Abstract
Lipoprotein lipase (LPL) is the rate-limiting enzyme for the import of triglyceride-derived fatty acids by muscle, for utilization, and adipose tissue (AT), for storage. Relative ratios of LPL expression in these two tissues have therefore been suggested to determine body mass composition as well as play a role in the initiation and/or development of obesity. To test this, LPL knockout mice were mated to transgenics expressing LPL under the control of a muscle-specific promoter (MCK) to generate induced mutants with either relative (L2-MCK) or absolute AT LPL deficiency (L0-MCK). L0-MCK mice had normal weight gain and body mass composition. However, AT chemical composition indicated that LPL deficiency was compensated for by large increases in endogenous AT fatty acid synthesis. Histological analysis confirmed that such up-regulation of de novo fatty acid synthesis in L0-MCK mice could produce normal amounts of AT as early as 20 h after birth. To assess the role of AT LPL during times of profound weight gain, L0-MCK and L2-MCK genotypes were compared on the obese ob/ob background. ob/ob mice rendered deficient in AT LPL (L0-MCK-ob/ob) also demonstrated increased endogenous fatty acid synthesis but had diminished weight and fat mass. These findings reveal marked alterations in AT metabolism that occur during LPL deficiency and provide strong evidence for a role of AT LPL in one type of genetic obesity.
Lipoprotein lipase (LPL), located on the capillary endothelium of extrahepatic tissues, catalizes the rate-limiting step in the hydrolysis of triglycerides (TGs) from circulating chylomicrons and very low density lipoprotein (for reviews, see refs. 1 and 2). Quantitatively, most LPL is found in adipose tissue (AT) and muscle, where the liberated free fatty acids are taken up and either stored or oxidized, respectively (3). It has been hypothesized that relative levels of LPL activity in AT and muscle determine how fat calories are partitioned toward storage or utilization, and that imbalances in tissue expression can therefore lead to obesity or weight loss (4, 5). Until the advent of induced mutant mice, experimental systems to directly test this hypothesis have not been available.
We have previously generated LPL knockout mice (6) as well as transgenic mice expressing human LPL exclusively in muscle (7). LPL-deficient mice are normal at birth, but develop lethal hypertriglyceridemia within the first day of life, at which point they have markedly reduced intracellular lipid stores. Transgenic mice that express high levels of human LPL in muscle showed increased muscle free fatty acid concentrations and increased numbers of fatty acid-metabolizing organelles (mitochondria and peroxisomes). Together these observations suggest that tissue LPL expression is a major determinant of fatty acid entry into cells. Unfortunately, the previous studies could not answer questions about the long-term metabolic consequences for the host of altered tissue expression of LPL. Neonatal death in the knockout mice precluded examination of tissue lipid accumulation in healthy adult animals. The transgenic mice expressing high levels of human LPL did loose weight, but developed myopathy and died. Their illness precluded definitive statements about the consequences of fat calorie apportionment between AT and muscle for body mass composition (BMC).
In the current study, we have rescued the LPL knockout trait by breeding in a relatively low-expressing muscle-specific human LPL transgene from a line that did not develop myopathy. The breeding scheme allowed us to compare wild-type mice (L2) with littermates containing the muscle-specific transgene and either two (L2-MCK) or no (L0-MCK) functional copies of the endogenous LPL gene (MCK, mouse creatine kinase). Effects of these geneotypes on the morbidly obese ob/ob background were also assessed. Comparisons of L2 with L0-MCK mice demonstrate that although AT LPL is not essential for adipose development, marked qualitative and quantitative (on the ob/ob background) differences in adipose stores are seen in its absence. By comparing L2 with the L2-MCK mice, relative overexpression of LPL in muscle was shown to have metabolic consequences, confirming that relative levels of muscle and AT LPL are physiologically important.
METHODS
Generation of Induced Mutant Mice on the Wild Type and the ob/ob Background.
The breeding strategy to produce mice deficient in AT LPL involved two crosses. First, LPL heterozygous knockout mice (mL1) and transgenic mice expressing human LPL (hLPL) exclusively in skeletal and cardiac muscle under control of the mouse MCK promoter [MCK-L (low expressor) renamed mL2/MCK-hLPL] were interbred to yield heterozygous knockout animals that contained the muscle-specific human transgene (mL1/MCK-hLPL). These were then backcrossed to mL1 mice to produce the following littermates for study (Table 1): mL2 mice (renamed L2) containing two functional copies of the mouse LPL gene (mLPL) with no transgene, mL2/MCK-hLPL mice (renamed L2-MCK) containing two functional copies of mLPL plus the muscle-specific hLPL transgene, and mL0/MCK-hLPL mice (renamed L0-MCK) containing no functional copies of mLPL with hLPL transgene expression exclusively in muscle. Tissue LPL activities were measured in the fed state (8 a.m.) as described (7). Skeletal muscle activities in L2, L2-MCK, and L0-MCK mice were 1.4 ± 0.5, 9.3 ± 1.4 (P < 0.05 vs. L2), and 9.3 ± 2.2 (P < 0.05 vs. L2), respectively. AT LPL activities were 9.4 ± 5.7, 17.1 ± 6.7, and 0.2 ± 0.2 (P < 0.05 vs. L2), respectively.
Table 1.
Summary of genotypes
| Genotype | Locus
|
||
|---|---|---|---|
| LPL | MCK-hLPL transgene | Ob | |
| L2 | +/+ | − | +/+ |
| L0-MCK | −/− | + | +/+ |
| L2-MCK | +/+ | + | +/+ |
| L2-MCK-ob/ob | +/+ | + | −/− |
| L0-MCK-ob/ob | −/− | + | −/− |
To generate and assess the effects of AT LPL deficiency on the ob/ob phenotype, L0-MCK mice bred to be homozygous for the MCK-hLPL transgene were crossed with female surrogates transplanted with ova from ob/ob mice (The Jackson Laboratories). All progeny were heterozygous for the LPL knockout, the MCK-hLPL transgene, and ob. These mice were then interbred to produce ob/ob male mice which contained the MCK-hLPL transgene and were either homozygous wild type (L2-MCK-ob/ob) or knockout (L0-MCK-ob/ob) at the LPL locus (Table 1). Genotypes were determined at the mouse LPL, the MCK-hLPL transgene, and the ob loci as previously described (6, 7). All mice were housed in a specific pathogen-free environment.
Growth Curves.
Mice of different genotypes were weaned at 3 weeks and then maintained on chow diet (4.5% fat) and weighed weekly.
BMC.
BMC was performed as described (5). Briefly, 4-month-old (wild-type background) and 7-month-old (ob/ob background) mice were killed by cervical dislocation and weighed (wet weight). To remove all body water, carcasses were placed in a 90°C convection oven until constant mass was observed. Total water content was calculated as pre-oven carcass weight minus post-oven carcass weight (dry weight). Carcasses were frozen briefly in liquid nitrogen and homogenized in a blender. One gram aliquots of homogenate were extracted for 3 h with chloroform/methanol (3:1) in a Soxhlet Extraction Apparatus to remove lipid. The remaining solids were dried over night at room temperature and weighed. Lipid was calculated as weight of sample before extraction minus weight of sample after extraction. Total body lipid was then calculated as lipid in dry carcass aliquot multiplied by total dry carcass weight. Lean body mass was calculated as weight before dehydration (wet weight) minus total lipid and water weights. All samples were done in duplicate.
Histologic Analysis.
At 20 h of life, pups were decapitated and blood and tail tips were removed for plasma and DNA analysis, respectively. Various tissues were then excised and prepared for analysis. For oil red O staining, animals were sagitally halved. One part was embedded in Tissue Tek on a cork plate, frozen in 5-methylbutan (Merck), and precooled in liquid nitrogen. Four-micrometer-thick sections were then cut and stained with oil red O. The remaining half was formalin fixed and embedded in paraffin wax by conventional techniques and stained with either hematoxylin-eosin, masson-trichrome, or periodic acid Schiff.
Fatty Acid Composition Analysis.
The fatty acid composition of the diet, epididymal AT, and plasma were performed as described (8). Briefly, following addition of C17:0 fatty acid TG, cholesterol ester, and phospholipid internal standards (Nu Check Prep, Elysian, MN, and Sigma), total lipid was immediately extracted with Folch solvent. The lipid fractions were separated by TLC using silica gel G plates and a solvent system of hexane/diethyl ether/glacial acetic acid, 60:40:1. The plate was sprayed with Rhodamine G and visualized with UV light. The bands of interest were scraped into tubes containing 2 ml of 5% methanolic hydrochloric acid. The tubes were capped with nitrogen, heated at 70°C for 2 h, and cooled. The fatty acid methyl ester were extracted with hexane after the addition of water, and run on a temperature programmed gas chromatograph (model 5890; Hewlett–Packard) equipped with a flame ionization detector and a 100 m × 0.25 mm SP2560 fused silica capillary column (Supelco) as described (8). Forty-three peaks were identified, corresponding to fatty acids 10:0 to 22:6n-3, and the weight percent for each fatty acid was calculated.
RESULTS
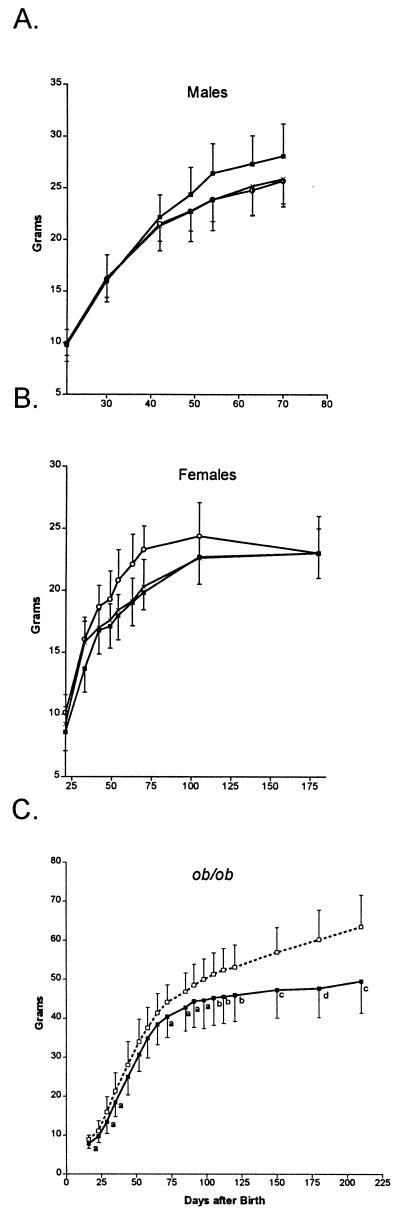
To assess the consequences of absolute (L0-MCK) and relative (L2-MCK) AT LPL deficiency on body weight, standard growth curves on chow diet were generated. Male (Fig. 1A) and female (Fig. 1B) mice of all groups gained weight at similar rates over the entire observation period. Several other cohorts of male littermates were weighed at 6–12 months of age, and no significant weight differences among the genotypes were ever observed (data not shown). L2-MCK female mice were slightly heavier at the start of the experiment (21 days) and maintained this difference (≈2 g) throughout the observation period. All three groups of female mice were weighed again at 6 months of age and found to be of similar weight (23 g). To assess the influence of AT LPL on weight gain in genetically programmed obesity, growth curves were also generated for L2-MCK and L0-MCK mice on the ob/ob background. As shown in Fig. 1C, as early as 16 days postpartum and through 65 days of age the L0-MCK-ob/ob mice showed a tendency to be lighter than their L2-MCK-ob/ob littermates. After day 65 the rate of weight gain in the L0-MCK-ob/ob mice fell off considerably compared with the L2-MCK-ob/ob mice. In the 4 months between days 90 and 210, L0-MCK-ob/ob mice gained only 5 g, stabilizing at 49 g, whereas L2-MCK-ob/ob mice gained 15 g and were still gaining weight at 64 g when the experiment was terminated.
Figure 1.
Growth curves. At 3 weeks of age, mice of different genotypes were weaned onto chow diet (4.5% fat) and were then weighed weekly. (A) Male L2 (×, n = 5), L2-MCK (○, n = 8), and L0-MCK (▪, n = 6) mice. (B) Female L2 (×, n = 7), L2-MCK (○, n = 8), and L0-MCK (▪, n = 10) mice. (C) L2-MCK-ob/ob (□, n = 20) and L0-MCK-ob/ob (▪, n = 16) mice. Values represent means ± SD. a, P < 0.05 (vs. L2-MCK-ob/ob); b, P < 0.005 (vs. L2-MCK-ob/ob); c, P < 0.0005 (vs. L2-MCK-ob/ob); d, P < 0.00005 (vs. L2-MCK-ob/ob).
BMC was determined on L2, L0-MCK and L2-MCK littermates, and no differences were found (Table 2). However, when L2-MCK-ob/ob mice were compared with age-matched L0-MCK-ob/ob mice, the entire difference in weight was found to be in the lipid mass, with equal water and lean body mass (Table 2).
Table 2.
BMC of transgenic and control mice
| Genotype | n | Weight, g | Water
|
Lipid
|
Lean body mass
|
|||
|---|---|---|---|---|---|---|---|---|
| Total, g | % | Total, g | % | Total, g | % | |||
| L2 | 5 | 25.6 ± 4.4 | 17 ± 3 | 67 ± 2 | 1.7 ± 0.3 | 7 ± 1 | 7 ± 1 | 27 ± 1 |
| L0-MCK | 6 | 24.6 ± 2.1 | 16 ± 2 | 67 ± 2 | 1.6 ± 0.4 | 7 ± 1 | 7 ± 1 | 27 ± 2 |
| L2-MCK | 7 | 25.9 ± 2.3 | 17 ± 1 | 67 ± 1 | 1.7 ± 0.3 | 7 ± 1 | 7 ± 1 | 27 ± 1 |
| L2-MCK-ob/ob | 5 | 67 ± 3 | 26 ± 3 | 39 ± 4 | 29 ± 5 | 43 ± 6 | 13 ± 1 | 19 ± 2 |
| L0-MCK-ob/ob | 4 | 52 ± 7a | 27 ± 5 | 51 ± 2a | 13 ± 0b | 26 ± 4 | 12 ± 2 | 22 ± 2c |
Two sets of animals were analyzed as described (6). L2, L2-MCK, and L0-MCK made up one group of littermates (3–4 months of age). L2-MCK-ob/ob and L0-MCK-ob/ob mice made up the second set (9 months of age). a, P < 0.005 (vs. L2-MCK-ob/ob), b, P < 0.0005 (vs. L2-MCK-ob/ob); c, P < 0.05 (vs. L2-MCK-ob/ob).
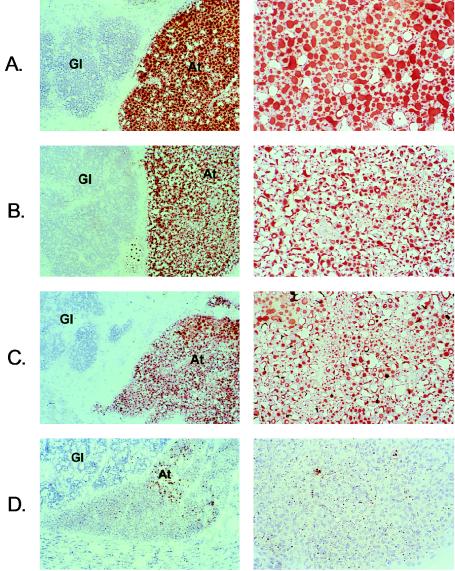
The normal BMC and gross appearance of AT in adult L0-MCK mice was unexpected in view of the critical role of LPL in AT fatty acid uptake and storage. In our previous study (6), L2 and LPL knockout (L0) mice had little to no AT at birth (cesarean section), but by 20 h of age the L2 mice had adipose stores, whereas the L0 mice did not. In the current study, L2, L0-MCK, L2-MCK, and L0 mice all contained only trace amounts of adipose stores at birth (data not shown). However, as shown in Fig. 2 Left (×70 magnification) by representative sections of periglandular AT, at 20 h of age all but the L0 mice had adequate AT at this and other sites. Careful histological examination (Fig. 2 Right, ×270 magnification) revealed relatively homogeneous, large, univacuolated adipocytes in L2 mice whereas both L0-MCK and L2-MCK mice had increased morphological heterogeneity with the appearance of many plurivacuolated adipocytes and a decrease in large univacuolated cells. Thus it appears that on a normal background, AT LPL deficiency has effects on cellular morphology but does not prevent AT accumulation, and animals can compensate for the deficit even as early as 20 h of age.
Figure 2.
Histologic analysis of AT. Typical periglandular AT from (A) L2, (B) L0-MCK, (C) L2-MCK, and (D) L0 pups at 20 h of life. (Left, ×70 magnification; Right, ×270 magnification focused on AT only.) Gl, glandular epithelium; At, adipose tissue.
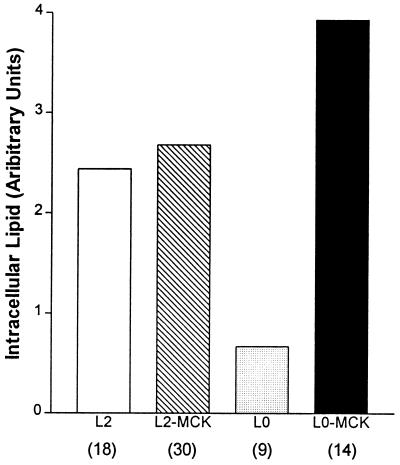
Intramuscular lipid accumulation was next assessed with different amounts of muscle LPL expression with and without AT LPL expression. Light microscopy was performed on muscle sections from 20-h-old L2, L2-MCK, L0-MCK, and L0 pups and intramuscular lipid content was assessed semiquantitatively (Fig. 3). When compared with muscle from L2 mice, L2-MCK mice had slightly (10–20%), whereas L0-MCK mice had markedly (60%) increased lipid. As previously reported, L0 mice had almost no intramuscular lipid. Thus the absence of AT LPL, more than total muscle LPL activity, determines muscle lipid accumulation.
Figure 3.
Semiquantitative analysis of intramuscular lipid droplets. Semiquantitative analysis of intramuscular lipid was performed “blindly” (without knowledge of genotype) by rating histological sections from one (least) to five (greatest) depending on the amount of intracellular lipid droplets. Values were then averaged for each genotype. Numbers in parentheses denote number of pups analyzed for each genotype.
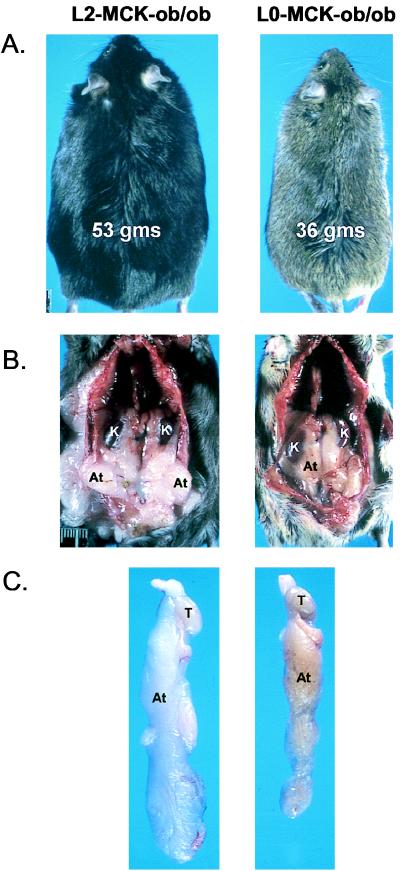
The gross appearances of AT in L2-MCK-ob/ob and L0-MCK-ob/ob mice were next compared (Fig. 4). Compatible with the decrease in weight and lipid mass, there was a gross decrease in perirenal and gonadal AT in L0-MCK-ob/ob mice as well as at other sites (data not shown). The AT also appeared brown with increased vascularity rather than white. Thus in genetically programmed obesity the lack of AT LPL impedes the accumulation of lipid in adipose stores.
Figure 4.
Gross appearance of ob/ob mice deficient in AT LPL. L2-MCK-ob/ob (Left) and L0-MCK-ob/ob (Right) mice and tissues were compared. (A) Typical male littermates at 7 months. Body weights are noted. (B) Perirenal and retroperitoneal fat depots. K, kidney; At, adipose tissue. (C) Gonadal fat pads. T, testes.
The chemical nature of the lipid stored in the AT of the induced mutant mice was determined to establish whether it was the result of dietary lipid import or endogenous synthesis. As shown in Table 3, dietary lipid contained a large amount of the essential fatty acid 18:2 (45.1%), and relatively little 16:1 (1.3%), a fatty acid that is synthesized endogenously from glucose. As expected, L2 mice had AT TG fatty acid composition with 18:2 of 35.0% and 16:1 of 4.6%, which largely reflects dietary fatty acid composition. Compared with diet, the slight decrease in 18:2 and increase 16:1 fatty acids is consistent with a small contribution of endogenously synthesized fatty acids in normal AT. In contrast, L0-MCK mice that lack AT LPL had a markedly different AT TG fatty acid profile with 18:2 of 4.7% and 16:1 of 20.6%. The 90% decrease in 18:2 and the 4-fold increase in 16:1 suggest that in the absence of AT LPL, adipose stores consist largely of endogenously synthesized rather than dietary fatty acid. In L2-MCK mice the AT TG fatty acid profile was intermediate between the L2 and L0-MCK mice with 18:2 of 25.8% and 16:1 of 9.5%. This suggests that in the presence of normal AT LPL activity relative over expression of muscle LPL steals fatty acid substrate, which turns on endogenous AT fatty acid synthesis. Assuming no selective metabolism of 18:2 fatty acids, the calculated fractions of endogenously synthesized fatty acids were 23%, 90%, and 43%, in L2, L0-MCK, and L2-MCK mice, respectively (8). As with the induced mutant mice on a normal background, L0-MCK-ob/ob mice had decreased 18:2 (13.7% versus 26.1%) and increased 16:1 (11.4% versus 6.4%), compared with L2-MCK-ob/ob mice (Table 3). This suggests that L0-MCK-ob/ob mice were capable of large amounts of endogenous fat synthesis, but not enough to keep up with the demands of the genetically programmed weight gain.
Table 3.
Mean fatty acid composition of diet and AT
| Fatty acid, %
|
|||||
|---|---|---|---|---|---|
| 18:2 | 9c:16:1 | 16:0 | 18:0 | 9c:18:1 | |
| Diet | 45.1 | 1.3 | 15.3 | 4.2 | 20.5 |
| AT TG | |||||
| L2 | 35.1 ± 1.5 | 4.6 ± 1.0 | 21.2 ± 1.1 | 3.0 ± 0.5 | 25.3 ± 1.2 |
| L0-MCK | 4.7 ± 0.8a | 20.6 ± 4.2b,c | 28.8 ± 1.5b | 3.3 ± 0.8 | 33.7 ± 5.7 |
| L2-MCK | 25.8 ± 3.2d | 9.5 ± 0.6b | 24.8 ± 1.6d | 3.0 ± 0.8 | 26.0 ± 2.2 |
| L2-MCK-ob/ob | 22.62 ± 2.24 | 6.4 ± 1.5 | 17.3 ± 0.7 | 1.5 ± 0.3 | 41.3 ± 1.4 |
| L0-MCK-ob/ob | 13.71 ± 1.39e | 11.4 ± 3.0f | 14.0 ± 2.2f | 1.5 ± 0.5 | 48.4 ± 2.7e |
The fatty acid composition of the diet and epididymal AT from fasted mice were performed as described (11).
a, P (vs. L2) < 0.000001; b, P (vs. L2) < 0.003; c, P (vs. L2-MCK) < 0.01; d, P (vs. L2) < 0.05; e, P(vs. L2-MCK-ob/ob) < 0.0001; f, P (vs. L2-MCK-ob/ob) < 0.01.
DISCUSSION
Until the advent of transgenic models, a cause-and-effect relationship between patterns of tissue-specific LPL expression and BMC has been difficult to definitively establish. Prior studies support a link by measuring increased AT LPL activity either in “obese” AT (3, 9) or in AT following weight reduction (10, 11). To vigorously address this question we have produced animals that contain particular tissue-specific patterns of LPL expression and looked at the effects of such manipulations on AT development. Specifically, the present study describes induced mutant mice in which altered muscle to AT LPL activity ratios lead to predicited patterns of fat calorie partitioning yet unexpected effects on energy storage. Mice deficient in AT LPL (L0-MCK) had normal weight gain and BMC on the wild-type background, with an AT fatty acid composition suggesting endogenous synthesis. Moreover, mice with an increased ratio of muscle to AT LPL (L2-MCK) had evidence of fatty acid substrate steal, but again BMC remained normal with evidence of increased endogenous AT fatty acid synthesis. On the background of genetically programmed obesity, ob/ob mice deficient in AT LPL had diminished fat mass, suggesting AT LPL could be rate limiting in fat accumulation under some circumstances. The implications of these findings are several fold.
In the absence of AT LPL the body is capable of synthesizing virtually its entire adipose store endogenously. In contrast with our findings, several clinical metabolic studies found little de novo synthesis of AT fatty acid and concluded that this pathway is limited and not of importance in humans (12–14). This raises the issue of possible species differences. However, compatible with our observations in mice, humans with type I hyperlipoproteinemia, who are LPL-deficient, have grossly normal fat stores (15). Moreover, data on AT fatty acid composition in these patients also suggests increased endogenous synthesis (L.C.H., unpublished data). In addition, a recent study by one of us (L.C.H.) has found that weight stable humans on a high carbohydrate diet are capable of increased de novo fatty acid synthesis (8). This was inferred by 13C-acetate studies as well as enrichment of very low density lipoprotein 16:0 and depletion of 18:2 on this diet, but the site of increased endogenous fatty acid synthesis could not be determined in this type of study. Thus significant endogenous fatty acid synthesis in humans can occur. The notion of the importance of endogenous fatty acid synthesis has also recently received support from studies by others in induced mutant mice. Transgenic mice that overexpress the glucose transporter Glut4 (16) in AT have increased fat stores and body weight whereas Glut4 knockout mice (17) have reduced body fat. Thus endogenous fatty acid biosynthesis, in this case driven by the amount of glucose substrate, can have a profound influence on AT synthesis and storage of fat. Thus, the dogma that the endogenous AT fatty acid biosynthesis pathway is limited and not important is probably incorrect. In fact under some circumstances it can even completely substitute for the provision of exogenous fatty acid to AT.
AT mass is precisely maintained even when the primary substrate for the synthesis of AT TG, exogenous fatty acid, is deprived. Thus compensatory mechanisms must have evolved to preserve adipose stores. These backup processes are finely controlled and in the adult animal result in normal adipose stores and, as judged by plasma leptin levels (data not shown), adipose function. The process commences early in life with qualitatively normal AT developing postnatally and present by 20 h of age. This implies that the maintenance of AT mass is essential to the survival of the organism. Interestingly, increased AT lipogenesis has also been recently described in rats having undergone several cycles of starvation–refeeding, again providing a potential survival advantage (18). The control for this process, be it central or local, needs to be elucidated.
Overexpression of LPL in muscle can lead to steal of fatty acid substrate away from AT, but does not result in decreased adipose mass due to endogenous AT fatty acid biosynthesis. Greenwood (4, 5) has postulated that the relative overexpression of AT compared with muscle LPL gives rise to obesity. This was inferred by studies in rodents and humans indicating relative overexpression of AT LPL in obesity conditions (19). Mechanistically it was suggested that the steal of substrate by AT either caused direct fat accumulation or by depriving other tissues of fat derived calories lead to increased appetite and excessive caloric consumption. In the current study we did not generate mice with increased AT to muscle LPL; rather we generated the opposite. In these animals (L2-MCK) there was indeed evidence of substrate steal by muscle, but compensatory increases in endogenous fatty acid synthesis in AT prevented alterations of BMC or food intake. Animals with an increased ratio of AT to muscle LPL activity are currently being generated to directly test the Greenwood hypothesis. However, the current experiments suggest that, in normal mice, a primary alteration in the ratio of muscle to AT LPL activity does not by itself alter weight gain or food consumption.
In ob/ob mice, a lack of AT LPL especially in older mice is associated with a flattening of the weight gain curve and less adipose mass than in mice where AT LPL is present. Under conditions of increased food intake and decreased metabolism that exist in ob/ob mice (19–21), AT LPL-deficient mice gain more weight than normal mice, but less than ob/ob mice with normal AT LPL. The simplest explanation is that endogenous synthesis of AT fatty acids can keep up to a certain point, but reaches some limit. Judging by the plateau in the weight gain curve in L0-MCK-ob/ob mice beyond 90 days a steady-state is then achieved with synthesis and removal pathways in balance. These data suggest that in some cases the amount of AT LPL may be limiting in the development of obesity.
LPL controls the entry of exogenous fatty acids into AT and muscle. LPL knockout mice, which die in the newborn period, have no adipose stores and profoundly decreased lipid in muscle cells at 20 h of age. Furthermore, animals with LPL in muscle only and those with an increased ratio of muscle to AT LPL have increased myocyte lipid with normal amounts of AT. Thus it is clear that muscle LPL can shunt calories, otherwise destined for fat, toward muscle. In fact, in previous studies, very high levels of LPL expression in the muscle of MCK-LPL transgenic mice lead to myopathy, presumably due to toxicity resulting from increased fatty acid entry (7). In the current study, the low level of muscle LPL transgene expression characteristic of the line used was not sufficient to cause toxicity and may have lead to sparing of glucose due to LPL-mediated availability of fatty acids to muscle. With regard to AT, the dramatic reduction in essential fatty acid 18:2 attests to LPL regulation of exogenous fat entry. The compensation in AT of L0-MCK, but not L0 mice, is presumably due to the availability of glucose substrate, via glucose sparing in muscle, which is not possible in L0 mice. Thus we can conclude that LPL is truly a gatekeeper for the entry of fatty acid into both muscle and AT.
In summary, the induced mutant mice reported herein reveal a fundamental role for LPL in tissue entry of fat-derived calories, but also indicate important backup pathways presumably necessary for the survival of the organism.
Acknowledgments
We thank A Strudl, A. Fuchsbichler, and I. Temmel for their expert technical assistance. This work was supported in part by grants S7110 and F00701 (R.Z.) from the Austrian Fonds zur Förderung der Wissenschaftlichen Forschung.
ABBREVIATIONS
- LPL
lipoprotein lipase
- hLPL
human LPL
- AT
adipose tissue
- BMC
body mass composition
- MCK
mouse creatine kinase
- TG
triglycerides
References
- 1.Goldberg I J. J Lipid Res. 1996;37:693–707. [PubMed] [Google Scholar]
- 2.Olivecrona G, Olivecrona T. Curr Opin Lipidol. 1993;4:187–196. [Google Scholar]
- 3.Eckel R H. In: Lipoprotein Lipase. Borensztajn J, editor. Chicago: Evener; 1987. pp. 79–132. [Google Scholar]
- 4.Greenwood, M. R. C. (1985) Int. J. Obesity 9 (Suppl. 1), 67–70. [PubMed]
- 5.Greenwood M R C. Int J Obesity. 1984;8:561–569. [PubMed] [Google Scholar]
- 6.Weinstock P H, Bisgaier C L, Aalto-Setala K, Radner H, Ramakrishnon R, Levak-Frank S, Essenberg A D, Zechner R, Breslow J L. J Clin Invest. 1995;96:2555–2568. doi: 10.1172/JCI118319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levak-Frank S, Radner H, Walsh A, Stollberger R, Knipping G, Heofler G, Sattler W, Weinstock P H, Breslow J L, Zechner R. J Clin Invest. 1995;96:976–986. doi: 10.1172/JCI118145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudgins L C, Hellerstein M K, Seidman C, Neese R A, Diakun J, Hirsch J. J Clin Invest. 1996;97:2081–2091. doi: 10.1172/JCI118645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruen R, Hietanen E, Greenwood M R C. Metabolism. 1979;27:1955–1966. doi: 10.1016/s0026-0495(78)80012-2. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz R S, Brunzell J D. J Clin Invest. 1981;67:1425–1430. doi: 10.1172/JCI110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckel R H, Yost T J, Jensen D R. Eur J Clin Invest. 1995;25:396–402. doi: 10.1111/j.1365-2362.1995.tb01720.x. [DOI] [PubMed] [Google Scholar]
- 12.Hellerstein M K, Christiansen M, Kaempfer S, Kletke C, Wu K, Reid J S, Hellerstein N S, Shackleton C H L. J Clin Invest. 1991;87:1841–1852. doi: 10.1172/JCI115206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aarsland A, Chinkes D, Wolfe R R. J Clin Invest. 1996;98:2008–2017. doi: 10.1172/JCI119005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarz J M, Neese R A, Turner S, Dare D, Hellerstein M K. J Clin Invest. 1996;96:2735–2743. doi: 10.1172/JCI118342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brun L D, Gagne C, Julien P, Tremblay A, Moorjani S, Bouchard C, Lupien P J. Metabolism. 1989;38:1005–1009. doi: 10.1016/0026-0495(89)90013-9. [DOI] [PubMed] [Google Scholar]
- 16.Tozzo E, Shepherd P R, Gnudi L, Kahn B B. Am J Physiol. 1995;268:E956–E964. doi: 10.1152/ajpendo.1995.268.5.E956. [DOI] [PubMed] [Google Scholar]
- 17.Katz E B, Stenbit A E, Hatton K, DePinho R, Charron M J. Nature (London) 1995;377:151–155. doi: 10.1038/377151a0. [DOI] [PubMed] [Google Scholar]
- 18.Kochan Z, Kabowska J, Swierczynski J. Metab Clin Exp. 1997;46:10–17. doi: 10.1016/s0026-0495(97)90160-8. [DOI] [PubMed] [Google Scholar]
- 19.Coleman D L. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 20.Coleman, D. L. (1982) Diabetes 31 (Suppl. 1), 1–6. [DOI] [PubMed]
- 21.Halaas J L, Gajiwala K S, Maffei M, Cohen S L, Chait B T, Rabinowitz D, Lallone R L, Burley S K, Friedman J M. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]