Abstract
In acute promyelocytic leukemia (APL), the typical t(15;17) and the rare t(11;17) translocations express, respectively, the PML/RARα and PLZF/RARα fusion proteins (where RARα is retinoic acid receptor α). Herein, we demonstrate that the PLZF and PML proteins interact with each other and colocalize onto nuclear bodies (NBs). Furthermore, induction of PML expression by interferons leads to a recruitment of PLZF onto NBs without increase in the levels of the PLZF protein. PML/RARα and PLZF/RARα localize to the same microspeckled nuclear domains that appear to be common targets for the two fusion proteins in APL. Although PLZF/RARα does not affect the localization of PML, PML/RARα delocalizes the endogenous PLZF protein in t(15;17)-positive NB4 cells, pointing to a hierarchy in the nuclear targeting of these proteins. Thus, our results unify the molecular pathogenesis of APL with at least two different RARα gene translocations and stress the importance of alterations of PLZF and RARα nuclear localizations in this disease.
Keywords: nuclear matrix, acute promyelocytic leukemia, interferon, translocation, yeast two-hybrid
Acute promyelocytic leukemia (APL) represents approximately 10% of all adult acute myeloid leukemias (1). The molecular pathogenesis of APL is, at least in part, associated with the disruption of the retinoic acid receptor α (RARα) gene through its fusion to one of four different loci (2–7). These translocations result in the expression of chimeric RARα fusion proteins that retain the DNA and ligand binding domains of the receptor and gain a dimerization domain from the fusion partner. Paradoxically, APL is the first human malignancy that may undergo complete remission in response to differentiation therapy with all-trans-retinoic acid (RA). The molecular basis of these remissions is still disputed.
The majority of APL cases, and all cases that consistently respond to RA treatment, possess the t(15;17) translocation that fuses the PML and RARα genes (3, 8–12). PML is a member of a functionally diverse gene family that encodes proteins characterized by the presence of a N-terminal C3HC4 RING-finger motif (13), followed by one or two cysteine-rich regions (B boxes) and a coiled-coil protein dimerization interface. The function of PML is unknown, but up-regulation of its expression by interferons (IFNs) (14–16) and its negative effect on cell growth and cellular transformation by cooperating oncogenes (17–19) suggest a role in growth control. The product of the wild-type PML gene is a phosphoprotein (20) that localizes both in the nucleoplasm and in the specific multiprotein structures called PML nuclear bodies (NBs) (20–24). The PML/RARα fusion protein, which is expressed in APL cells as a result of t(15;17), contains all predicted PML structural motifs and is able to delocalize the wild-type PML and other NB components onto discrete microspeckled nuclear structures (21–24). It is still unclear which role, if any, disruption of NBs and/or establishment of microspeckled structures play in cellular transformation. Nevertheless, complete restoration of NBs upon RA treatment in NB4 cells, but not in RA-resistant sublines (which remain microspeckled), strongly suggests a perturbation of NBs in the pathogenesis of APL (23, 25).
Three other APL-associated translocations of the RARα gene have been characterized at the molecular level. The t(5;17)(q35;q21) (5), t(11;17)(q23;q21) (6), and t(11;17)(q13;q21) (7) fuse RARα to nucleophosmin (NPM), promyelocytic leukemia zinc (Zn) finger (PLZF), and nuclear mitotic apparatus (NuMA) genes, respectively. In all cases RARα fusion proteins are expressed that structurally resemble PML/RARα. All four fusion proteins possess identical RARα sequences, which include the DNA binding region C, the ligand binding domain E, and N-terminal protein–protein interaction motifs derived from PML, PLZF, NuMA, and NPM proteins. Nevertheless, at least in the case of PLZF/RARα-associated APL, patients and primary PLZF/RARα-positive APL cells in culture do not respond to RA (26). The molecular basis for this difference in clinical response of the disease is not understood.
Recently, we have shown that in analogy to PML, the PLZF protein possesses a speckled nuclear localization (27, 28) and its overexpression leads to suppression of cell growth (A.R., Rita Shaknovich, J.L., S.W., and A.Z., unpublished data). PLZF belongs to a protein family characterized by the presence of a BTB/POZ domain, involved in dimerization and transcriptional repression (29, 30). Moreover, most of these proteins contain several Krüppel-like Zn fingers, some of which are involved in sequence-specific DNA binding (29, 31). PLZF/RARα contains the N-terminal BTB/POZ domain and two out of nine Krüppel-like Zn fingers fused upstream of the RARα sequences. When transiently expressed in mammalian cells, this protein also appears to be, at least in part, localized to discrete nuclear domains (32).
The similarity in the intranuclear localization of PML and PLZF, as well as their RARα fusions, prompted us to investigate whether PML and PLZF are present in the same nuclear compartments and might be functionally related. Herein we provide evidence for an interaction and nuclear colocalization between these two proteins. Apart from identifying a new PML partner, these findings strengthen the importance of NB disturbances in APL and unify the molecular models for its pathogenesis.
MATERIALS AND METHODS
Coimmunoprecipitation.
Transfections of COS6 cells with 0.5 μg of PLZFflag (27) and/or 0.5 μg of PML (8) mammalian expression vectors were carried out in 60-mm tissue culture plates (see below). The total amount of transfected expression vector was kept constant at 1 μg in all experiments. Approximately 18 h after transfection cells were labeled for 4 h with [35S]methionine/cysteine (200 μCi; 1 Ci = 37 GBq), then lysed with 800 μl of lysis buffer [250 mM NaCl/10 mM Tris⋅HCl, pH 7.4/1 mM EDTA/0.1 mM Na3VO4/0.5% Nonidet P-40/2% phenylmethylsulfonyl fluoride/arrotonin (75 μg/ml)/soybean trypsin inhibitor (75 μg/ml)/leupeptin (75 μg/ml)/bestatin (75 μg/ml)], and 700 μl of the cell lysate was incubated at 4°C for 2 h with 2 μg of anti-FLAG antibody (Kodak) [preadsorbed for 2 h at 4°C on 50% slurry of protein G-Sepharose beads (Pharmacia) in lysis buffer]. Proteins were eluted from the beads with Laemmli-loading buffer and electrophoresed on SDS/PAGE gels. Resolved proteins were transferred to a solid support and blotted with an anti-PML mouse monoclonal antibody (see below).
Immunofluorescence.
Immunofluorescence was performed essentially as described (21, 28). Polyclonal rabbit anti-PML (21) and the monoclonal mouse anti-PLZF (Rita Shaknovich, J.L., S.W., and A.Z., unpublished results) antibodies were used at a 1:400 and 1:100 dilutions in Tris-buffered saline (TBS), respectively. Confocal microscopy was as described (21).
Cell Transfection.
Transient and semistable transfections were performed in COS6 or CHO cells by using Lipofectin (Life Technologies) according to the manufacturer’s instructions. In short, cells were plated at 50% or 30% (semistable) confluence in a 25-cm2 flask and transfected in Optimem medium with 3 μg of plasmid overnight. The next day serum was added, and 48 h (transient) or 5–6 days (semistable) later, cells were examined for expression. COS6 and CHO cells were cultured in DMEM with 10% fetal calf serum, and KG1 and NB4 cells were cultured in RPMI 1640 medium with 10% retal claf serum. For IFN treatment, cells were incubated for 2 days with IFN-α or γ at 1000 units/ml. For RA treatment, 1 day after transfection PLZF/RARα-transfected cells were split 1:2, treated or not with 10−6 M RA, and harvested 24–48 h later.
Glutathione S-Transferase (GST) “Pull-Down” Experiments.
GST–PLZF protein was prepared as described (32). For “pull-down” experiments, 1 μg of protein on glutathione-Sepharose beads (Pharmacia) and 3 μl of a given 35S-labeled in vitro-translated product made with TNT-coupled reticulocyte lysate (Promega) were used. All conditions were as described (32).
Yeast Two-Hybrid Assay.
The Y190 strain of Saccharomyces cerevisiae (33), which harbors integrated lacZ reporter gene under control of the Gal4-responsive promoter, was transformed by using the lithium acetate method (34) with 1 μg of yeast expression vectors, pASI and pACTII (35), containing either PLZF or PML cDNAs fused in-frame to the Gal4 DNA binding and activation domains, respectively. Liquid and solid media, as well as conditions used for yeast growth, were as described (36). For β-galactosidase assays, randomly picked colonies were grown in a liquid culture to an OD600 of approximately 1.0. Yeast cells were lysed and assayed for β-galactosidase activity by using standard procedures (37). Units of β-galactosidase activity were calculated by the formula 1000 × (OD420/t × V × OD600), where t is incubation time in min and V is the volume of extract used for the assay in ml.
Western Blotting.
Western blot analysis was performed as described (21). The polyclonal anti-PLZF (27, 38) and monoclonal anti-PML (M.-C. Guillemin and H. de Thé, unpublished results) antibodies were used at 1:500 and 1:200 dilutions in TBS, respectively. Anti-p68 kinase antibody (Ribogene, Hayward, CA) was used at 1:10,000 dilution.
RESULTS
Colocalization Between PML and PLZF.
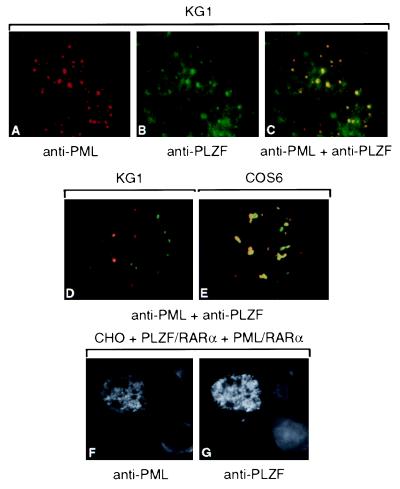
Given the parallels between the PLZF and PML proteins, we investigated the localization of PLZF and PML in a number of different systems. In the nuclei of KG1 cells, various degrees of colocalization between endogenous PLZF and PML proteins were observed, ranging from a small number of doubly positive NBs (Fig. 1D) to total colocalization (Fig. 1 A–C). Similar results were found in K562 or HL60 cells (data not shown). Subsequently, we tried to reproduce these results in cotransfection experiments. In COS6 cells, transiently transfected with PLZF and PML expression vectors, complete colocalization was rarely observed; nevertheless in each experiment, cells could be found in which the two proteins totally colocalized (Fig. 1E). However, in CHO cells semistably transfected with both genes colocalization was constantly found (data not shown), suggestive of a time/expression-dependent targeting process.
Figure 1.
PML and PLZF localize to the same nuclear domains. Colocalization (yellow) in KG1 cells of endogenous PML (red) and PLZF (green) proteins onto NBs by confocal microscopy (A–C). In a subpopulation of KG1 cells, only partial colocalization between PML and PLZF was detected (D). The complete colocalization is reproducible in rare COS6 cells, transiently cotransfected with PML and PLZF expression vectors (E). PML/RARα and PLZF/RARα colocalize after transient cotransfection onto the same microspeckled nuclear domains (F and G).
Interaction Between PML and PLZF.
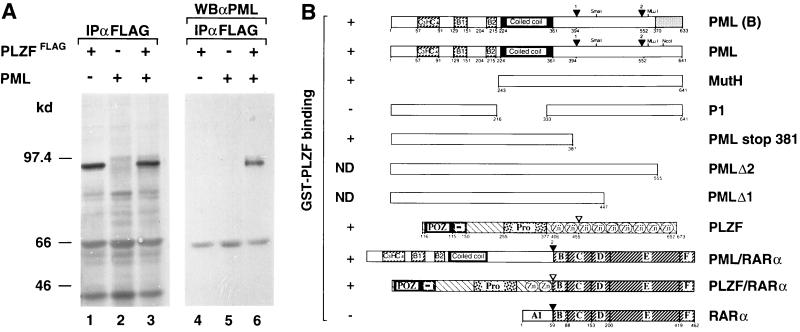
As most of the PML protein is associated with the nuclear matrix (39) and thus requires stringent conditions for extraction that destroy noncovalent interactions, coimmunoprecipitation of the endogenous PML and PLZF proteins proved technically very difficult. Nevertheless, we were able to readily coimmunoprecipitate PLZF and PML proteins from nuclear extracts of transiently cotransfected COS6 cells (Fig. 2A). This suggested that at least in this artificial system, a fraction of the two proteins are engaged in the same complex.
Figure 2.
Interaction between the PML and PLZF proteins. (A) Coimmunoprecipitation of epitope-tagged PLZF (PLZFFLAG) and PML, transiently coexpressed in COS6 cells. 35S-labeled proteins were precipitated with anti-FLAG antibodies (lanes 1–3), resolved by SDS/PAGE, and Western blotted with monoclonal anti-PML antibody (lanes 4–6). Both PML and PLZF proteins migrate at approximately 97 kDa. (B) Summary of the GST pull-down experiments demonstrating in vitro interaction of GST–PLZF and in vitro-translated 35S-labeled PML/RARα, different PML isoforms, and deletion mutants as indicated. Plus and minus signs indicate binding and no interaction, respectively. ND, not done. Various proteins are represented schematically with key restriction endonuclease recognition sites indicated. Different functional regions of each protein are indicated by different patterns and numbers correspond to the first and last amino acid flanking the given region. Positions of various PML deletion mutants are as indicated.
The interaction is apparently direct because we were also able to show specific binding between in vitro-translated 35S-labeled PML, as well as PML/RARα, with bacterially produced GST–PLZF (summarized in Fig. 2B). A number of in vitro-translated PML mutants and a different C-terminal isoform of PML [herein referred to as PML(B) (10)] also interacted to a similar degree with the PLZF protein in vitro (Fig. 2B). These data implicate the coiled-coil region of PML in the interaction with PLZF. Note, that this same region has also been shown to play a role in PML homodimerization (40).
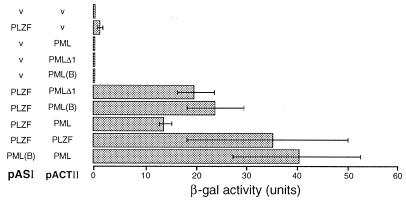
The PLZF–PML interaction was also assessed in vivo by using the yeast two-hybrid system. As can been seen in Fig. 3, PLZF [fused to the DNA binding domain of Gal4] interacted with the wild-type PML (fused to the Gal4 activation domain). As previously shown, PML and PLZF formed PML–PML and PLZF–PLZF homodimers (32, 40). The C-terminally truncated PML, as well as PML(B), also interacted with PLZF in this assay, corroborating the results obtained from in vitro experiments. However, the interaction between PML and PLZF was unidirectional because it only could be detected when PLZF was fused to the DNA binding domain and PML to the activation domain of Gal4 (data not shown). The directionality of interaction in the two-hybrid system is not unique to this case because it has been demonstrated for other proteins including Myc and Max (41).
Figure 3.
Yeast two-hybrid assay for in vivo interaction between the PLZF and PML proteins. Various proteins (see Fig. 2B for schematic representation) were expressed as fusions with either Gal4 DNA binding domain (pASI) or activation domain (pACTII) in yeast cells containing a lacZ reporter under the control of a Gal4-responsive promoter. Transfected empty vector control is indicated as v. Interaction between the proteins results in activation of lacZ expression and is detected by assaying for the β-galactosidase activity. Above results correspond to mean of four experiments.
Localization of PLZF/RARα and PML/RARα Fusion Proteins.
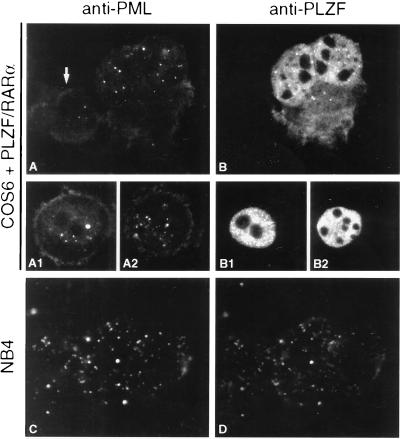
Previously, we and others had shown that the PML/RARα fusion protein exhibits a dominant negative behavior upon the localization of endogenous PML and, so far, on all the other NB-associated antigens (10, 21–24, 42). Therefore, it was interesting to determine whether the nuclear localization of PLZF would also be affected by the PML/RARα fusion protein. In the t(15;17)-positive NB4 APL cell line, PML/RARα delocalize the endogenous PLZF proteins onto the microspeckles (Fig. 4 C and D). After RA treatment of NB4 cells, the PML pattern is restored and coincides exactly with the normal PLZF distribution (data not shown). These results are readily reproducible in transient or semistable transfections (data not shown) and are consistent with the requirement of the PML coiled-coil domain for in vitro interaction with PLZF. As suggested (32), also PLZF/RARα displays a different fluorescence pattern when compared with the wild-type PLZF (Figs. 4 B, B1, and B2 and 5A1); in both transient or semistable transfections, the fusion protein’s pattern is microspeckled. The differences in PLZF/RARα staining patterns among various cells examined within the same transfection experiment (Fig. 4, B–B2) were probably due to the varying levels of PLZF/RARα expression and/or to a given cell’s stage in the cell cycle.
Figure 4.
Expression of the PLZF/RARα fusion protein does not dominantly affect the localization of endogenous PML. COS6 cells transiently transfected with PLZF/RARα expression vector and stained with anti-PML (A–A2) and anti-PLZF (B–B2) antibodies; arrow indicates an untransfected cell. Note the different aspects of the PLZF/RARα localization ranging from almost uniform (B2) to microspeckled (B1) and speckled in a uniform background (B). The PML/RARα fusion protein dominantly affects the localization of endogenous PLZF proteins in the NB4 cell line (C and D).
In cells transfected with a PLZF/RARα expression vector (Fig. 4 A–A2) or in primary APL blasts from a patient with a t(11;17)(q23;q21) translocation (data not shown), normal nuclear localization patterns of the endogenous PML were observed. This strongly suggests that at least some of the seven C-terminal Zn fingers of PLZF are required for its interaction with PML and that the PLZF/RARα fusion protein does not affect the PML localization.
As the microspeckled patterns of both PML/RARα and PLZF/RARα resembled each other, we transiently cotransfected vectors encoding each of these fusion proteins. As demonstrated in Fig. 1 F and G, both proteins appear to colocalize to the same nuclear domains. Remarkably, in sharp contrast to the PLZF–PML colocalization, which was rare under transient but common under semistable expression conditions, this colocalization was very frequently observed in transient systems. Furthermore, a PLZF/RARα mutant that lacks the BTB/POZ domain colocalized with the PML/RARα protein, whereas a mutant that lacks the BTB/POZ domain and the two N-terminal Krüppel-like Zn fingers of the PLZF protein or just the two Zn-finger motifs did not (data not shown). These results are in agreement with in vitro binding data of various PLZF deletion mutants to GST–PML that also indicate the requirement of the PLZF Zn fingers, but not the BTB/POZ-domain, for interaction with PML (data not shown). The two Krüppel-like Zn fingers in the PLZF/RARα fusion protein were also required for its full dominant negative effect on the wild-type RARα activity (32).
If PLZF/RARα and PML/RARα colocalization involves a physical contact between these proteins, the above results could suggests that the two N-terminal Zn fingers also participate in the PML–PLZF interaction. PLZF/RARα expression has no effect on localization of the endogenous PML, suggesting that some of the C-terminal PLZF Zn fingers may also be necessary for interaction with the wild-type PML. Therefore, we propose three nonexclusive hypotheses to explain why PML/RARα and PLZF/RARα can interact and/or colocalize: (i) conformational changes that allow an interaction exclusively between the first two Zn fingers, (ii) interaction via the two N-terminal Zn fingers stabilized by the strong DNA binding of the RARα DNA binding domains, and (iii) alternatively, the microspeckled pattern could be explained by a transport of fusion proteins to the same microspeckles as homodimers PLZF/RARα and PLZF/RARα or PML/RARα and PML/RARα but not as heterodimers PLZF/RARα and PML/RARα.
RA Causes Degradation of PLZF/RARα.
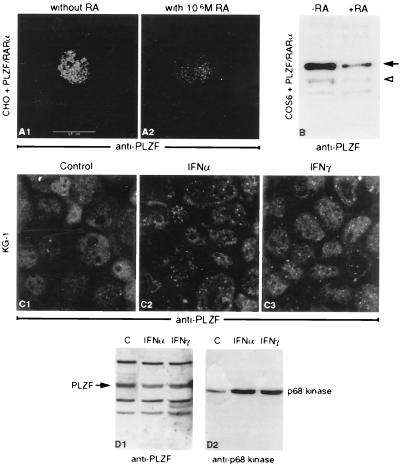
We also investigated the effect of RA treatment on localization of PLZF/RARα in transfected cells. COS6 cells transiently expressing PLZF/RARα (or semistable CHO cells), when treated with RA, displayed decreased staining with anti-PLZF antibody (Fig. 5 A1 and A2) and a lower number of PLZF/RARα-positive cells (data not shown). Western blot analysis of extracts derived from treated and untreated cells indicated sharply decreased levels of PLZF/RARα protein (Fig. 5B). Although we cannot exclude the possibility that RA causes elimination of cells expressing PLZF/RARα or inhibits its expression, it is most likely that the PLZF/RARα protein is degraded in RA-treated cells. This conclusion is further supported by marked reduction of PLZF/RARα levels in t(11;17)-positive APL cells after RA treatment (data not shown). These findings are remarkably similar to the recently published results regarding the PML/RARα protein (43–45). It is paradoxical, however, that both fusion proteins are degraded upon RA treatment but that only the t(15;17)-associated APL can be treated by in vivo RA administration (26).
Figure 5.
Exposure to 10−6 M RA lowers both the number and the labeling intensity of PLZF/RARα expressing cells (A1 and A2). PLZF/RARα protein quantities (arrows) in COS6 cells transiently transfected with a PLZF/RARα expression vector, and treated (+RA) or not (−RA) with 10−6 M RA for 24 h (B); the nonspecific band (arrowhead) serves as an internal control. Immunofluorescence with anti-PLZF antibodies of KG1 cells not treated (C1) and treated with IFN-α (C2) or IFN-γ (C3). No apparent induction of the PLZF protein can be detected upon IFN treatment (D1), whereas IFN-inducible p68 kinase is clearly induced (D2).
PML Recruits PLZF onto the PML NB.
Given the inducibility of PML by IFNs, we investigated whether the expression of the PLZF gene is regulated by these signaling molecules. Although IFN-α and IFN-γ substantially increased both the number and intensity of PLZF-positive NBs (Fig. 5 C1–C3), Western blot analysis revealed an increase in the levels of the IFN-induced p68 kinase but not of the PLZF protein (Fig. 5 D1 and D2). The increased PLZF fluorescence in the NBs is most likely due to the recruitment of nucleoplasmic PLZF onto the NBs by increasing amounts of PML after IFN treatment. Given our recent demonstration that arsenic aggregates PML onto “NBs,” the behavior of PLZF after arsenic exposure was investigated. As previously shown for NB-associated proteins (45), together with arsenic, IFN leads to a dramatic aggregation of PLZF and PML onto the bodies, but alone has little or no effect (data not shown).
DISCUSSION
RARα is one of a number of genes that are frequently targeted by chromosomal translocations encountered among different hemopoietic malignancies that are associated with expression of fusion gene products (46). For example, more than 10 different translocations involving MLL gene have been described (47). As with the majority of MLL rearrangements, genes that are fused with the RARα appear to have very little in common except that they all provide RARα with a heterologous dimerization interface, allowing for formation of stable homodimers of the fusion proteins, and may all be involved in some aspects of cell growth control (see above). It is not clear at the present time whether the non-RARα sequences of various chimeric proteins play an active or passive role(s) in transformation. Retinoids and retinoid receptors affect not only differentiation of hemopoietic cells but also cell growth and apoptosis (48, 49). One could therefore argue that the entire APL phenotype, block of differentiation and inhibition of programmed cell death, is a result of a disruption in retinoid signaling by the RARα chimeric proteins.
The first evidence against this hypothesis stems from the fact that in several cellular systems PML is a potent growth suppressor (17–19), and the second stems from the ability of the PML/RARα chimeric protein to delocalize different components of NBs onto microspeckled nuclear structures, a phenomenon reversible in RA-sensitive APL cells upon their differentiation in response to RA (21–24, 50). Moreover, in RA-resistant NB4 mutants that only differentiate in the presence of cAMP, RA followed by cAMP administration, but not RA alone, restores both NB structure and differentiation (25). Furthermore, the dramatic role of arsenic trioxide, which targets PML and PML/RARα onto NBs and induces apoptosis in APL cells (45), strongly suggests a contribution of PML and NB disruption in the pathogenesis of this disease.
Our experiments identify, to our knowledge, the first PML-associated protein for which physical interaction was actually demonstrated. This is in contrast with the recent work on the Pic-1 protein (51) where coimmunoprecipitation and GST “pull down” were not feasible, questioning a non-enzyme-mediated direct interaction with PML. Colocalization and interaction between PML and PLZF strongly suggests a functional relationship for these two proteins. In the context of their respective RARα chimeras, PML and PLZF may also affect the same nuclear processes. This is strongly supported by the fact that both PML/RARα and PLZF/RARα localize in the microspeckled nuclear structures. Some of these microspeckles are sites of transcription (52) and could be important targets for the transforming activities of the fusions. Our studies of PLZF protein indicate that it functions as a DNA binding transcriptional repressor (unpublished data) and that the BTB/POZ domain is required for the repressing effect. In this respect, it is interesting that a Krüppel-like Zn-finger protein with a related transcriptional repression domain, KRAB, interacts with a PML-related RING-finger protein, TIF1β (53), and this interaction is required for transcriptional repression by KRAB proteins (54–56). Note, in this respect that a PML family member, Rpt-1, is a transcriptional repressor of several promoters (57) and that PML appears to enhance the transcription by the progesterone receptor (58). In an analogous manner, PML could participate in transcriptional regulation by the PLZF protein. Alternatively, as suggested by the sharp increase in NB-associated PLZF fluorescence after IFN treatment (and furthermore arsenic), PML could shuttle bound proteins, such as PLZF and SP100 (45), from the nucleoplasm onto the NBs and in this way modulate transcriptional regulation by the PLZF protein.
Interestingly, in NB4 cells PML/RARα delocalizes the wild-type PLZF, whereas PLZF/RARα has no effect on the localization of the wild-type PML, pointing to the importance of alterations in PLZF nuclear localization. In this respect, it is worth noting that the pattern of PLZF expression during hemopoiesis suggests that its product may play a role in myeloid development (28) and perturbation of this potential role by the PML/RARα chimera may contribute to leukemogenesis. Whereas all the data presented herein for PLZF/RARα are derived from studies using transfected cells, results obtained from recent analyses of cells derived from a t(11;17)-positive APL patient (to be reported elsewhere) completely corroborate conclusions drawn in this manuscript. It is remarkable that the two different RARα gene translocations lead to the expression of fusion proteins that colocalize in the same microspeckled nuclear structures and that the common feature of the leukemias involving either PML [t(15;17)] or PLZF [t(11;17)] is the presence of PLZF and RARα sequences in these microspeckles. Although several aspects deserve additional analysis, our findings that PLZF and PML (which both have growth suppressive/apoptotic properties) interact and colocalize in the nucleus, unify not only the cellular targets of the two fusions but also the pathophysiology of APL.
Acknowledgments
We thank Michel Schmid for help with confocal analysis, Bernard Boursin for photography, Dr. Leanne Wiedemann for comments, Dr. Alan Ashworth for advice on the yeast two-hybrid system, Philip Strutt for technical assistance, and Dr. Pierre Chambon for gift of PML(B) expression vector. Drs. Laurent Degos and Mel Greaves are acknowledged for support and stimulating discussions. A.Z. also thanks Dr. Peter O’Hare for comments and discussion. This research was supported by the Leukaemia Research Fund of Great Britain, National Institutes of Health Grant CA-59936–01, and the Centre National de la Recherche Scientifique. J.Z. was supported by th Samuel Waxman Cancer Research Foundation, and S.D., S.-J.C., and Z.D. were supported by the National Natural Science Foundation of China and Shanghai Life Science Centre.
ABBREVIATIONS
- RAR
retinoic acid receptor
- PLZF
promyelocytic leukemia zinc-finger gene
- PML
promyelocytic leukemia gene
- APL
acute promyelocytic leukemia
- RA
all-trans-retinoic acid
- NB
nuclear body
- IFN
interferon
- GST
glutathione S-transferase
References
- 1.Warrell R, de Thé H, Wang Z, Degos L. N Engl J Med. 1993;329:177–189. doi: 10.1056/NEJM199307153290307. [DOI] [PubMed] [Google Scholar]
- 2.Borrow J, Goddart A, Sheer D, Solomon E. Science. 1990;249:1577–1580. doi: 10.1126/science.2218500. [DOI] [PubMed] [Google Scholar]
- 3.de Thé H, Chomienne C, Lanotte M, Degos L, Dejean A. Nature (London) 1990;347:558–561. doi: 10.1038/347558a0. [DOI] [PubMed] [Google Scholar]
- 4.Alcalay M, Zangrilli D, Pandolfi P, Longo L, Mencarelli A, Giacomucci A, Rocchi M, Biondi A, Rambaldi A, Lo Coco F, Diverio D, Donti E, Grignani F, Pelicci P. Proc Natl Acad Sci USA. 1991;88:1977–1981. doi: 10.1073/pnas.88.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redner R L, Rush E A, Faas S, Rudert W A, Corey S J. Blood. 1996;87:882–886. [PubMed] [Google Scholar]
- 6.Chen Z, Brand N, Chen A, Chen S, Tong J, Wang Z, Waxman S, Zelent A. EMBO J. 1993;12:1161–1167. doi: 10.1002/j.1460-2075.1993.tb05757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells R A, Kamel-Reid S. Blood. 1996;88:S1449. (abstr.). [Google Scholar]
- 8.de Thé H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean A. Cell. 1991;66:675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- 9.Kakizuka A, Miller W, Jr, Umesono K, Warrell R, Jr, Frankel S R, Murty V V, Dmitrovsky E, Evans R M. Cell. 1991;66:663–74. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- 10.Kastner P, Perez A, Lutz Y, Rochette-Egly C, Gaub M-P, Durand B, Lanotte M, Berger R, Chambon P. EMBO J. 1992;11:629–642. doi: 10.1002/j.1460-2075.1992.tb05095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goddard A D, Borrow J, Freemont P S, Solomon E. Science. 1991;254:1371–1374. doi: 10.1126/science.1720570. [DOI] [PubMed] [Google Scholar]
- 12.Pandolfi P P, Grignani F, Alcalay M, Mencarelli A, Biondi A, LoCoco F, Grignani F, Pelicci P G. Oncogene. 1991;6:1285–1292. [PubMed] [Google Scholar]
- 13.Freemont P, Hanson I, Trowsdale J. Cell. 1991;64:483–484. doi: 10.1016/0092-8674(91)90229-r. [DOI] [PubMed] [Google Scholar]
- 14.Stadler M, Chelbi-Alix M K, Koken M H M, Venturini L, Lee C, Saïb A, Quignon F, Pelicano L, Guillemin M-C, Schindler C, de Thé H. Oncogene. 1995;11:2565–2573. [PubMed] [Google Scholar]
- 15.Chelbi-Alix M K, Pelicano L, Quignon F, Koken M H M, Venturini L, Stadler M, Pavlovic J, Degos L, de Thé H. Leukemia. 1995;9:2027–2033. [PubMed] [Google Scholar]
- 16.Lavau C, Marchio A, Fagioli M, Jansen J, Falini B, Lebon P, Grosveld F, Pandolfi P P, Pelicci P G, Dejean A. Oncogene. 1995;11:871–876. [PubMed] [Google Scholar]
- 17.Koken M H M, Linares-Cruz G, Quignon F, Viron A, Chelbi-Alix M K, Sobczak-Thépot J, Juhlin L, Degos L, Calvo F, de Thé H. Oncogene. 1995;10:1315–1324. [PubMed] [Google Scholar]
- 18.Mu Z M, Chin K V, Liu J H, Lozano G, Chang K S. Mol Cell Biol. 1994;14:6858–6867. doi: 10.1128/mcb.14.10.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J-H, Mu Z-M, Chang K-S. J Exp Med. 1995;181:1965–1973. doi: 10.1084/jem.181.6.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang K-S, Fan Y-H, Andreeff M, Liu J, Mu Z-M. Blood. 1995;85:3646–3653. [PubMed] [Google Scholar]
- 21.Koken M H M, Puvion-Dutilleul F, Guillemin M C, Viron A, Linares-Cruz G, Stuurman N, de Jong L, Szostecki C, Calvo F, Chomienne C, Degos L, Puvion E, de Thé H. EMBO J. 1994;13:1073–1083. doi: 10.1002/j.1460-2075.1994.tb06356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniel M-T, Koken M, Romagné O, Barbey S, Bazarbachi A, Stadler M, Guillemin M-C, Degos L, Chomienne C, de Thé H. Blood. 1993;82:1858–1867. [PubMed] [Google Scholar]
- 23.Dyck J A, Maul G G, Miller W H, Chen J D, Kakizuka A, Evans R M. Cell. 1994;76:333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 24.Weis K, Rambaud S, Lavau C, Jansen J, Carvalho T, Carmo-Fonseca M, Lamond A, Dejean A. Cell. 1994;76:345–356. doi: 10.1016/0092-8674(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 25.Duprez E, Lillehaug J R, Naoe T, Lanotte M. Oncogene. 1996;12:2451–2459. [PubMed] [Google Scholar]
- 26.Licht J D, Chomienne C, Goy A, Chen A, Scott A A, Head D R, Michaux J L, Wu Y, DeBlasio A, Miller W H, Jr, Zelenetz A D, Willman C L, Chen Z, Chen S-J, Zelent A, Macintyre E, Veil A, Cortes J, Kantarjian H, Waxman S. Blood. 1995;85:1083–1094. [PubMed] [Google Scholar]
- 27.Licht J D, Shaknovich R, English M A, Melnick A, Li J-Y, Reddy J C, Dong S, Chen S-J, Zelent A, Waxman S. Oncogene. 1996;12:323–336. [PubMed] [Google Scholar]
- 28.Reid A, Gould A, Brand N, Cook M, Strutt P, Li J, Licht J, Waxman S, Krumlauf R, Zelent A. Blood. 1995;86:4544–4552. [PubMed] [Google Scholar]
- 29.Bardwell V J, Treisman R. Genes Dev. 1994;8:1664–1677. doi: 10.1101/gad.8.14.1664. [DOI] [PubMed] [Google Scholar]
- 30.Albagli O, Dhordain P, Deweindt C, Lecocq G, Leprince D. Cell Growth Differ. 1995;6:1193–1198. [PubMed] [Google Scholar]
- 31.Sitterlin D, Tiollais P, Transy C. Oncogene. 1997;14:1067–1074. doi: 10.1038/sj.onc.1200916. [DOI] [PubMed] [Google Scholar]
- 32.Dong S, Zhu J, Reid A, Strutt P, Guidez F, Zhong H-J, Wang Z-Y, Licht J, Waxman S, Chomienne C, Zelent A, Chen S-J. Proc Natl Acad Sci USA. 1996;93:3624–3629. doi: 10.1073/pnas.93.8.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 34.Gietz R D, Woods R A. In: Molecular Genetics of Yeast: A Practical Approach. Johnston J R, editor. Oxford: Oxford Univ. Press; 1994. pp. 121–134. [Google Scholar]
- 35.Durfee T, Becherer K, Chen P-L, Yeh S-H, Yang Y, Kilburn A E, Lee W-H, Elledge S J. Genes Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- 36.Johnston J R. In: The Practical Approach Series. Rickwood D, Hames B D, editors. Oxford: Oxford Univ. Press; 1994. [Google Scholar]
- 37.Guarente L. In: Methods in Enzymology. Wu R, Grossman L, Moldave K, editors. Vol. 101. New York: Academic; 1983. pp. 181–191. [DOI] [PubMed] [Google Scholar]
- 38.Cook M, Gould A, Brand N, Davies J, Strutt P, Shaknovich R, Licht J, Waxman S, Chen Z, Gluecksohn-Waelsch S, Krumlauf R, Zelent A. Proc Natl Acad Sci USA. 1995;92:2249–2253. doi: 10.1073/pnas.92.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stuurman N, de Graaf A, Floore A, Josso A, Humbel B, de Jong L, van Driel R. J Cell Sci. 1992;101:773–784. doi: 10.1242/jcs.101.4.773. [DOI] [PubMed] [Google Scholar]
- 40.Perez A, Kastner P, Sethi S, Lutz Y, Reibel C, Chambon P. EMBO J. 1993;12:3171–3182. doi: 10.1002/j.1460-2075.1993.tb05986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Estojak J, Brent R, Golemis E A. Mol Cell Biol. 1995;15:5820–5829. doi: 10.1128/mcb.15.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korioth F, Gieffers C, Maul G G, Frey J. J Cell Biol. 1995;130:1–13. doi: 10.1083/jcb.130.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raelson J V, Nervi C, Rosenauer A, Benedetti L, Monczak Y, Pearson M, Pelicci P G, Miller W H. Blood. 1996;88:2826–2832. [PubMed] [Google Scholar]
- 44.Yoshida H, Kitamura K, Tanaka K, Omura S, Miyazaki T, Hachiya T, Ohno R, Naoe T. Cancer Res. 1996;56:2945–2948. [PubMed] [Google Scholar]
- 45.Zhu J, Koken M H M, Quignon F, Chelbi-Alix M K, Degos L, Wang Z Y, Chen Z, de Thé H. Proc Natl Acad Sci USA. 1997;94:3978–3983. doi: 10.1073/pnas.94.8.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabbitts T H. Nature (London) 1994;372:143–149. doi: 10.1038/372143a0. [DOI] [PubMed] [Google Scholar]
- 47.Rowley J D. Semin Cancer Biol. 1993;4:377–385. [PubMed] [Google Scholar]
- 48.Nagy L, Thomazy V A, Shipley G L, Fesus L, Lamph W, Heyman R A, Chandraratna R A, Davies P J. Mol Cell Biol. 1995;15:3540–3551. doi: 10.1128/mcb.15.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smeland E B, Rusten L, Jacobsen S E, Skrede B, Blomhoff R, Wang M Y, Funderud S, Kvalheim G, Blomhoff H K. Blood. 1994;84:2940–2945. [PubMed] [Google Scholar]
- 50.Zhu J, Shi X-G, Chu H-Y, Tong J-H, Wang Z-Y, Naoe T, Waxman S, Chen S-J, Chen Z. Leukemia. 1995;9:302–309. [PubMed] [Google Scholar]
- 51.Boddy M N, Howe K, Etkin L D, Solomon E, Freemont P S. Oncogene. 1996;13:971–982. [PubMed] [Google Scholar]
- 52.Grande M A, van der Kraan I, van Steensel B, Schul W, de Thé H, van der Voort H T M, de Jong L, van Driel R. J Cell Biochem. 1996;63:280–291. doi: 10.1002/(sici)1097-4644(19961201)63:3<280::aid-jcb3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 53.Le Douarin B, Nielsen A L, Garnier J-M, Ichinose H, Jeanmougin F, Losson R, Chambon P. EMBO J. 1996;15:6701–6715. [PMC free article] [PubMed] [Google Scholar]
- 54.Moosmann P, Georgiev O, le Douarin B, Bourquin J-P, Schaffner W. Nucleic Acids Res. 1996;24:4859–4867. doi: 10.1093/nar/24.24.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim S-S, Chen Y-M, O’Leary E, Witzgall R, Vidal M, Bonventre J V. Proc Natl Acad Sci USA. 1996;93:15299–15304. doi: 10.1073/pnas.93.26.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Friedman J R, Fredericks W J, Jensen D E, Speicher D W, Huang X P, Neilson E G, Rauscher F J. Genes Dev. 1996;10:2067–2078. doi: 10.1101/gad.10.16.2067. [DOI] [PubMed] [Google Scholar]
- 57.Patarca R, Scharwtz J, Singh R P, Kong K T, Murphy E, Anderson Y, Sheng F Y, Singh P, Johnson K A, Guarnagia S M, Durfee T, Blattner F, Cantor H. Proc Natl Acad Sci USA. 1988;85:2733–2737. doi: 10.1073/pnas.85.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guiochon-Mantel A, Savouret J F, Quignon F, Delabre K, Milgrom E, de Thé H. Mol Endocrinol. 1995;9:1791–1803. doi: 10.1210/mend.9.12.8614415. [DOI] [PubMed] [Google Scholar]