Abstract
We describe mutations of three genes in Arabidopsis thaliana—extra cotyledon1 (xtc1), extra cotyledon2 (xtc2), and altered meristem programming1 (amp1)—that transform leaves into cotyledons. In all three of these mutations, this transformation is associated with a change in the timing of events in embryogenesis. xtc1 and xtc2 delay the morphogenesis of the embryo proper at the globular-to-heart transition but permit the shoot apex to develop to an unusually advanced stage late in embryogenesis. Both mutations have little or no effect on seed maturation and do not affect the viability of the shoot or the rate of leaf initiation after germination. amp1 perturbs the pattern of cell division at an early globular stage, dramatically increases the size of the shoot apex and, like xtc1 and xtc2, produces enlarged leaf primordia during seed development. These unusual phenotypes suggest that these genes play important regulatory roles in embryogenesis and demonstrate that the development of the shoot apical meristem and the development of the embryo proper are regulated by independent processes that must be temporally coordinated to ensure normal organ identity.
Keywords: heterochrony, embryogenesis, pattern formation, seed development
Embryogenesis in seed plants consists of several discrete processes (1–4). During the early, morphogenetic phase of embryogenesis, the primary axis of the plant is established, one or more cotyledons are formed, and the primordia of the root and shoot systems are initiated. The embryo subsequently undergoes a process of maturation in which it acquires the capacity for autonomous growth, accumulates nutritional reserves, becomes desiccation tolerant, and then enters a period of dormancy. Upon germination, the seedling uses its stored reserves, and the shoot and root meristems resume growth. Recent work has shown that although these events usually occur in a predictable temporal sequence, they are not part of a linear developmental pathway (4). For example, in several species, precocious germination leads to the simultaneous expression of genes that are normally expressed only during embryo maturation and genes that are normally expressed only during germination (reviewed in ref. 1). Similarly, the leafy cotyledon1-2 mutation in Arabidopsis causes plants to express germination-specific genes without completely suppressing the expression of a storage protein gene that is normally expressed during the maturation phase of embryogenesis (5). Conversely, the raspberry mutant of Arabidopsis thaliana is blocked at a globular stage of embryogenesis, but expresses genes that are normally activated much later (6).
To study how morphogenetic events are temporally coordinated during embryogenesis, we screened for mutations of A. thaliana that mimicked the cotyledon-leaf phenotype of precociously germinated Brassica napus embryos (7, 8). We identified three mutations that have this phenotype. Here we describe the effect of these mutations on the morphogenesis of the embryo and provide evidence that the effect of these mutations on leaf identity is the result of a change in the relative timing of the development of the shoot meristem and embryo proper.
MATERIALS AND METHODS
Mutagenesis and Screening.
Five hundred milligrams of Landsberg erecta (Ler) seeds (approximately 25,000 seeds) were incubated at room temperature in 50 ml of water overnight on a shaker. Thirty-four microliters of diepoxybutane (Sigma) in 40 ml of water was added (for a final concentration of 11 mM), and seeds were shaken in the light at 150 rpm for 4 hr. Seeds were rinsed five times with 1 liter of water in a 2-liter flask (5 min each rinse) and distributed in flats of Metromix 200 (Scotts, Marysville, OH) at a density of about 1,000 seeds per flat. Seeds were harvested from individual M1 plants, and approximately 20 M2 progeny from each M1 plant were screened about 2 weeks after germination for plants in which one or more of the first two leaves had fewer than five trichomes. This criterion was used to identify extra cotyledons in all subsequent experiments; under our growing conditions, the first two leaves of wild-type Ler plants normally have about 11 trichomes on their adaxial (i.e., upper) surface. The mutations isolated in this screen were backcrossed to Ler five times to eliminate second-site mutations, although no obvious additional mutations were present in the original M2 families.
Mapping.
an1, dis1, and pt seed stocks were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus, OH). amp1-1 was provided by A. Chaudhury (Commonwealth Scientific and Industrial Research Organization, Canberra, Australia), and seeds of the emb mutants were provided by D. Meinke (Oklahoma State University, Stillwater, OK). xtc1 and xtc2 were mapped relative to an1 and dis1 by screening for recombinants among the xtc plants in the F2 progeny of self-pollinated xtc an1/XTC AN1 and xtc dis1/XTC DIS1 plants.
Timed Pollinations.
Plants were grown at 22°C under continuous illumination in controlled environment rooms (Conviron, Winnipeg, Canada) at approximately 80% relative humidity. Floral buds were emasculated and pollinated immediately with pollen of the same genotype. Pollinations were carried out over several days, but only plants that were pollinated on the same day were harvested at each time point. Although there was some variation in the stage of the embryos in different siliques of the same genotype, there was no overlap between mutant and wild-type plants, except very early in development.
Histology.
The morphology of mutant and wild-type embryos was examined in whole mount preparations and in sectioned material. Embryos were fixed at various times after pollination in 3:1 acetic acid/ethanol, and then cleared with modified Hoyer’s solution (7.5 g of gum arabic, 5 ml of glycerin, 100 g of chloral hydrate, 30 ml of water) for up to several days. To visualize the vascular pattern of leaves and cotyledons, seedlings fixed in 3:1 acetic acid/ethanol were stained with 0.005% toluidine blue in 0.001 M sodium acetate. Light and electron microscopy were performed on specimens fixed overnight in 3% glutaraldehyde/1.5% acrolein/1.5% paraformaldehyde, pH 6.8, postfixed for 2 hr in 1% OsO4, and embedded in Spurr’s resin.
RESULTS
The Isolation and Genetic Characterization of xtc1, xtc2, and amp1(pt).
To identify mutations that affect the transition from embryonic to vegetative development, approximately 1,500 diepoxybutane-mutagenized M2 families were screened for abnormalities in the first two leaves. Two mutations that cause one or both of these leaves to resemble cotyledons were identified in this screen—extra cotyledon 1 (xtc1) and extra cotyledon 2 (xtc2). We subsequently found that the primordia timing mutation, which we determined to be an allele of the altered meristem programming1 (amp1) gene, has a similar effect on leaf identity. In these mutants, leaves that are partially transformed into cotyledons (extra cotyledons) can be readily distinguished from true cotyledons, because they are located in the position of the first two leaves on the shoot (i.e., at 90° relative to the cotyledons and at higher node), they emerge after cotyledons but before the first two leaf primordia of wild-type plants, and they are smaller than cotyledons and often irregular in shape (Fig. 1). Fully expanded extra cotyledons resemble cotyledons in that they have few or no trichomes and have a simple venation pattern similar to that of cotyledons (Fig. 2). Moreover, immature primordia of extra cotyledons (examined 2 days after imbibition) possess large numbers of starch grains, protein bodies and lipid bodies—storage products that are normally found in cotyledons, but not in leaves (Fig. 3) (1, 9).
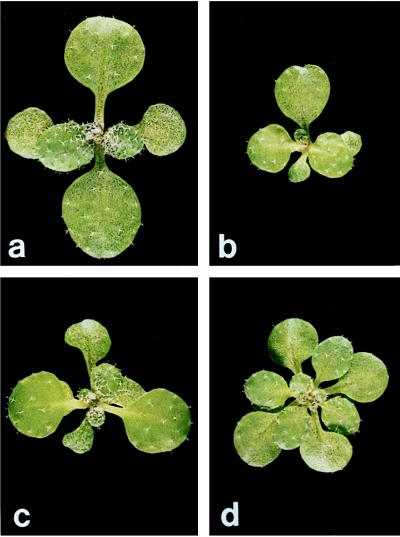
Figure 1.
The phenotype of (a) wild-type, (b) xtc1, (c) xtc2, and (d) amp1(pt) seedlings 2 weeks after germination. Leaves 1 and 2 are at the top and bottom of each figure. The true cotyledons, which are oriented horizontally, are smaller in mutant than in wild-type plants and are hidden by leaf primordia in b, c, and d.
Figure 2.
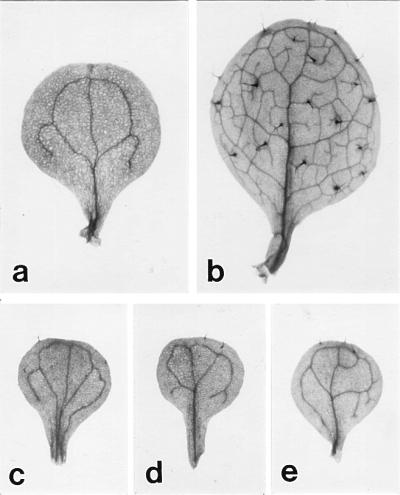
The morphology of a (a) cotyledon and (b) first leaf of a wild-type plant, and the first leaf of (c) xtc1, (d) xtc2, and (e) amp1(pt) seedlings. The first leaves of mutant plants have few or no trichomes, and they have a simple venation pattern like that of cotyledons.
Figure 3.
Electron micrographs of (a) a wild-type cotyledon and (b) a wild-type first leaf primordium and the first leaf primordia of (c) xtc1, (d) xtc2, and (e) amp1(pt) seedlings 2 days after imbibition. Wild-type leaf primordia are almost completely devoid of storage products, whereas cotyledon-like leaf primordia have large numbers of lipid bodies, starch grains, and the remnants of storage protein bodies. The cotyledon in a is at a much more advanced stage of development than the leaf primordia. (Scale bar = 5 μm.)
The extra-cotyledon phenotype of xtc1 and xtc2 shows variable penetrance and expressivity. On average, only 30% (n = 2,034) of the progeny of xtc1/xtc1 plants and 70% (n = 1,744) of the progeny of xtc2/xtc2 plants have extra cotyledons. Most plants have only one such structure, which may be either completely or only partially cotyledon-like. F1 progeny (n = 165) from crosses between these mutants were wild type, demonstrating that they are not allelic. This result was confirmed by the observation that these genes map to different sites on chromosome 1. xtc1 and xtc2 map between the visible markers angustifolia (an) and distorted trichomes1 (dis1) in the order and with the approximate map distances: an1—6—xtc2—3—xtc1—10—dis1. The SSLP (10) marker nga63 maps between xtc2 and xtc1, but its distance relative to these genes has not been accurately determined. Crosses between xtc1 and xtc2 and other defective embryo mutants (emb142, emb173, and emb176) that map between an1 and dis1 demonstrated that xtc1 and xtc2 are not allelic to any of these loci.
The extra-cotyledon phenotype of amp1(pt) in a Ler genetic background is almost completely penetrant and is more extreme than that of either xtc1 or xtc2. Seventy percent (n = 349) of amp1(pt) plants produce at least one leaf that is completely devoid of trichomes, and all plants produce at least one leaf with fewer than five trichomes. In addition, 26% (n = 416) of amp1(pt) seedlings have an additional true cotyledon; this is also characteristic of the amp1–1 allele (11). Additional true cotyledons can be readily distinguished from cotyledon-like leaves, because they are identical in shape and size to true cotyledons and emerge at the same time, at the same node, and at 120° orientation relative to other true cotyledons. amp1-1 has been mapped to the bottom of chromosome 3, between ap3 and tt5 (11).
The Extra-Cotyledon Phenotype Is Due to a Change in the Relative Timing of Leaf and Embryo Development.
The developmental basis of the extra-cotyledon phenotype initially was suggested by the precocious appearance of transformed leaves (Table 1). At 22°C under continuous light, the first two leaf primordia of wild-type seedlings do not emerge until 5 days after imbibition (DAI), whereas in mutant plants these leaf primordia frequently emerge 3 or 4 DAI. The phenotype of the first two leaves in mutant plants is correlated with their time of emergence. Leaves that are visible 3 DAI are completely devoid of trichomes and have a simple venation pattern; leaves that emerge at progressively later times have increasing numbers of adaxial trichomes. A histological examination of sections of mature seeds demonstrated that 5 of 11 xtc1 embryos, 8 of 10 xtc2 embryos, and 16 of 19 amp1(pt) embryos had enlarged leaf primordia. In mature wild-type embryos, the primordia of the first two leaves sometimes protrude slightly from the shoot meristem, but are more typically arrested before this stage. These numbers are consistent with the penetrance of the extra-cotyledon phenotype of these mutants and suggested that the transformation of leaves into cotyledons in these mutants might result from the precocious production of leaf primordia during embryogenesis. To explore this hypothesis, we examined the morphology of wild-type and mutant embryos at various times after pollination (Fig. 4). Both xtc1 and xtc2 produce embryos with phenotypes ranging from those arrested at a globular-to-heart stage of development to phenotypically wild-type embryos. The following descriptions pertain to the intermediate phenotype characteristic of the majority of mutant embryos.
Table 1.
The number of trichomes on the upper surface of leaves that emerge on various DAI
| Genotype | Date of leaf emergence DAI
|
|||
|---|---|---|---|---|
| 3 | 4 | 5 | 6 | |
| Wild type | 10.1 ± 0.4 | |||
| xtc1 | 0 | 5.9 ± 0.7 | 11.7 ± 0.5 | 11.3 ± 0.5 |
| xtc2 | 0 | 8.7 ± 0.8 | 11.0 ± 0.4 | |
| pt | 0 | 3.7 ± 0.5 | ||
| pt;psd | 4 ± 0.2 | 3.4 ± 0.2 | ||
DAI was measured starting from the day that seeds were transferred to a growth chamber after 3 days on moist soil at 4° C. Only plants that had fully expanded cotyledons by 3 DAI were scored. These data represent the number of adaxial trichomes on leaf 1 or leaf 2. In wild-type plants, these leaves emerge simultaneously at 5 DAI. In other genotypes, one or both of these leaves emerge at various times after imbibition. Values are ± the SEM.
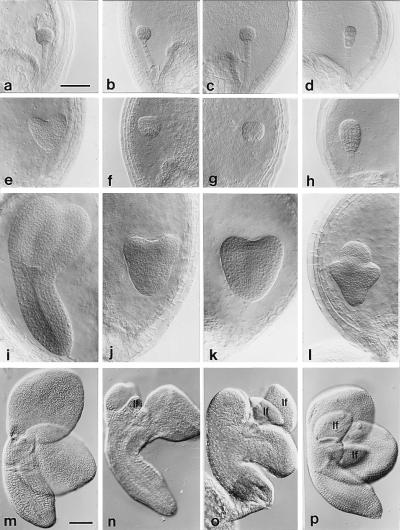
Figure 4.
Wild-type and mutant embryos at 4 days (a, b, c, and d), 5 days (e, f, g, and h), 6 days (i, j, k, and l) and 10 days (m, n, o, and p) after pollination. (a, e, i, and m) Wild type. (b, f, j, and n) xtc1. (c, g, k, and o) xtc2. (d, h, l, and p) amp1(pt). lf, leaf primordium. (Scale bar: a–l = 50 μm; m–p = 100 μm.)
In wild-type plants, the embryo undergoes a transition from an undifferentiated globular structure to heart-shaped embryo with well defined shoot and root poles and cotyledon primordia between 4 and 5 days after pollination (DAP) (Fig. 4e). The differentiation of the shoot meristem begins during this transition (12, 13). xtc1 embryos are similar to wild-type embryos 4 DAP (Fig. 4b) but fail to undergo the globular-to-heart transition at 5 DAP (Fig. 4f). Mutant embryos produce well developed cotyledon primordia and a shoot apical meristem primordium 1 day later (Fig. 4j), but the embryo is often somewhat elongated at this stage, and cotyledons frequently develop asynchronously and somewhat abnormally. xtc1 embryos continue to increase in size at the same rate as wild-type embryos after this initial delay (data not shown) and develop enlarged meristems and leaf primordia by 8 DAP, which then grow to an unusually large size by 10 DAP (Fig. 4n). xtc2 embryos also resemble wild-type embryos early in development (Fig. 4c) but do not undergo the globular-to-heart transition until wild-type embryos are at a bending cotyledon stage (Fig. 4 g and k). Although they continue to grow throughout the remainder of seed development, the cotyledons and hypocotyl of xtc2 embryos do not expand fully, so that mutant embryos often retain the morphology of late heart stage or early torpedo stage embryos. Despite the arrested development of the embryo proper, histological analysis of xtc2 embryos demonstrates that they produce an unusually large shoot apical meristem and leaf primordia by 8 DAP (data not shown). The size of the primordia at 10 DAP is shown in Fig. 4o. Thus, both of these mutations perturb the morphogenesis of the embryo at the globular-to-heart transition, but permit the shoot meristem to develop to an advanced stage by the end of embryogenesis.
amp1(pt) also dissociates the development of the shoot meristem from the development of the embryo proper, but in a different way than xtc1 or xtc2. In wild-type embryos, the apical cell divides in a stereotypical pattern to produce the globular embryo proper whereas the basal cell divides transversely to produce the suspensor (15). In amp1(pt) embryos, cell division in both the suspensor and the embryo proper is highly abnormal. Early in development, cells in the apical regions of the suspensor often undergo a series of vertical, oblique, and transverse divisions (Fig. 4d), producing an elongated rather than a spherical embryo proper. At about 5 DAP, cells in the entire apical half of the embryo acquire the dense cytoplasmic appearance typical of cells in the shoot meristem, and the embryo proper begins to expand laterally (Fig. 4h). Two or three cotyledons arise near the midline of the embryo 1 day later (Fig. 4l). The shoot meristem is prominent in amp1(pt) embryos at 8 DAP, but the overall size of the embryo proper is reduced by comparison to both xtc1 and xtc2. As in the case of these mutants, leaf primordia arise in amp1(pt) embryos between 8 and 10 DAP (Fig. 4p).
To test the hypothesis that the effect of amp1(pt) on leaf identity is due to the precocious development of the shoot meristem, we examined the effect of the paused (psd) mutation on the amp1(pt) phenotype. psd delays the initiation of the first two leaves, but has no effect on the rate of leaf initiation once these leaves are produced (16). Observations of cleared, mature psd/psd; amp1(pt)/amp1(pt) double mutant embryos demonstrated that they do not have enlarged leaf primordia (data not shown); furthermore, the first two leaves of these double mutants emerge about 2 days after the cotyledon-like leaves in amp1(pt) plants (Table 1). As predicted, the extra-cotyledon phenotype of amp1(pt) is strongly suppressed in amp1(pt)/amp1(pt); psd/psd double mutants (Table 1; Fig. 5). Although the first leaves of double mutants are similar in size to the cotyledon-like leaves of amp1(pt) plants, their trichome density and venation pattern resembles that of normal first leaf (compare Fig. 2b and Fig. 5b). This result supports the conclusion that the extra-cotyledon phenotype of amp1(pt) is due to the precocious development of the shoot meristem, rather than being a direct effect of amp1(pt) on leaf identity. We did not test the effect of psd on xtc1 and xtc2 because of their highly variable penetrance and expressivity. It is significant, however, that the phenotype of the first leaves in these mutants is correlated with their time of emergence, with cotyledon-like leaves emerging sooner than phenotypically normal first leaves (Table 1). Assuming that the time at which a leaf primordium emerges after germination is directly related to its size in the embryo, this observation is consistent with the conclusion that the precocious development of leaves during embryogenesis is responsible for their cotyledon-like phenotype.
Figure 5.
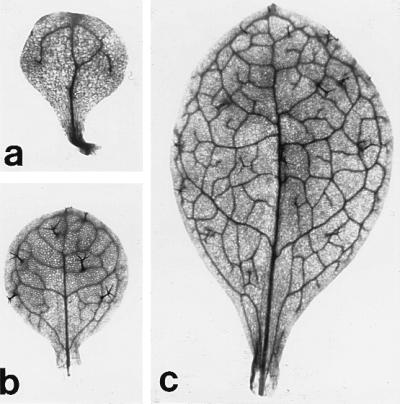
The first leaf of (a) amp1(pt)/amp1(pt), (b) amp1(pt)/amp1(pt); psd/psd, and (c) psd/psd mutant plants. The double mutant leaf in b resembles a normal first leaf in having well developed trichomes and a complex venation pattern.
xtc1, xtc2, and amp1(pt) Do Not Affect the Viability of the Shoot.
All three of these mutations have pleiotropic effects on shoot development after germination, but mutant plants are vigorous, and they flower within about 2 days of wild-type plants (Fig. 6). xtc1 and xtc2 have no effect on the rate of leaf initiation after germination (data not shown). However, amp1(pt) produces a significant increase in the rate of leaf initiation, as has been previously reported for other mutant alleles of this locus (11, 16, 17). The rosette and the inflorescence stem of xtc1 plants are smaller than in wild-type plants; in addition, the inflorescence stem lacks epicuticular wax (i.e., it has a cer phenotype). Crosses between xtc1 and cer1, which maps near xtc1, demonstrated that these mutations are not allelic. xtc1 also has a number of effects on flower development. In particular, xtc1 causes flower buds to open prematurely, sometimes produces an increase in carpel number, and reduces female fertility. Some cer mutations are semisterile under conditions of low humidity. To determine if this is the basis of the semisterility of xtc1, we enclosed plants in plastic bags during inflorescence development to increase the relative humidity, a treatment that previously has been shown to restore fertility to cer mutations (18). This treatment did not markedly increase the fertility xtc1, implying that the semisterility of this mutation is probably not due to its effect on epicuticular wax production.
Figure 6.
Mature wild-type and mutant plants. (Left to Right) Ler, xtc1, xtc2, and amp1(pt).
Although xtc2 seedlings are initially smaller that wild-type seedlings and sometimes have slightly abnormal phyllotaxis, this mutation has no other obvious effects on vegetative development. The inflorescence morphology of xtc2 plants is also normal, but mutant flowers are semisterile because they shed little or no pollen. xtc2 also may produce an increase in carpel number and prevent carpel fusion, but the expressivity of these phenotypes is quite variable.
The postembryonic phenotype of amp1(pt) is similar to that of the previously described amp1-1 allele (11). As noted above, amp1(pt) increases the rate of leaf initiation after germination and reduces apical dominance. amp1(pt) also produces a general reduction in the size of leaves, inflorescence stems, and floral organs. amp1(pt) occasionally produces fasciated inflorescence stem, but does not affect the number of floral organs or have any obvious effect on the pattern of floral development.
DISCUSSION
Previous studies have demonstrated that genes involved in the physiological maturation and subsequent germination of an embryo are part of several independent developmental pathways that must be properly coordinated if the embryo is to develop normally (2, 4). Evidence also shows that the morphogenesis of different parts of the embryo may depend on the coordination of otherwise independent processes. In particular, mutant alleles of twn (19), sus1, sus2, and sus3 (20) disrupt the development of the embryo proper and cause the suspensor either to initiate a secondary embryo or to undergo a pattern of cellular differentiation typical of the embryo proper. This phenotype suggests that the development of the suspensor is regulated by the embryo proper, and that in the absence of the embryo proper the suspensor is capable of displaying a much greater developmental potential than it does normally (21). The developmental relationship between other parts of the embryo (cotyledons, hypocotyl, shoot apical meristem, and root apical meristem) is less clear. Mutations that delete the shoot apical meristem in Arabidopsis (12) and petunia (22) have little or no effect on other aspects of embryogenesis, implying that pattern formation in the embryo proper does not depend on information provided by the shoot apical meristem. Most of the “pattern formation” mutations in Arabidopsis have global effects on the morphogenesis or growth of the embryo, although they generally affect one particular part of the embryo more than another (23–26). Whether this reflects a general requirement for these genes throughout the embryo or is a consequence of interactions between different parts of the embryo remains to be determined. The mutants described here are interesting because they suggest that although the development of the embryo proper does not depend on the shoot meristem, the developmental state of the embryo does influence the development of the shoot meristem. Thus, the temporal coordination of the development of the shoot apex and the embryo proper may be important during the early morphogenesis of the shoot.
This conclusion is based on the observation that in xtc1, xtc2, and amp1(pt) the transformation of leaves into cotyledons is associated with a change in the relative timing of the development of the embryo proper and the shoot apex. The dissociation of shoot meristem development and the development of the embryo proper occurs in slightly different ways in amp1(pt), xtc1, and xtc2. In amp1(pt) embryos, the shoot meristem develops to an unusually large size at the globular stage of development, well before the shoot meristem is fully developed in wild-type embryos. By contrast, xtc1 and xtc2 delay the development of the embryo proper and subsequently permit the shoot meristem to develop to a greater extent that it would under normal conditions. The result of this dissociation of shoot and embryo proper development is that leaf primordia develop precociously during embryogenesis. Evidence that the transformation of leaf primordia into cotyledons is a consequence of their precocious development rather than being a direct effect of these mutations on leaf identity is provided by the fact that the psd mutation suppresses the extra-cotyledon phenotype of amp1(pt), and by the observation that the phenotype of the first two leaves of mutant plants is correlated with their time of emergence. Thus, leaf primordia that develop precociously during embryogenesis (and therefore emerge precociously after germination) are more likely to become transformed into cotyledons than leaves whose development is delayed.
The mutants described here are phenotypically similar to Brassica napus embryos that have been forced to germinate precociously (7, 8), but differ from these B. napus seedlings in several respects. First, whereas B. napus seedlings continue to produce extra cotyledons for several plastochrons after germination, this does not appear to be true for xtc1 and xtc2. Both of these mutants produce no more than two extra cotyledons, and these structures always emerge precociously relative to the first two leaves of wild-type plants; leaf primordia that emerge at the same time as wild-type leaves (i.e., 5 DAI) are completely normal. We conclude, therefore, that the effect of these mutations on leaf identity is confined to leaves initiated before germination. The situation is less clear in the case of amp1(pt) because this mutation may produce seedlings in which four or more leaves have a reduced number of trichomes. We occasionally have observed more than two enlarged leaf primordia in mature mutant embryos, but the complex morphology of these embryos has made it difficult to accurately determine their number of leaf primordia. It is possible that amp1(pt) plants produce extra cotyledons both before and after germination. Second, whereas the extra cotyledons produced in B. napus are frequently mosaic structures, consisting of discrete longitudinal sectors of leaf-like and cotyledon-like tissue (8), the extra cotyledons produced by the mutants described here appear to be homogeneous; these structures are either completely cotyledon-like or display a general reduction in trichome density or vascular complexity rather that having discrete leaf-like and cotyledon-like domains. Finally, it should be emphasized that amp1(pt), xtc1, or xtc2 probably do not act by inducing a germination program because all three mutations have desiccation-tolerant seeds. In summary, the transformation of leaves into cotyledons appears to occur by at least three mechanisms (precocious germination, precocious development of the shoot apex, and the delayed development of the embryo proper), all of which result in a change in the relative timing of shoot and embryo proper development.
Changes in developmental timing (heterochrony) have been widely invoked as a source of novelty in evolution (27, 28), but the significance of this phenomenon in plant evolution is still unclear. Genetic analyses of heterochrony in plants have focused on the phenomenon of shoot maturation, which involves characteristic changes in the morphology, anatomy, and physiology of leaves produced at different stages in shoot development (29, 30). Based on the results of this study, it is reasonable to propose that the character of the first few leaves of a plant may be influenced by the developmental stage of the embryo at the time each of these leaves were initiated. In fact, in maize (31–34) there is evidence that the development of the first two leaves may be regulated differently than leaves produced later in embryogenesis. It is fascinating that the number of leaf primordia produced during seed development (i.e., the number of leaves in the plumule) varies widely in different species. As far as we know, the evolutionary significance of this interspecific variation in plumule leaf number has not been carefully investigated. If leaves produced during embryogenesis, or at particular times in embryogenesis, are fundamentally different from leaves produced at other times, then variation in the duration or rate of meristem development during embryogenesis may generate novel types of leaves and provide an explanation for this general and poorly understood aspect of plant biology.
Although amp1-1 and its alleles, hauptling (S. Ploense, personal communication) and cop2 (17), are known to have an effect on the number of true cotyledons and on the rate of leaf initiation after germination (11, 16, 17, 35), the effect of amp1 mutations on the morphogenesis of the embryo and on leaf identity has not been previously described. amp1(pt) differs from other mutations that increase the size of the meristem (36–38) in that it does not increase floral organ number and only infrequently produces stem fasciation. The significance of this observation remains to be demonstrated because we do not know that amp1(pt) increases the size of the shoot meristem during the postembryonic growth of the shoot. In any event, the highly pleiotropic effects of this mutation imply that AMP1 functions in many aspects of plant development and is not confined in its activity to the shoot apical meristem. Chaudhury et al. (14) have shown that the level of cytokinin in amp1-1 mutants is six times greater than in wild-type plants and attribute the phenotype of this mutation to this elevated level of cytokinin. The precocious development of the shoot meristem in amp1(pt) mutants is consistent with this hypothesis, because cytokinin has long been known to promote shoot meristem development (39, 40). However, it is notable that amp1(pt) mutants do not produce excessive amounts of anthocyanin. Because an increase in anthocyanin production is one of the most characteristic effects of exogenously applied cytokinin (41), it remains to be determined whether the elevated level of cytokinin in amp1-1 is responsible for its mutant phenotype or is a secondary effect of this mutation.
xtc1 and xtc2 represent a novel class of embryonic mutations. Many mutations that arrest embryogenesis or perturb the morphogenesis of the embryo have been described (3, 35), but we are unaware of any previously described mutations that delay embryogenesis at the globular-to-heart transition but do not have major effects on the viability of the embryo or the development of the shoot after germination. This intriguing phenotype suggests that XTC1 and XTC2 may function to regulate this important developmental transition. A more detailed analysis of the effect of xtc1 and xtc2 on embryonic gene expression, as well as the isolation and characterization of additional alleles of these genes, should provide a clearer picture of their role in Arabidopsis embryogenesis.
Acknowledgments
We thank Abby Telfer, Krista Bollman, and Maja Bucan for their comments on the manuscript. This work was supported by a postdoctoral fellowship from the American Cancer Society (L.J.C.) and by a grant from the National Institutes of Health.
ABBREVIATIONS
- DAP
days after pollination
- DAI
days after imbibition
References
- 1.Crouch M L. In: Developmental Biology: A Comprehensive Synthesis. Browder L, editor. Vol. 5. New York: Plenum; 1988. pp. 367–404. [Google Scholar]
- 2.Goldberg R B, Paiva G D, Yadegari R. Science. 1994;266:605–614. doi: 10.1126/science.266.5185.605. [DOI] [PubMed] [Google Scholar]
- 3.Meinke D W. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:369–394. [Google Scholar]
- 4.McCarty D R. Ann Rev Plant Physiol Plant Mol Biol. 1995;46:71–93. [Google Scholar]
- 5.West M A, Yee K M, Danao J, Zimmerman J L, Fischer R L, Goldberg R B, Harada J J. Plant Cell. 1994;6:1731–1745. doi: 10.1105/tpc.6.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yadegari R, Paiva G R D, Laux T, Koltunow A M, Apuya N, Zimmerman J L, Fischer R L, Harada J J, Goldberg R B. Plant Cell. 1994;6:1713–1729. doi: 10.1105/tpc.6.12.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkelstein R R, Crouch M L. Planta. 1984;162:125–131. doi: 10.1007/BF00410208. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez D E. Development (Cambridge, UK) 1997;124:1149–1157. doi: 10.1242/dev.124.6.1149. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg R B, Barker S J, Perez-Grau L. Cell. 1989;56:149–160. doi: 10.1016/0092-8674(89)90888-x. [DOI] [PubMed] [Google Scholar]
- 10.Bell C J, Ecker J R. Genomics. 1994;19:137–144. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhury A M, Letham S, Craig S, Dennis E S. Plant J. 1993;4:907–916. [Google Scholar]
- 12.Barton M K, Poethig R S. Development (Cambridge, UK) 1993;119:823–831. [Google Scholar]
- 13.Long J A, Moan E I, Medford J I, Barton M K. Nature (London) 1996;379:66–69. doi: 10.1038/379066a0. [DOI] [PubMed] [Google Scholar]
- 14.Medford J I, Behringer F J, Callos J D, Feldmann K A. Plant Cell. 1992;4:631–643. doi: 10.1105/tpc.4.6.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansfield S G, Briarty L G. Can J Bot. 1991;69:461–476. [Google Scholar]
- 16.Telfer A, Bollman K M, Poethig R S. Development (Cambridge, UK) 1997;124:645–654. doi: 10.1242/dev.124.3.645. [DOI] [PubMed] [Google Scholar]
- 17.Lehman A, Black R, Ecker J R. Cell. 1996;85:183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- 18.Preuss D, Lemieux B, Yen G, Davis R W. Genes Dev. 1993;7:974–985. doi: 10.1101/gad.7.6.974. [DOI] [PubMed] [Google Scholar]
- 19.Vernon D M, Meinke D W. Devel Biol. 1994;165:566–573. doi: 10.1006/dbio.1994.1276. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz B, Yeung E C, Meinke D W. Development (Cambridge, UK) 1994;120:3235–3245. doi: 10.1242/dev.120.11.3235. [DOI] [PubMed] [Google Scholar]
- 21.Yeung E C, Meinke D W. Plant Cell. 1993;5:1371–1381. doi: 10.1105/tpc.5.10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Souer E, Houwelingen A V, Kloos D, Mol J, Koes R. Cell. 1996;85:159–170. doi: 10.1016/s0092-8674(00)81093-4. [DOI] [PubMed] [Google Scholar]
- 23.Shevell D E, Leu W-M, Gillmor C S, Xia G, Feldmann K A, Chua N-H. Cell. 1994;77:1051–1062. doi: 10.1016/0092-8674(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 24.Berleth T, Jürgens G. Development (Cambridge, UK) 1993;118:575–587. [Google Scholar]
- 25.Mayer U, Büttner G, Jürgens G. Development (Cambridge, UK) 1993;117:149–162. [Google Scholar]
- 26.Torres-Ruiz R, Lohner A, Jürgens G. Plant J. 1996;10:1005–1016. doi: 10.1046/j.1365-313x.1996.10061005.x. [DOI] [PubMed] [Google Scholar]
- 27.Gould S J. Ontogeny and Phylogeny. Cambridge, MA: Belknap; 1977. [Google Scholar]
- 28.Lord E M, Hill J P. In: Development as an Evolutionary Process. Raff R A, Raff E C, editors. New York: Liss; 1987. pp. 41–70. [Google Scholar]
- 29.Lawson E, Poethig R S. Trends Genet. 1995;11:263–268. doi: 10.1016/s0168-9525(00)89072-1. [DOI] [PubMed] [Google Scholar]
- 30.Hackett W P, Murray J R. In: Maturation and Rejuvenation in Woody Species. Ahuja M R, editor. Dordrecht, the Netherlands: Kluwer; 1993. pp. 93–105. [Google Scholar]
- 31.Evans M M S, Passas H J, Poethig R S. Development (Cambridge, UK) 1994;120:1971–1981. doi: 10.1242/dev.120.7.1971. [DOI] [PubMed] [Google Scholar]
- 32.Moose S P, Sisco P H. Plant Cell. 1994;6:1343–1355. doi: 10.1105/tpc.6.10.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moose S P, Sisco P H. Genes Dev. 1996;10:3018–3027. doi: 10.1101/gad.10.23.3018. [DOI] [PubMed] [Google Scholar]
- 34.Bongard-Pierce D K, Evans M M S, Poethig R S. Int J Plant Sci. 1996;157:331–340. [Google Scholar]
- 35.Jürgens G, Ruiz R A T, Berleth T. Annu Rev Genet. 1994;28:351–371. doi: 10.1146/annurev.ge.28.120194.002031. [DOI] [PubMed] [Google Scholar]
- 36.Leyser H M O, Furner I J. Development (Cambridge, UK) 1992;116:397–403. [Google Scholar]
- 37.Clark S E, Running M P, Meyerowitz E M. Development (Cambridge, UK) 1993;119:397–418. doi: 10.1242/dev.119.2.397. [DOI] [PubMed] [Google Scholar]
- 38.Clark S E, Running M P, Meyerowitz E M. Development (Cambridge, UK) 1995;121:2057–2067. [Google Scholar]
- 39.Skoog F, Miller C O. Symp Soc Exp Biol. 1957;11:118–131. [PubMed] [Google Scholar]
- 40.Grayburn W S, Green P B, Stuchek G. Plant Physiol. 1982;69:682–686. doi: 10.1104/pp.69.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deikman J, Hammer P E. Plant Physiol. 1995;108:47–57. doi: 10.1104/pp.108.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]