single antibiotic selection, or sequentially transfected with one chain cDNA under a single antibiotic selection, cloned, and then transfected with the other cDNA under a different antibiotic selection. The following cytotoxic levels were determined for 293 cells and were maintained in culture medium of transfected cells, starting 2–3 days after transfection: 1 μg/ml puromycin, 500 μg/ml G418, and/or 100 μg/ml hygromycin.
Protein Purification, Antibodies, SDS/PAGE, Protein Immunoblotting, and Electron Microscopy.
(i) Laminin-1 (EHS) was purified as described (12). FLAG-tagged recombinant protein was purified by monoclonal anti-FLAG M2 affinity chromatography (Eastman Kodak Scientific Imaging Systems, New Haven, CT) and elution with FLAG peptide, as described in the manufacturer’s instructions. (ii) Polyclonal antibodies specific for laminin fragment E8 (distal long arm and proximal G domain) and laminin fragment E4 (β1-chain domains VI-V) were generated by immunizing rabbits with the respective fragments, followed by affinity purification on a Sepharose CL-4B column coupled to the immunizing protein. Fragment-specific antibodies were then cross-absorbed against E3 (distal moiety of the C-terminal G domain and E1′ (short arm fragment with α and γ short arms), respectively. E8-specific antibody reacted with the C-terminal moieties of all three chains in Western blots. Anti-E8 specific for the βγ chains (anti-E8-βγ) was prepared by affinity chromatography on immobilized E8-βγ chains and then cross-absorbed against immobilized E8-α chain (11). E4-specific antibody was utilized to immunoprecipitate laminin through its N-terminal β chain. The antibody was found to react with E4, but not to react and precipitate E1′, E8, or E3. (iii) SDS/PAGE was carried out, unless otherwise indicated, in 3.5–12% linear gradient gels (9). Immunoblotting and rotary shadow electron microscopy were performed as described (12, 13). Immunoprecipitations were conducted by incubating 5 ml of conditioned medium with 10 μg/ml anti-E4 followed by 25 μl of protein A-Sepharose, or with 25 μl of anti-FLAG agarose beads. Beads were washed with PBS prior to SDS/PAGE.
RESULTS
Expression of Laminin Solitary α Chain and β and γ Chains.
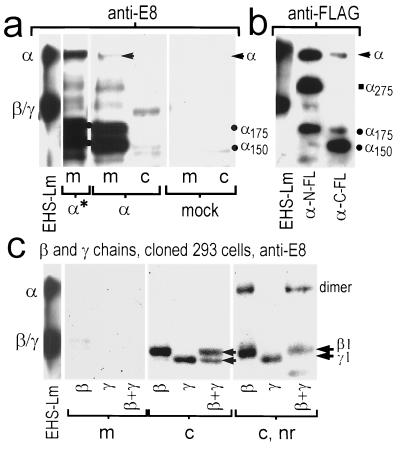
To study the fate of individual chains in the absence of their normal partners, we selected a transfectable cell line with low endogenous laminin chain expression. In initial evaluations of wild-type 293 cells no secreted laminin was detected in conditioned medium analyzed by SDS/PAGE and stained with Coomassie blue or in Western blots with anti-E8 antibody. Only trace levels of endogenous laminin β chain, confined almost entirely to the cell fraction, were detected with anti-E4 or when we overexposed immunoblots using anti-E8 antibody. Expression of the α chain alone (Fig. 1a) by cloned cells (secreted fraction) revealed a mixture of intact (≈380-kDa) and smaller species (principally 175 and 150 kDa). For α-transfected cells, the full-size α-chain band was detected as a minor species, with abundant amounts of the smaller species. The medium, in contrast to cell fraction, contained the great majority of the recombinant protein, indicating that most α-chain protein was secreted. The nature of the α chain cleaved products was analyzed by transiently expressing the α chain containing the FLAG epitope sequence at the N or C terminus, and detecting protein with FLAG-specific antibody (Fig. 1b). For N-terminal tagged α chain, bands migrating at ≈380, 275 (α275), and 175 (α175) kDa were detected. For C-terminal FLAG α chain, bands migrating at ≈380, 175, and 150 kDa (α150) were detected. Since the presence of FLAG epitope fixes the location of one end, and since the predicted protein mass of the α1 chain is ≈380 kDa with carbohydrate) it could be deduced that the cleavage site for all three faster migrating bands lies within the coiled-coil region of the α chain. Wild-type cells were both transiently and stably transfected with cDNA coding for the β and γ chains and evaluated in immunoblots (Fig. 1c and data not shown). Expression of prominent β and γ species, corresponding to the expected sizes of ≈200 kDa and ≈190 kDa, respectively, were detected with anti-E8 antibody under reducing conditions. In the case of cells either transiently or stably transfected with the β plus γ cDNAs, two anti-E8-reacting bands, migrating closely together, were observed. Regardless of whether cells expressed laminin β, γ, or β plus γ, all, or nearly all, of these protein chains were localized in the intracellular fraction. Under nonreducing conditions, what appears to be a little less than half of the β chain migrated slower at twice the molecular mass, reflecting the presence of some disulfide-linked dimer. In contrast, none of the γ chain, when expressed singly, was observed to form a dimer or dimers. For cells stably expressing both the β and γ chains, what appears to be the majority of the laminin chains migrated as a dimer under nonreducing conditions, with consumption of all of the monomeric γ-chain species, but with a fraction of remaining monomeric β chain. While the dimeric species might contain a mixture of ββ and βγ dimers, the higher ratio of monomer to dimer and absence of monomeric γ chain suggest that βγ dimers are favored over ββ dimers. Overall, these data suggest that the laminin β and γ chains, whether expressed singly or together, cannot be secreted and that ββ and βγ, but not γγ, dimers assemble in the intracellular compartment.
Figure 1.
Expression of laminin solitary α chain and β and γ chains in 293 cells. Human 293 cells were transfected with DNAs encoding mouse laminin β, γ, β + γ, or with carrier DNA (mock). Conditioned culture medium (secreted fraction, m) and soluble cell lysate (c) were collected and analyzed in immunoblots under reducing or nonreducing (lanes indicated with nr) conditions using anti-E8 or anti-FLAG antibodies. (a) Wild-type 293 cells were stably (α*) and transiently (α) transfected with α cDNA. Medium from cloned transfected cells revealed a mixture of intact (arrow), and 175-kDa/150-kDa (dots) laminin-α-reacting species. Intact α chain, most secreted, was detected after transient transfection; however, most of the transiently expressed protein was noted to migrate at 175 and 150 kDa, suggesting proteolytic processing. No laminin bands were detected after mock transfections. EHS-laminin standard is shown in the left lane (note that the β and γ chains of EHS laminin are not resolved due to carbohydrate micro-heterogeneity). (b) Laminin α-pCIS, modified to contain the FLAG sequence at the N or C terminus, was transiently expressed. The differential intensities of FLAG (FL) epitope reactivity (all bands reacted with anti-laminin, not shown) indicated that the 275-kDa, 175-kDa, and 150-kDa bands were degradation products containing either the N or C terminus, and with cleavage in the coiled-coil domain. (c) Cellular expression, but not secretion, of prominent β and γ chains, and low amounts of smaller (degraded) species, was observed. When analyzed under nonreducing conditions, β and βγ dimers, but not γγ dimers, were detected.
Expression of the α Chain with β and γ Chains.
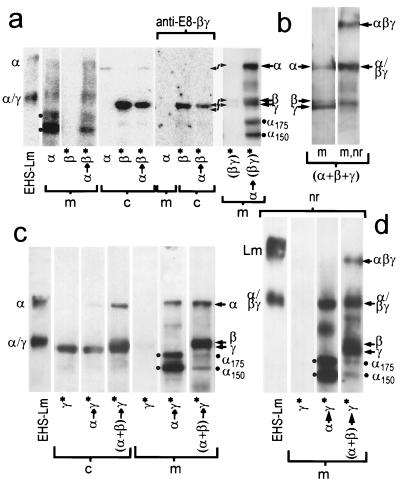
To determine whether the α-chain expression was required for β- and γ-chain assembly and secretion. α-pCIS was transfected into wild-type, β stable, γ stable, and βγ stable clones (Fig. 2). When the α chain was transiently expressed in β cells, the α chain was detected as a secreted product (medium) while the β chain was detected only in the cell lysate (Fig. 2a). The α chain could not drive secretion of the β chain, and there was no evidence for chain association. In contrast, when βγ-expressing cloned cells were transiently transfected with α DNA, intact α- and β/γ-chain secretion was detected, with increased full-size chain and a decreased fraction of α175 and α150 degradation products. When cells were simultaneously transfected under transient conditions with the α, β, and γ laminin cDNA constructs (Fig. 2b), three full-sized α, β, and γ chains were identified. In analysis under nonreducing conditions, a band was detected corresponding in migration to native disulfide-stabilized trimeric laminin. However, in some transfections, only the α chain with degraded bands was observed in medium (not shown). We considered the possibility that the transfection efficiencies varied from experiment to experiment such that in some cases most cells each expressed all three chains (with assembly and secretion), while in other cases different cells expressed the different chains (with assembly failure).
Figure 2.
Expression of the α chain with β and γ chains. (a) 293 cells, wild type and stably expressing β (β*), or both β and γ [(βγ)*], were transiently transfected with α-pCIS (arrow points to transfection target cell) and evaluated for recombinant protein production with anti-E8 (or, where indicated, the βγ-specific reagent). With α/β coexpression, the α chain was detected in the medium while all of the β chain was confined to the cell fraction. In contrast, α transfection of βγ-expressing cells resulted in secretion of all three chains and in an increase in the fraction of intact α-chain. (b) Wild-type 293 cells were simultaneously transiently transfected with the α, β, and γ cDNAs. Expression and secretion of all three chains, with little degraded product, was detected with anti-E8 antibody, and a fraction of the secreted protein migrated in the expected position of disulfide-linked trimeric laminin. (c and d) Laminin γ cells (γ*) were transiently transfected with α DNA or were cotransfected with α and β DNA. Under reducing conditions the γ chain remained intracellular when expressed alone or coexpressed with only α (α→γ*). However, when α was coexpressed with the β and γ chains [(α+β)→γ*)], a broad βγ complex (double arrows) was detected in medium along with intact α chain (single arrow). Again, the α chain was secreted regardless of whether the β and γ were expressed; however, the chain was largely intact (≈380 kDa) when all three chains were expressed, but considerably cleaved (two dots) when expressed only with the γ chain. Under nonreducing conditions (nr, d), a fraction of the α chain migrated similar to that trimeric native EHS-laminin only when all three chains were expressed, indicating some recombinant chain was disulfide-linked to its partners.
When the α chain was transiently expressed in γ-stable cells (Fig. 2c), the α chain was secreted while the γ chain remained within the cell. There was no evidence for the formation of stable αγ dimers. A small difference noted here is that the fraction of intact α chain was increased, possibly due to some protective effect of the intracellular γ chain (also observed in stable αγ clones). When stable γ cloned cells were cotransfected with the α- and β-chain cDNAs, expression of the three chains was detected. In this situation, a heavy band, interpreted as corresponding to incompletely resolved β and γ chain species, as well as intact α chain, could be detected in medium under reducing conditions. Under nonreducing conditions (Fig. 2d) a band migrating similar to authentic EHS laminin (αβγ) was now observed, in addition to antibody-reacting bands corresponding to the migration of disulfide-linked βγ dimers [seen also with the EHS control; positions as described (19)] and non-disulfide-linked dimers. Thus, expression of all three chains was required for assembly of disulfide-linked trimer and secretion of this trimer. Furthermore, some of the recombinant α chain was disulfide-linked to its βγ partners, and some non-disulfide-linked trimers appeared to have formed as well. Transfection of the γ cells with the α plus β chains resulted in a substantial reduction of cleaved α chain.
Characterization of Clones Expressing αγ and αβγ mRNA and Protein.
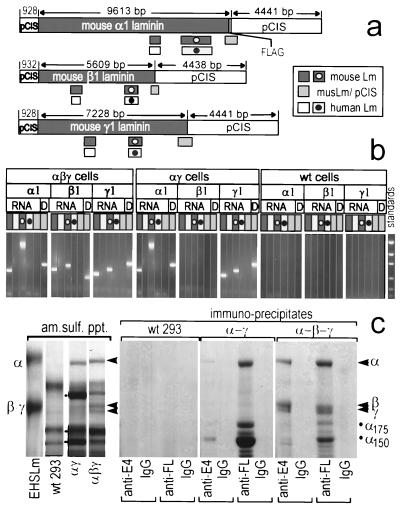
We considered the possibility that endogenous human mRNA/protein might make significant contributions to recombinant laminin assembly and secretion, particularly in the circumstance of two- and three-chain expression. Transfection of human cells with constructs containing mouse cDNAs permitted the use of RT-PCR primers that were both chain- and species-specific to demonstrate mRNA expression of the transfected chains and any endogenous human homologues. Different regions of each construct were chosen for amplification (Fig. 3 and data not shown). Primers specific to the pCIS construct backbone, both upstream (not shown) and downstream of each inserted cDNA, and to the inserted mouse cDNA demonstrated the presence of the construct in the transfected cells by PCR of genomic DNA. Furthermore, since the DNA from an integrated construct would provide an RT-PCR template identical to that of the reverse-transcribed mRNA transcript and be represented in high concentration in any contaminating genomic DNA of an RNA preparation, the same primer combinations were used as negative amplification controls to demonstrate that the product bands observed in the RT-PCR did not come from contaminating construct DNA. The 5′ and 3′ ends of the laminin cDNA of interest were also targeted for amplification. RT-PCR of the mouse αβγ-transfected cell lines clearly demonstrated the production of mouse α1, β1, and γ1 mRNA with no detectable levels of endogenous human α1, β1, or γ1 mRNA. The αγ-transfected cells showed production of mouse α1 and γ1. The absence of endogenous laminin mRNA, however, was not total. When a 4-fold higher level of total RNA per RT-PCR was used, trace amounts of endogenous human β1 in particular, but also, some γ1 and possibly α1, could now be detected (data not shown).
Figure 3.
mRNA and protein characterization of cells expressing αγ and αβγ chains. (a) RT-PCR amplification products from the ORF (dark gray bars) of mouse α1, β1, and γ1 cDNAs, from pCIS extending into the ORFs (light gray bars), and from corresponding human α1, β1, and γ1 chains (white bars) were prepared from αβγ, αβ, and wild-type (wt) cells, using either total RNA (DNase-treated) or genomic DNA (D) as template. The human primers amplified the expected products from human placental RNA but not from midterm mouse embryo RNA. Conversely, the mouse primers amplified the expected products from mouse RNA but not from human RNA (data not shown). (b) The 293 cell products were electrophoresed on agarose gels to analyze recombinant and endogenous chain-specific mRNA expression (coded bars matched to map; standards were 2.0, 1.2, 0.8, 0.4, and 0.2 kb). The pCIS-specific products revealed that recombinant DNAs are present and that the RNA was not contaminated with these DNAs. As expected, recombinant mouse chains were expressed in the αγ and αβγ clones and not in wild-type cells. (c) Coomassie blue-stained polyacrylamide gel, reducing conditions, showing corresponding immunoblots with anti-E8 from media of wild-type cells and cells stably expressing mouse laminin αγ chains and αβγ chains. Secreted proteins from the media were precipitated with ammonium sulfate or immunoprecipitated with anti-E4 (β1-specific) antibody, rabbit IgG control, anti-FLAG (FL) antibody, or mouse IgG control. EHS laminin 1 (5 μg) was used as standard.
Serum-free conditioned medium was obtained from γ cells either transfected with the α1-C-FLAG cDNA or cotransfected with the α- plus β-chain cDNAs and further cloned under double-antibiotic selection. Expressed protein was characterized by immunoprecipitation and Coomassie blue staining (Fig. 3c). As noted before with the α-transient/γ-stable cells, the α chain from the αγ cells was detected as a mixture of intact and cleaved product in medium while the γ-chain remained in the cell lysate. The γ chain was detected in the cell but not in conditioned medium. In marked contrast, the triple-chain-transfected cells secreted all three chains. When protein was ammonium sulfate precipitated from media conditioned by wild-type cells, αγ-transfected cells, and αβγ-transfected cells, no bands corresponding in migration to the laminin chains were seen in the wild-type medium by Coomassie blue staining. In contrast, an α band and darkly staining faster-migrating bands (indicated with dots adjacent to the third lane in the left part of Fig. 3c) were noted in medium conditioned by αγ cells. Most striking was medium from αβγ cells, in which prominent intact α, β, and γ chains were detected. Immunoprecipitation with anti-E4 and anti-FLAG antibody confirmed the nature of the bands in a Coomassie blue-stained gel. While anti-E4 antibody did not precipitate protein from wild-type conditioned medium, and precipitated minor bands from the αγ cell medium, it precipitated three major bands from the triple-chain-transfected medium with intact α, β, and γ migrations. Anti-FLAG antibody furthermore precipitated intact α and 175-kDa and 150-kDa degraded α bands from the αγ cells, but no β or γ chain could be seen. In contrast, anti-FLAG immunoprecipitates of the αβγ cells contained major bands corresponding in migration to the α, β, and γ chains plus considerably reduced amounts (compared with αγ cells) of the α-chain degradation products. The interpretation of the chains in the ammonium sulfate precipitates and immunoprecipitates was confirmed in Western immunoblots (not shown), and we calculated that the medium contained ≈1 μg/ml recombinant protein.
Characterization of Recombinant Laminin α Subunit and Recombinant Laminin Structures.
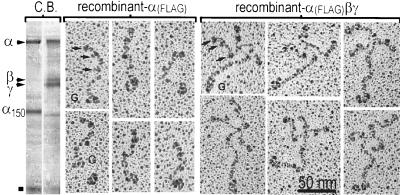
Conditioned medium from αγ stably transfected cells was purified by FLAG affinity chromatography and analyzed by SDS/PAGE (Fig. 4 Left, Coomassie blue stained). The free α-chain protein from the αγ cells consisted of intact α and the α150 fragment (and a band migrating at ≈66 kDa, indicated with ▪, probably medium albumin). The electron microscopic appearance of the α-chain preparation from the αγ cells was strikingly different from that of trimeric laminin in that there were a great number of flexible linear rods bearing globular domains. These rods, ≈80 nm in length, contained a large elongated globule at one end and three smaller globules at the other end, spaced apart such that the most included globule was 40–50 nm along the length of the rod. The larger globule corresponded to G domain, and the rod segment with globules corresponded to the α-chain short arm, given its similarity with the α-chain short arm in native laminin (13). Since 80 nm is shorter than the combined length of the α-chain short and long arms (≈120 nm), it seems likely that the coiled region of the recombinant α chain is in a compact self-folded state. Such a thickened single chain coiled-coil region is not, in contrast, observed with the β chain (20). A second population of molecules, approximately equal to the first in number, and consisting of globular structures (15–20 nm dimension), sometimes comma-shaped, was noted as well (not shown). These were identical in appearance to recombinant distal α1 chain containing the distal portion of the coiled-coil and G domains (11) and were interpreted as representing the C-terminal 150-kDa degradation product. The recombinant laminin was observed to consist of intact α chain, β chain, and γ chain by SDS/PAGE (Fig. 4 Right). The αβγ chain preparation, by rotary-shadow electron microscopy, was seen to consist of fields of cruciform structures whose morphology could not be distinguished from that of native laminin.
Figure 4.
Rotary-shadowed electron micrographs of recombinant laminin α subunit and heterotrimeric laminin 1. Secreted FLAG-tagged recombinant α chain and laminin were affinity purified, rotary shadowed, analyzed in Coomassie blue (C.B.)-stained gels (Left), and examined by electron microscopy (Center and Right). The free α chain (Center) was a linear structure with a normal short arm (arrows indicate globules) and shortened long arm terminating in G domain. The fully recombinant αβγ chain complex (Right) had the typical appearance of laminin.
DISCUSSION
Laminin 1, like the other members of the laminin family, is a large and complex multidomain glycoprotein assembled from three different subunits. Through a suitable choice of mammalian cell recipient, expression vector, epitope tag, and sequential transfection protocol, we established a novel strategy to express different combinations of recombinant laminin chains in a near-null background and therefore were able to dissect rules of association and export to the extracellular space. From these studies with 293 cells, and interpreted in the context of earlier work, a working hypothesis of assembly/secretion was developed (Fig. 5). When expressed alone, the α chain was found to be secreted as a monomer. Purified protein was visualized by electron microscopy as linear molecules containing a normal α-chain short arm connected to a foreshortened long arm-like structure terminating in a G domain. Some of this protein was cleaved within the coiled-coil region, probably due to exposure of part of the normally buried heptad sequence to intracellular and perhaps extracellular proteases. The β and γ chains, whether expressed singly or together, were not secreted and remained intracellular. The β chain formed intracellular dimers, whereas the γ chain did not. When both chains were expressed, βγ dimers formed and appeared to be preferred. The α chain did not drive secretion of the γ chain in the absence of the β chain, and similarly did not drive secretion of the β chain in the absence of the γ chain. However, if β and γ chain were expressed, then all three assembled into a trimer which was secreted into the medium, accompanied by a substantial decrease of α-chain degradation.
Figure 5.
Working hypothesis of laminin subunit assembly and secretion. Expression by 293 cells of laminin β or γ chain alone results in intracellular retention of non-disulfide-linked chain with dimerization of the β chain, but not γ chain. β and γ coexpression results in intracellular retention of heterodimers. Expression of α chain results in secretion of monomeric chain with partial proteolytic cleavage: assembly with β without γ, or γ without β, does not occur. Coexpression of all three chains results in secretion of intact trimeric laminin. The α chain, the only chain which can be secreted free, drives secretion of paired βγ chains.
The coiled-coil domain of the long arm is crucial for assembly of the three chains of laminin (5–10). Disulfide bonds bridge and stabilize all three chains in the most proximal region and join the β and γ chains in the most distal region of the long arm. Biosynthetic labeling of F9 cells revealed the presence of a βγ intermediate during laminin synthesis (16), and in vitro studies conducted with C-terminal coiled-coil segments of the three chains revealed that the β and γ chain segments form coiled-coil dimers which can reorganize to form αβγ segment heterotrimers upon addition of the α-chain fragment (7–10). In studies using synthetic and prokaryotic recombinant fragments, it furthermore was found that both ββ and βγ dimers can form, with the later preferred over the former (9, 10, 21). In addition, weaker homo-associations of α and γ chains could be detected (21). The apparent decrease of chain specificity was attributed to the relatively short length of the protein with the expectation this would not be the case for the full-length chains. It was therefore a surprise to discover that ββ as well as βγ dimers assemble in vivo, and that even for full-length chains, the heterodimeric intermediate is favored, albeit not strongly. However, the other in vitro associations were not found in the transfected cells, and our data show that cellular export of β and γ chains and their complexes lacking the α chain is forbidden in 293 cells.
Our analysis has shown that the α chain can be expressed and secreted as a single chain and that, once associated with the β and γ chains, it can drive their secretion as well. This suggests that the α chain possesses properties unique to its sequence and structure that are not present in its chain partners. The determinants that prevent secretion in the β and γ chains are unlikely to lie within the proximal short arms, since recombinant β1(VI-IV) is secreted by 293 cells (unpublished observations) and it seems more likely that the impediment to β and γ secretion lies in the region of the coiled-coil itself. While still an open question, it is possible to at least identify candidate laminin α-chain secretion determinants. In addition to the α chain being the largest of the three chains, it is the most basic, particularly in the G domain (12). The α chain also appears to be the most highly glycosylated (5, 22), with many of these sites lying within the coiled-coil. One possibility is that the carbohydrate structure uniquely present in the α chain, and in particular in the N-terminal moiety of the coiled-coil, permits its secretion. While one might suspect that these bulky carbohydrate groups might also affect coiling, self-assembly studies using C-terminal peptides lacking carbohydrate suggest that carbohydrate is not a major determinant in this regard (21). The same full-length α cDNA, inserted into baculovirus, resulted in α chain expression but failed to result in secretion following infection of Sf9 insect cells (data not shown). In contrast, these same cells expressed and secreted recombinant α G domain with or without the distal (C-terminal) 2/5 of the coiled-coil sequence (11, 12). These insect cells possess the necessary post-translational enzymes required for disulfide isomerization and addition of N-linked carbohydrate, but they lack the ability to convert the mannose-type oligosaccharide to complex oligosaccharide (11). We suggest that the complex carbohydrate of the α chain is one determinant of cellular export of free α chain and assembled laminin.
We have observed that the β and γ chains require α-chain co-expression for their secretion. There is, furthermore, evidence to suggest that the phenomenon is not confined to 293 cells (23–26). Suppression of laminin α-chain expression in epithelial cells grown on fibroblasts resulted in ablation of laminin secretion with intracellular accumulation of the β and γ chains (24), and the laminin α1 chain appeared to be the limiting factor in the secretion of β and γ chains in adrenal cortical cells, with intracellular retention of βγ chains expressed in molar excess (26). These findings make it unlikely, as once proposed, that there exist laminins that lack an α chain (23). An unexpected and contrasting finding of the current study was that the α chain, expressed in the absence of its normal β- and γ-chain partners, was secreted as a free monomer. We would predict this event when the intracellular α-chain concentration exceeds that of available βγ complex, raising the possibility that tissue cells may, under some circumstances, secrete free α chain. If so, the molecules would have a considerably different set of functions from that of laminin. Our findings also suggest that whole laminin secretion may be controlled by the α-chain gene product. It is interesting to note that over a decade ago Cooper and MacQueen (27) proposed that the α chain might regulate the extracellular secretion and basement membrane accumulation of laminin; they found that the α chain of laminin was expressed before the β and γ chains at the 16-cell morula stage, when extracellular laminin first appeared. In adrenal cortex, corticotropin (ACTH) was found to up-regulate laminin-1 secretion by inducing α-chain synthesis in the presence of a constant intracellular molar excess of β and γ chains (26). Because laminin extracellular accumulation precedes other basement membrane components in some developmental stages (28), we speculate that regulation of the α chain may be used to trigger basement membrane assembly.
In conclusion, we have applied a recombinant strategy to the analysis of laminin assembly and secretion, revealing new rules of assembly as well as confirming some, but not all, conclusions based on in vitro studies. We have also shown that it is possible to generate biochemical quantities of a fully recombinant trimeric extracellular matrix molecule for study. This is, as far as we can determine, the first time that such a multisubunit extracellular glycoprotein has been assembled or handled as a recombinant entity. From earlier efforts, we concluded that these goals could not be achieved with prokaryotic or baculovirus expression systems: these other systems appeared to be unable to provide the necessary post-translational modifications needed for secretion. The mammalian expression strategy we employed overcame these difficulties and now can be exploited to further analyze assembly as well as to elucidate and map the different functions of laminin isoforms which are largely unavailable as isolated components. It furthermore opens an avenue to dissect the molecular bases of normal laminin functions and to evaluate altered functions in laminins bearing mutations as found in such disorders as the merosin-deficient congenital muscular dystrophies.
Acknowledgments
We thank Genentech for providing pCIS. This study was supported by Grant R01-DK36425 (P.D.Y.) from the National Institutes of Health, a technical development grant from BioStratum (P.D.Y.), and a grant from the American Heart Association (J.J.O.).
ABBREVIATION
- RT
reverse transcriptase
References
- 1.Yurchenco P D. In: Extracellular Matrix Assembly and Structure. Yurchenco D P, Birk D, Mecham R, editors. New York: Academic; 1994. pp. 351–388. [Google Scholar]
- 2.Timpl R, Brown J C. BioEssays. 1996;18:123–132. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- 3.Engvall E, Wewer U M. J Cell Biochem. 1996;61:493–501. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C493::AID-JCB2%3E3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 4.Burgeson R E, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinman H, Martin G R, Meneguzzi G, Paulsson M, Sanes J, Timpl R, Tryggvason K, Yamada Y, Yurchenco P D. Matrix Biol. 1994;14:209–211. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 5.Beck K, Hunter I, Engel J. FASEB J. 1990;4:148–160. doi: 10.1096/fasebj.4.2.2404817. [DOI] [PubMed] [Google Scholar]
- 6.Beck K, Dixon T W, Engel J, Parry D A D. J Mol Biol. 1993;231:311–323. doi: 10.1006/jmbi.1993.1284. [DOI] [PubMed] [Google Scholar]
- 7.Hunter I, Schulthess T, Bruch M, Beck K, Engel J. Eur J Biochem. 1990;188:205–211. doi: 10.1111/j.1432-1033.1990.tb15391.x. [DOI] [PubMed] [Google Scholar]
- 8.Hunter I, Schulthess T, Engel J. J Biol Chem. 1992;267:6006–6011. [PubMed] [Google Scholar]
- 9.Utani A, Nomizu M, Timpl R, Roller P P, Yamada Y. J Biol Chem. 1994;269:19167–19175. [PubMed] [Google Scholar]
- 10.Nomizu M, Utani A, Beck K, Otaka A, Roller P P, Yamada Y. Biochemistry. 1996;35:2885–2893. doi: 10.1021/bi951555n. [DOI] [PubMed] [Google Scholar]
- 11.Sung U, O’Rear J J, Yurchenco P D. J Cell Biol. 1993;123:1255–1268. doi: 10.1083/jcb.123.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yurchenco P D, Cheng Y-S. J Biol Chem. 1993;268:17286–17299. [PubMed] [Google Scholar]
- 13.Colognato-Pyke H, O’Rear J J, Yamada Y, Carbonetto S, Cheng Y-S, Yurchenco P D. J Biol Chem. 1995;270:9398–9406. doi: 10.1074/jbc.270.16.9398. [DOI] [PubMed] [Google Scholar]
- 14.Brandenberger R, Kammerer R A, Engel J, Chiquet M. J Cell Biol. 1996;135:1583–1592. doi: 10.1083/jcb.135.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki M, Kato S, Kohno K, Martin G R, Yamada Y. Proc Natl Acad Sci USA. 1987;84:935–939. doi: 10.1073/pnas.84.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki M, Yamada Y. J Biol Chem. 1987;262:17111–17117. [PubMed] [Google Scholar]
- 17.Sasaki M, Kleinman H K, Huber H, Deutzmann R, Yamada Y. J Biol Chem. 1988;263:16536–16544. [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 19.Morita A, Sugimoto E, Kitagawa Y. Biochem J. 1985;229:259–264. doi: 10.1042/bj2290259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pikkarainen T, Schulthess T, Engel J, Tryggvason K. Eur J Biochem. 1992;209:571–582. doi: 10.1111/j.1432-1033.1992.tb17322.x. [DOI] [PubMed] [Google Scholar]
- 21.Kammerer R A, Antonsson P, Schulthess T, Fauser C, Engel J. J Mol Biol. 1995;250:64–73. doi: 10.1006/jmbi.1995.0358. [DOI] [PubMed] [Google Scholar]
- 22.Fujiwara S, Shinkai H, Deutzmann R, Paulsson M, Timpl R. Biochem J. 1988;252:453–461. doi: 10.1042/bj2520453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein G, Ekblom M, Fecker L, Timpl R, Ekblom P. Development. 1990;110:823–837. doi: 10.1242/dev.110.3.823. [DOI] [PubMed] [Google Scholar]
- 24.De Arcangelis A, Neuville A P, Boukamel R, Lefebvre O, Kedinger M, Simon-Assmann P. J Cell Biol. 1996;133:417–430. doi: 10.1083/jcb.133.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsui C, Wang C K, Nelson C F, Bauer E A, Hoeffler W K. J Biol Chem. 1995;270:23496–23503. doi: 10.1074/jbc.270.40.23496. [DOI] [PubMed] [Google Scholar]
- 26.Pellerin S, Keramidas M, Chambaz E M, Feige J-J. Endocrinology. 1997;138:1321–1327. doi: 10.1210/endo.138.3.4962. [DOI] [PubMed] [Google Scholar]
- 27.Cooper A R, MacQueen H A. Dev Biol. 1983;96:467–471. doi: 10.1016/0012-1606(83)90183-5. [DOI] [PubMed] [Google Scholar]
- 28.Kusche-Gullberg M, Garrison K, MacKrell A J, Fessler L I, Fessler J H. EMBO J. 1992;11:4519–4527. doi: 10.1002/j.1460-2075.1992.tb05553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]