Human nicotinamide phosphoribosyltransferase has been crystallized using microseeding methods and X-ray diffraction data have been collected at 2.0 Å resolution.
Keywords: NMPRTase, NAD biosynthetic pathway
Abstract
In the NAD biosynthetic pathway, nicotinamide phosphoribosyltransferase (NMPRTase; EC 2.4.2.12) plays an important role in catalyzing the synthesis of nicotinamide mononucleotide from nicotinamide and 5′-phosphoribosyl-1′-pyrophosphate. Because the diffraction pattern of the initally obtained crystals was not suitable for structure analysis, the crystal quality was improved by successive use of the microseeding technique. The resultant crystals diffracted to 2.0 Å resolution. These crystals belonged to space group P21, with unit-cell parameters a = 60.56, b = 106.40, c = 82.78 Å. Here, the crystallization of human NMPRTase is reported in the free form; the crystals should be useful for inhibitor-soaking experiments on the enzyme.
1. Introduction
Nicotinamide phosphoribosyltransferase (NMPRTase) catalyzes the synthesis of nicotinamide mononucleotide (NMN) from nicotinamide (NM) and 5′-phosphoribosyl-1′-pyrophosphate (PRPP), thus playing an important role in the cyclic biosynthetic pathway of nicotinamide adenine dinucleotide (NAD) in the human body (Magni et al., 2004 ▶). NAD performs crucial roles in living cells by participating widely in redox reactions and also in signal transduction. In the latter, NAD is degraded and consumed by NAD cyclase, ADP ribosyltransferase etc. (Guse et al., 1996 ▶; Ziegler, 2000 ▶). Thus, in order to maintain the concentration of NAD, organisms have a system that recruits NAD in addition to the pathway for its de novo biosynthesis. Nicotinamide (NM), a degradation product of NAD, is reused in cells via the following two pathways. In the first path, NM is transformed to nicotinic acid (NA) by nicotinamide deaminase and then to nicotinic acid mononucleotide (NAMN) by phosphoribosyltransferase (NAPRTase), ultimately leading to NAD. However, in the human body, nicotinamide deaminase is only found in the case of infection by malarial parasites (Zerez et al., 1990 ▶); it is not found in healthy humans, so this path does not generally contribute. In the second path, the main recruitment path, NM reacts with 5′-phosphoribosyl-1′-pyrophosphate (PRPP) to form nicotinamide mononucleotide (NMN), which then leads to NAD.
It would be expected that the inhibition of NMPRTase would cause exhaustion of NAD and disorder of the NAD-requiring systems of mitochondrial respiratory and energy metabolism. This is supported by a report on a new anticancer drug, FK866 (Hasmann & Schemainda, 2003 ▶), which causes cell apoptosis by inhibiting this enzyme.
NMPRTase has also been identified as pre-B-cell colony-enhancing factor (PBEF; Samal et al., 1994 ▶), a growth factor for early stage B cells, and has recently further been identified as visfatin, a cytokine that is secreted from visceral fat tissues. It has attracted attention as the ‘second insulin’ as visfatin binds to the insulin receptor and acts to lower the blood-sugar level (Fukuhara et al., 2005 ▶).
Recently, crystal structures of NMPRTase/PBEF/visfatin have been reported (Khan et al., 2006 ▶; Kim et al., 2006 ▶; Wang et al., 2006 ▶). Eight structures have been described: three murine structures (free form, PDB codes 2h3b and 2gvl; complex with NMN, 2h3d), three rat structures (free form, 2g95; complex with NMN, 2g96; complex with FK866, 2g97) and two human structures (complex with NMN, 2gvg; complex with FK866, 2gvj). However, the free form of human NMPRTase has not yet been reported.
In this paper, we report the first crystallization of the free form of human NMPRTase using the microseeding technique (Thaller et al., 1981 ▶; Stura, 1999a ▶,b ▶; Bergfors, 2003 ▶), with the aim of structure determination of the enzyme itself and further analysis of its reaction mechanism by conducting inhibitor-soaking experiments on the enzyme.
2. Materials and methods
2.1. Expression and purification of recombinant human NMPRTase
A cDNA encoding human NMPRTase was cloned into the EcoI and SalI sites of the expression vector pGEX6p1 (GE Healthcare). The plasmid was introduced into Escherichia coli BL21 (DE) cells and the cell culture was grown at 310 K in Luria–Bertani medium for 2 h and then cooled to 291 K. Growth continued at 291 K to an optical density OD600 of 0.5–0.6. Induction by the addition of IPTG to a final concentration of 0.1 mM was carried out at 291 K, followed by 3 h incubation. Cells were harvested by centrifugation, collected, resuspended in PBS (0.1 M phosphate-buffered saline pH 7.4) and broken by ultrasonication after the addition of PMSF and DTT to a final concentration of 1 mM each. The resulting lysate was ultracentrifuged and the supernatant was then applied onto glutathione Sepharose 4B beads (GE Healthcare). After washing the column with 60 ml (60 times the bed volume) of PBS followed by 15 ml Tris buffer (30 mM Tris–HCl, 150 mM NaCl, 1 mM DTT pH 8.5), the bound protein was treated with PreScission Protease (GE Healthcare) at 277 K for 8 h to cleave the GST tag. Finally, the protein was purified by gel-filtration chromatography with Tris buffer (20 mM Tris–HCl pH 8.5, 150 mM NaCl, 1 mM DTT).
2.2. Crystallization and X-ray data collection
The concentration of the NMPRTase solution was 10 mg ml−1 in Tris buffer (20 mM Tris–HCl pH 8.5, 150 mM NaCl, 1 mM DTT). The solution was obtained by concentration by ultrafiltration on a Microcon YM-10 (Millipore) and was stored at 277 K. Initial screening was performed using the sitting-drop vapour-diffusion method in a 96-well plate (Hampton Research Inc.) with a commercially available screening kit, Index HT (Hampton Research Inc.), at 277 K. Using each solution from the kit, drops were made by mixing 1 µl NMPRTase solution with 1 µl reservoir solution and were sealed against 80 µl reservoir solution. Crystals first appeared within a week under condition E8 of Index HT, which contained 35% pentaerythritol propoxylate (5/4 PO/OH) and 200 mM KCl pH 7.5. Optimization of this condition was carried out. The following experiments were performed using the hanging-drop vapour-diffusion method in 24-well plates (Hampton Research Inc.). Each well was filled with 500 µl reservoir solution. Drops consisting of 1 µl NMPRTase solution and 1 µl reservoir solution were placed on 22 mm siliconized circular cover slides and set to equilibrate against these reservoir solutions at 277 K. The concentrations of precipitant and of salt and the pH of the reservoir solution were varied within the ranges 30–40%, 0–200 mM and 6–9, respectively. Using the condition 38% pentaerythritol propoxylate (5/4 PO/OH) and 200 mM KCl pH 9.0, crystals similar to that shown in Fig. 1 ▶ were obtained reproducibly. However, these crystals (referred to in the following as the first crystals) were not suitable for structure analysis. Improvement of the quality and size of the crystals was achieved using the microseeding method. The first crystals were crushed in a drop using Micro-Tools (Hampton Research Inc.). The drop containing these seed crystals was transferred into 200 µl 50% aqueous pentaerythritol propoxylate (5/4 PO/OH) solution (Hampton Research Inc.). This solution containing seed crystals was used as the stock solution in the following experiments. 2, 4, 6, 8 and 10 µl aliquots of the stock solution were mixed with 500 µl of 38% pentaerythritol propoxylate (5/4 PO/OH) and 200 mM KCl pH 9.0 (Fitzgerald & Madsen, 1986 ▶; Luft & DeTitta, 1999 ▶). Each mixed solution was left to stand at 277 K for 1 h for pre-equilibration and then used as the reservoir solution for the next stage. In the second stage, drops made by mixing 1 µl NMPRTase solution with 1 µl of each reservoir solution were equilibrated against 500 µl of the above-mentioned reservoir solution at 277 K. At this stage, crystals (referred to as the second crystals) appeared within a week. Further improvement of crystal quality and size were achieved by using the microseeding method again.
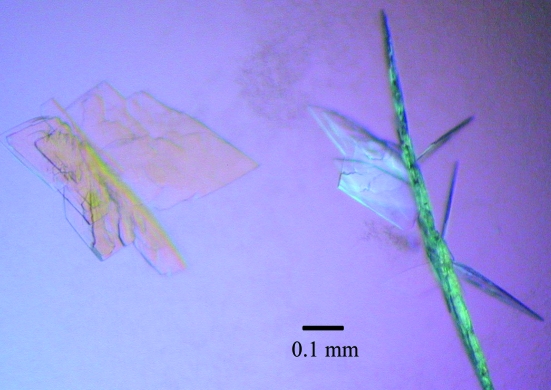
Figure 1.
The initial crystals of human NMPRTase.
In the third stage, crystallization was carried out in a similar way to the method used to obtain the second crystals by preparing the stock solution using the second crystals; suitable crystals were thus obtained (referred to as the third crystals).
Diffraction data were collected from the third crystals in a nitrogen stream at 100 K under cryoconditions [45% pentaerythritol propoxylate (5/4 PO/OH), Tris buffer pH 8.0–9.0 and 200 mM KCl] at beamline BL38B1, SPring-8 (Hyogo, Japan) using a Rigaku Jupiter 210 CCD detector. A total of 180 frames were collected with a rotation angle of 1° and a crystal-to-detector distance of 170 mm. Diffraction images from the CCD detector system were processed using DENZO and SCALEPACK from the HKL-2000 package (Otwinowski & Minor, 1997 ▶) and were further processed using the CCP4 program suite (Colaborative Computational Project, Number 4, 1994 ▶).
3. Results and discussion
NMRPTase plays a critical role in the synthesis of NMN from NM and RPPP in the NAD biosynthetic pathway. Therefore, we attempted to elucidate the three-dimensional structure of human NMPRTase in the free form. We first established the overexpression system for human NMPRTase in E. coli with the plasmid pGEX6p1 and then purified the protein. Crystals of human NMPRTase were initially obtained using the Index HT screening kit. However, these crystals (the first crystals) grew as thin plates stacked on top of each other (Fig. 1 ▶). The diffraction patterns from these first crystals were very poor; we only obtained diffraction spots in one direction because of the thinness of the crystals. In order to improve the quality of the crystals, we carried out microseeding using the first crystals as seeds for subsequent crystallization (Stura, 1999a ▶,b ▶; Bergfors, 2003 ▶). The size of the crystals grown in the seeded experiments depended on the degree of dilution of the stock solution; the size decreased as the ratio of the stock solution increased. Single crystals (the second crystals) with a thickness of ∼0.05 mm were obtained when the stock solution was mixed with the reservoir solution [38% pentaerythritol propoxylate (5/4 PO/OH) and 200 mM KCl pH 9.0] at a ratio of 2–4 µl to 500 µl. The second crystals diffracted to a resolution of 2.5 Å.
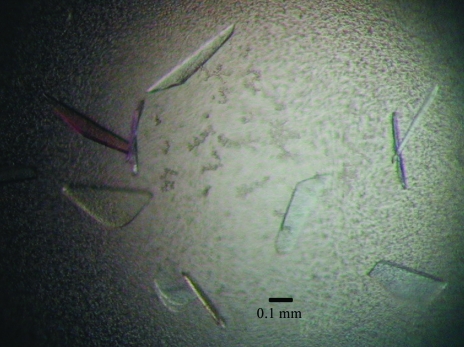
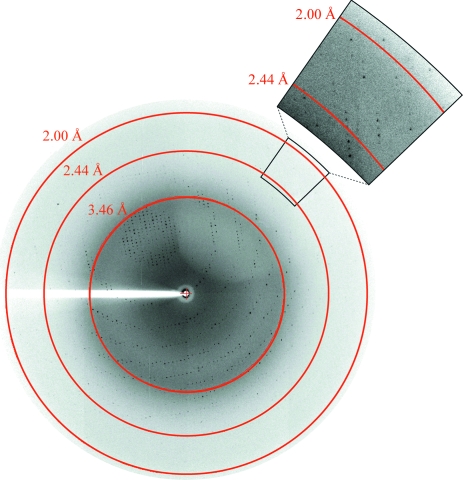
In order to grow large single crystals, microseeding was carried out once more using the second crystals, utilizing the ‘Repeated Seeding Technique’ proposed by Thaller et al. (1981 ▶). Repeatedly applying this microseeding technique, we obtained satisfactory crystals (the third crystals) when the stock solution was mixed with the reservoir solution at a ratio of 2–4 µl to 500 µl. These third crystals, with dimensions of 0.5 × 0.3 × 0.05 mm (Fig. 2 ▶), were suitable for data collection. Although they were rather thin, they diffracted X-rays to 2.0 Å resolution (as shown in Fig. 3 ▶) using the synchrotron-radiation source at SPring-8. Thus, we succeeded in improving the resolution of the crystals from 4.5 to 2.0 Å by repeating the microseeding twice.
Figure 2.
Crystals of human NMPRTase from the third crystallization stage.
Figure 3.
Diffraction image of a crystal of human NMPRTase. An enlarged region with increased contrast is shown at the top right.
The diffraction data indicated that the crystals of NMPRTase in the free form belong to space group P21, with unit-cell parameters a = 60.56, b = 106.40, c = 82.78 Å, β = 96.50°. The data-collection statistics are summarized in Table 1 ▶. Assuming the presence of two human NMPRTase molecules in the asymmetric unit, the Matthews coefficient V M is 2.4 Å3 Da−1, corresponding to a solvent content of 48.5%, which is within the range typical for protein crystals. The result that human NMPRTase exists in a dimeric form in the crystal is supported by the fact that the molecule has been revealed to have a dimeric form in solution by ultracentrifugation analysis with sedimentation-equilibrium measurements (data not shown).
Table 1. Data-collection statistics for human NMPRTase.
Values in parentheses are for the highest resolution shell.
| Temperature (K) | 100 |
| Space group | P21 |
| Unit-cell parameters (Å) | a = 60.56, b = 106.40, c = 82.78 |
| Molecular per ASU | 2 |
| VM (Å3 Da−1) | 2.4 |
| Solvent content (%) | 48.5 |
| Resolution limits (Å) | 50.0–2.0 (2.07–2.00) |
| No. of unique/observed reflections | 70989/250483 |
| Average redundancy | 1.9 (1.8) |
| Completeness (%) | 97.2 (93.2) |
| Rmerge† | 0.078 (0.377) |
R
merge = 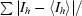
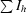 , where 〈I
h〉 is the average intensity of reflection h and symmetry-related reflections.
, where 〈I
h〉 is the average intensity of reflection h and symmetry-related reflections.
We are now determining the three-dimensional structure of human NMPRTase in the free form by the molecular-replacement method using the structure of murine NMPRTase (PDB code 2h3b) as the search model.
Acknowledgments
The authors thank Drs K. Hasegawa and H. Sakai of Japan Synchrotron Radiation Research Institute (JASRI). The X-ray data collection was carried out with the approval of the organizing committee of SPring-8 (proposal No. 2006A1719-NL-np-P3k).
References
- Bergfors, T. (2003). J. Struct. Biol.142, 66–76. [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. [Google Scholar]
- Fitzgerald, P. M. D. & Madsen, N. B. (1986). J. Cryst. Growth, 76, 600–606.
- Fukuhara, A. et al. (2005). Science, 307, 426–430. [DOI] [PubMed] [Google Scholar]
- Guse, A. H., Silva, C. P., Weber, K., Ashamu, G. A., Potter, B. V. & Mayr, G. W. (1996). J. Biol. Chem.271, 23946–23953. [DOI] [PubMed] [Google Scholar]
- Hasmann, M. & Schemainda, I. (2003). Cancer Res.63, 7436–7442. [PubMed] [Google Scholar]
- Khan, J. A., Tao, X. & Tong, L. (2006). Nature Struct. Mol. Biol.13, 582–588. [DOI] [PubMed]
- Kim, M. K., Lee, J. H., Kim, H., Park, S. J., Kim, S. H., Kang, G. B., Lee, Y. S., Kim, J. B., Kim, K. K., Suh, S. W. & Eom, S. H. (2006). J. Mol. Biol.362, 66–77. [DOI] [PubMed] [Google Scholar]
- Luft, J. R. & DeTitta, G. T. (1999). Acta Cryst. D55, 988–993. [DOI] [PubMed] [Google Scholar]
- Magni, G., Amici, A., Emanuelli, M., Orsomando, G., Raffaelli, N. & Ruggieri, S. (2004). Cell. Mol. Life Sci.61, 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Samal, B., Sun, Y., Stearns, G., Xie, C., Suggs, S. & McNiece, I. (1994). Mol. Cell. Biol.14, 1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stura, E. A. (1999a). Crystallization of Nucleic Acids & Proteins, edited by A. Ducruix & R. Giegé, pp. 177–206. Oxford University Press.
- Stura, E. A. (1999b). Protein Crystallization, edited by T. Bergfors, pp. 139–153. La Jolla, CA, USA: International University Line.
- Thaller, C., Weaver, L. H., Eichele, G., Wilson, E., Karlsson, R. & Jansonius, J. N. (1981). J. Mol. Biol.147, 465–469. [DOI] [PubMed] [Google Scholar]
- Wang, T., Zhang, X., Bheda, P., Revollo, J. R., Imai, S. & Wolberger, C. (2006). Nature Struct. Mol. Biol.13, 661–662. [DOI] [PubMed]
- Zerez, C. R., Roth, E. F. Jr, Schulman, S. & Tanaka, K. R. (1990). Blood, 75, 1705–1710. [PubMed] [Google Scholar]
- Ziegler, M. (2000). Eur. J. Biochem.267, 1550–1564. [DOI] [PubMed] [Google Scholar]