The M. tuberculosis protein Rv0765c was cloned, expressed, purified and crystallized. In an attempt to improve the quality of the crystals of Rv0765c, the protein was modified by reductive methylation. The methylated protein crystallized in a new crystal form with profoundly improved diffraction properties.
Keywords: reductive methylation, Rv0765c, Mycobacterium tuberculosis
Abstract
Rv0765c from Mycobacterium tuberculosis was cloned and heterologously expressed in Escherichia coli. It was purified using affinity and size-exclusion chromatographic techniques and crystallized. The native protein crystallized in a hexagonal crystal form which diffracted to 7 Å resolution. In an attempt to improve the quality of the Rv0765c crystals, the protein was modified by reductive methylation using dimethylaminoborane and formaldehyde. The modified protein crystallized under different conditions in a tetragonal crystal form, from which diffraction data could be collected to a resolution of 3.2 Å. In both crystal forms of Rv0765c, the asymmetric unit contained two copies of the protein molecule.
1. Introduction
Tuberculosis (TB) is a prevalent infectious disease with high morbidity and mortality which is caused by Mycobacterium tuberculosis (Mtb). As estimated by the World Health Organization, one-third of the world’s population is infected with Mtb and new cases are occurring at a rate of one per second (World Health Organization, 2006 ▶). One in ten latent infections will progress to active TB which, if left untreated, kills more than half of its victims (Onyebujoh & Rook, 2004 ▶). TB mostly occurs in developing countries, but a rising number of people in the industrialized world develop this disease because their immune systems are compromised, e.g. by administration of immunosuppressive drugs, drug abuse or HIV/AIDS. Furthermore, the number of multiple drug-resistant TB cases is increasing (World Health Organization, 2004 ▶).
A major boost to Mtb biology was provided by the completion of the genome sequence of the best-characterized virulent strain of Mtb (H37Rv; Cole et al., 1998 ▶; Camus et al., 2002 ▶). It revealed that the Mtb genome codes for 3995 open reading frames. Among the gene products encoded by H37Rv, a group of proteins were identified in microarray experiments as being differentially expressed in the lungs of patients and hence were considered to potentially be important for the persistence and pathogenicity of Mtb (Rachman, Strong, Schaible et al., 2006 ▶; Rachman, Strong, Ulrichs et al., 2006 ▶). The Germany-based X-MTB consortium (http://www.xmtb.org), with participants from Hamburg, Berlin and Munich, selected targets for the structure-based design of compounds for novel intervention strategies against TB. Rv0765c, a protein consisting of 275 amino-acid residues with a molecular weight of 29.1 kDa, belongs to this group and has been shown by genome-wide expression profiling experiments to be 3.4-fold upregulated in Mtb residing in granulomas compared with Mtb grown from in vitro cultures. The upregulation is further increased to 7.8-fold in Mtb residing in the pericavity and distant lung (Rachman, Strong, Schaible et al., 2006 ▶; Rachman, Strong, Ulrichs et al., 2006 ▶).
Based on sequence similarity, Rv0765c has been annotated as a putative oxidoreductase and is most likely to belong to the short-chain dehydrogenase/reductase family (Cole et al., 1998 ▶). It is conserved within the Mycobacterium family, exhibiting 100% amino-acid identity to the M. bovis protein Mb0788c (Garnier et al., 2003 ▶) and 75% and 72% identity to the M. smegmatis protein MSMEG_5862 and the M. avium protein MAV_0709, respectively (EMBL/GenBank/DDBJ databases). Homologues of Rv0765c are present in many other mycobacteria and even in unrelated bacterial species. Among human proteins, carbonic reductase 4 (Ota et al., 2004 ▶) shows 27% identity and 46% similarity to Rv0765c for a 230 amino-acid overlap. The best structurally characterized homologues of Rv0765c found to date are clavulanic acid dehydrogenase from Streptomyces clavuligerus (MacKenzie et al., 2007 ▶; PDB codes 2jah and 2jap), which shows 33% identity for a 242-residue sequence overlap, and β-ketoacyl reductase from Escherichia coli (Price et al., 2001 ▶; PDB code 1io1), with 34% identity for a 231-residue overlap. Elucidation of the three-dimensional structure of Rv0765c will thus provide valuable information towards characterizing its precise function. Once its function is known and an enzymatic assay has been established, high-throughput screening of potential novel inhibitors of the enzyme should become feasible.
Modification of surface residues by point mutations (when a plausible model is available) or by random mutagenesis (when there is no such information available) have already proven to be powerful tools for changing protein solubility and inducing crystallization or altering crystal packing (Derewenda & Vekilov, 2006 ▶). Chemical modification of the accessible amino groups, in which primary amines (lysine residues and the N-terminus) are modified to tertiary amines, constitutes yet another methodology, which was originally introduced by Means & Feeney (1968 ▶) and subsequently successfully used for crystallization by Rayment et al. (1993 ▶) and Rypniewski et al. (1993 ▶). Reductive methylation is a Schiff-base reaction (Gidley & Sanders, 1982 ▶). Dimethylation of lysine side chains reduces their interaction with solvent and hence may enhance the ability of the protein to form crystals. Examples have been reported in which reductive methylation has indeed induced crystallization (Schubot & Waugh, 2004 ▶; Rayment et al., 1993 ▶), improved the diffraction properties of crystals (Kobayashi et al., 1999 ▶; Kurinov et al., 2000 ▶) or altered the arrangement of the molecules in the crystal (Rypniewski et al., 1993 ▶). Recently, this methodology has gained increasing popularity (Walter et al., 2006 ▶).
In this report, we describe the cloning, purification, crystallization and initial X-ray analysis of Rv0765c. In addition, we show that successful reductive methylation of Rv0765c has a significant influence on the quality of the crystals obtained.
2. Experimental methods
2.1. Cloning
Genomic DNA from the H37Rv strain of Mtb was used as a template for the polymerase chain reaction. The following forward and reverse primers were used: 5′-AAAAGGTCTCACATGCCACGCTTCGAACCTCACCCCGCCCG-3′ and 5′-AAAACTCGAGTTAGCCCGGCATCCCCTCTTCGCCCAGTACTAGC-3′, respectively. The amplified fragment containing the 5′-BsaI and 3′-XhoI restriction sites (shown in bold in the primer sequences) was digested and ligated into the pETM-10 expression vector (EMBL) digested with the restriction enzymes NcoI and XhoI. The restriction enzyme BsaI was used to overcome problems inherent to the presence of an internal NcoI cleavage site in the Rv0765c gene. BsaI treatment results in the sticky end of the desired sequence, which in the presented case is the sequence after digestion with NcoI (italicized). The pETM-10 vector adds an N-terminal His6 tag to the expressed recombinant protein. The construct was sequenced to confirm the cloning of the Rv0765c gene sequence in frame.
2.2. Expression and purification
The recombinant plasmid was used to transform E. coli Rosetta(DE3)pLysS cells (Novagen). Cells from an overnight 150 ml pre-culture were grown in LB broth medium containing chloramphenicol (34 µg ml−1) and kanamycin (30 µg ml−1) at 310 K and 210 rev min−1. The culture was induced with 1 mM isopropyl β-d-thiogalactopyranoside (IPTG) at an OD600 of approximately 0.8 at 293 K. After induction, the culture was incubated for about 20 h at 293 K and 210 rev min−1 and then harvested. The cells were frozen and stored at 253 K until further processing. 1 g cell pellet was dissolved in 5 ml buffer A [50 mM Tris pH 8.0, 200 mM NaCl, 5 mM 2-mercaptoethanol and one complete Mini EDTA-free Protease Inhibitor Cocktail tablet (Roche) per 25 ml] and then lysed by sonication three times for 4 min using 0.3 s pulses at 277 K. The cell debris was pelleted by centrifugation for 45 min at 277 K and 20 000 rev min−1. The crude lysate was filtered through a 0.22 µm membrane and loaded onto a 5 ml Hi-Trap Chelating HP column (GE Healthsciences) charged and equilibrated with Ni2+ and buffer A, respectively. In order to remove unbound proteins, the column was first washed with five column volumes of buffer A and then with five column volumes of buffer B (50 mM Tris pH 8.0, 200 mM NaCl, 50 mM imidazole, 5 mM 2-mercaptoethanol). The protein was eluted by running a linear gradient from 50 to 400 mM imidazole (in buffer B). One half of the Rv0765c sample was subsequently purified by gel filtration (Superdex 200, 16/60 column, GE Healthsciences) using buffer C (50 mM Tris pH 8.0, 200 mM NaCl, 3 mM DTT) for both equilibration and elution. The peak fractions were analyzed by SDS–PAGE, pooled and concentrated to 12 mg ml−1 for crystallization trials. The second half of the Rv0765c sample was used for reductive-methylation experiments (see §2.3). The purity of native Rv0765c was judged using 10% SDS–PAGE stained with Coomassie Brilliant Blue.
2.3. Reductive methylation
The methylation experiment was based on the protocols described by Rypniewski et al. (1993 ▶) and Walter et al. (2006 ▶). The protein was transferred to 50 mM HEPES pH 8.0 and 200 mM NaCl (buffer D) by a three-step centrifugation buffer exchange using a filter with a 10 kDa molecular-weight cutoff. It was concentrated to 5 mg ml−1 and for each 1 ml of solution, 20 µl 1 M dimethylaminoborane (DMAB) was added followed by 40 µl 1 M formaldehyde. The addition of DMAB and formaldehyde was repeated after 2 h. After another 2 h, 10 µl 1 M DMAB was added and the reaction was incubated for 18 h. To terminate the reaction, DMAB and formaldehyde were removed via a three-step centrifugation buffer exchange using buffer D and a filter with a 10 kDa molecular-weight cutoff. All steps were performed at 277 K. The methylated protein was subsequently purified by gel filtration using the same conditions as used for native Rv0765c. The peak fractions were analyzed by SDS–PAGE, pooled and concentrated to 12 mg ml−1. Although methylation has sometimes been reported to alter the behaviour of the protein, the methylated Rv0765c essentially behaved similarly to the native protein. The methylation was verified via MALDI–TOF mass spectrometry (data not shown). The purity of methylated Rv0765c was judged as described above for the native protein.
2.4. Crystallization of Rv0765c
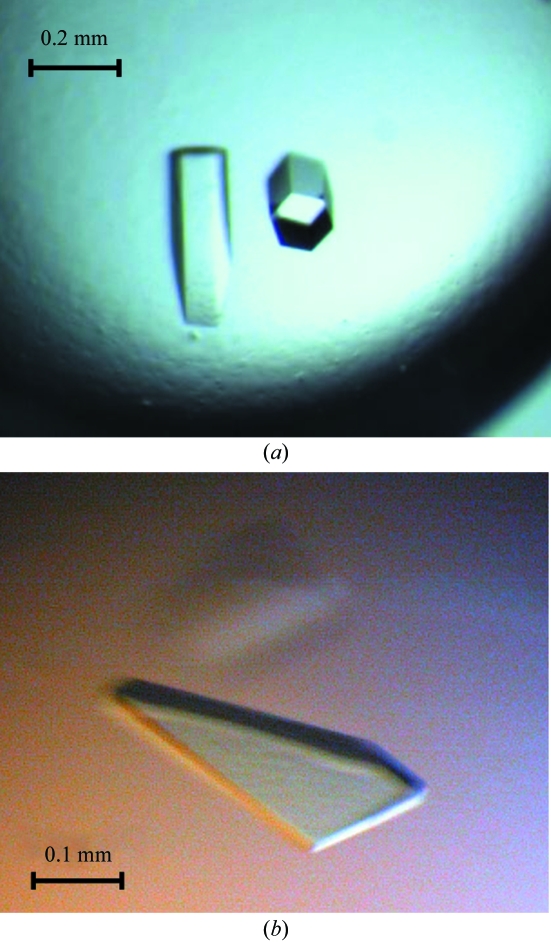
Native Rv0765c from Mtb was crystallized using the hanging-drop vapour-diffusion method at 292 K. Initial crystallization screening for the native protein was performed using commercially available sparse-matrix screens (Jancarik & Kim, 1991 ▶) from Hampton Research. In condition No. 19 of Crystal Screen, composed of 200 mM CaCl2, 28%(v/v) PEG 400 and 100 mM HEPES pH 7.5, the protein crystallized in the form of a shower of very thin needles. The crystals were too small and were not suitable for characterization using a diffraction experiment. To improve the quality of the crystals, optimization was performed using Additive Screens (Hampton Research). The best result was obtained in the presence of 2%(w/v) benzamidine, where the droplet contained 1.2 µl protein plus 0.3 µl additive plus 1.5 µl reservoir solution equilibrated against 1 ml reservoir solution. Further optimization showed that Rv0765c crystallizes from various PEGs (molecular weights 200–4000 Da) and over a wide pH range (6.5–8.0). The crystals appeared after one week and required another 2–3 weeks to grow to final size (Fig. 1 ▶ a).
Figure 1.
Single crystals of Rv0765c from M. tuberculosis. (a) Hexagonal crystals of the native protein; (b) tetragonal crystals of methylated Rv0765c.
Methylated Rv0765c initially crystallized in condition No. A4 of Jena Bioscience Screen No. 10 composed of 800 mM sodium/potassium tartrate and 100 mM HEPES pH 7.5, as well as in several other conditions containing various PEGs. Optimization resulted in the growth of crystals that were suitable for diffraction experiments from 900 mM sodium/potassium tartrate and 100 mM HEPES pH 8.0 supplemented with 0.7%(v/v) n-butanol or 4%(v/v) acetonitrile as additives (droplets mixed as mentioned above). The plate-like crystals appeared after two weeks and took another 2–3 weeks to grow to maximum size (Fig. 1 ▶ b).
2.5. Diffraction data collection and processing
A crystal of native Rv0765c with dimensions 0.40 × 0.15 × 0.15 mm grown from 185 mM CaCl2, 100 mM HEPES pH 7.5, 20%(v/v) PEG 550 MME and 2%(w/v) benzamidine was mounted in a nylon-fibre loop and flash-cooled to 100 K in a nitrogen-gas stream. Diffraction data were collected on beamline X13 (EMBL Hamburg, c/o DESY, Germany) using a MAR CCD 165 mm detector.
A diffraction experiment using the methylated Rv0765c crystals was performed on beamline X12 (EMBL Hamburg, c/o DESY, Germany) equipped with a MAR CCD 225 mm detector. Low-temperature data were collected from a methylated Rv0765c crystal of dimensions 0.40 × 0.30 × 0.05 mm grown from 900 mM sodium/potassium tartrate and 100 mM HEPES pH 8.0 supplemented with 4%(v/v) acetonitrile. Cryoprotection was performed for approximately 5 s in reservoir solution complemented with 30%(v/v) glycerol.
Both data sets were indexed and integrated using DENZO (Otwinowski & Minor, 1997 ▶) and scaled using SCALEPACK (Otwinowski & Minor, 1997 ▶). The redundancy-independent merging R factor R r.i.m. as well as the precision-indicating merging R factor R p.i.m. (Weiss, 2001 ▶) were calculated using the program RMERGE (available from http://www.embl-hamburg.de/~msweiss/projects/msw_qual.html or from MSW upon request). Intensities were converted to structure-factor amplitudes using the program TRUNCATE (French & Wilson, 1978 ▶; Collaborative Computational Project, Number 4, 1994 ▶). The optical resolution was calculated using the program SFCHECK (Vaguine et al., 1999 ▶) and the self-rotation function was calculated using the program MOLREP (Collaborative Computational Project, Number 4, 1994 ▶; Vagin & Teplyakov, 1997 ▶). Owing to the high noise level in the data as well as the observed strong anisotropy in both crystal forms, the maximum resolution for calculating the self-rotation function had to be limited to 12 Å for the hexagonal crystal form and to 7 Å for the tetragonal crystal form, respectively. Table 1 ▶ summarizes the data-collection and processing statistics for both crystal forms.
Table 1. Data-collection and processing statistics.
Values in parentheses correspond to the highest resolution shell.
| Crystal form | Hexagonal | Tetragonal |
|---|---|---|
| No. of crystals | 1 | 1 |
| Beamline | X13 | X12 |
| Wavelength (Å) | 0.8080 | 0.9184 |
| Crystal-to-detector distance (mm) | 385 | 350 |
| Total rotation range (°) | 180 | 180 |
| Temperature (K) | 100 | 100 |
| Space group | P6222 or P6422 | P42212 |
| Unit-cell parameters (Å) | a = b = 136.7, c = 135.5 | a = b = 106.6, c = 104.3 |
| Resolution limits (Å) | 40.0–7.0 (7.12–7.00) | 40.0–3.2 (3.26–3.20) |
| Mosaicity (°) | 1.4 | 0.8 |
| Total No. of reflections | 23672 | 133981 |
| Unique reflections | 1305 | 10389 |
| Redundancy | 18.1 (18.6) | 12.9 (11.3) |
| I/σ(I) | 25.4 (6.1) | 25.4 (3.0) |
| Completeness (%) | 99.9 (100) | 99.1 (90.7) |
| Rmerge (%) | 13.3 (68.2) | 9.5 (69.4) |
| Rr.i.m. (%) | 13.8 (33.9) | 9.9 (72.3) |
| Rp.i.m. (%) | 3.2 (7.7) | 2.7 (20.2) |
| Overall B factor from Wilson plot (Å2) | n.d. | 59 |
| Optical resolution (Å) | 4.7 | 2.4 |
3. Results and discussion
Expression of Rv0765c in E. coli Rosetta(DE3)pLysS cells resulted in approximately 85% of the protein in the soluble fraction and the remainder in inclusion bodies. After the two-step chromatographic procedure, the final yield of the pure protein was approximately 10 mg from 1 l culture. The protein eluted from the gel-filtration column with an apparent molecular weight of approximately 120 kDa, which suggests that the protein is tetrameric in solution. This was confirmed by DLS, SLS and small-angle X-ray scattering (data not shown). The purity of the sample prepared for crystallization was at least 95% as estimated by SDS–PAGE.
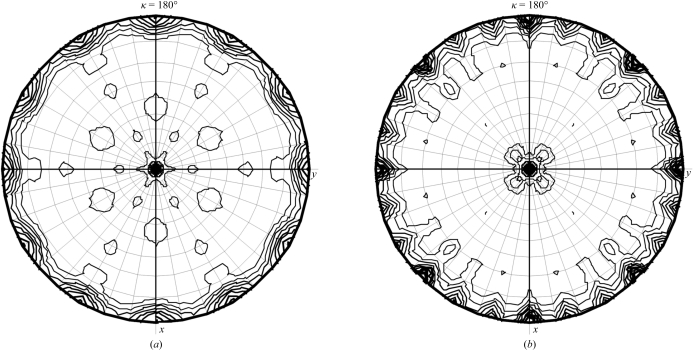
Fig. 1 ▶ shows crystals of native and methylated Rv0765c from Mtb grown from their optimized crystallization conditions. Native Rv0765c crystals belong to the hexagonal crystal system, space group P6222 or P6422, with unit-cell parameters a = b = 136.7, c = 135.5 Å. Although the crystals diffracted to 4 Å resolution along the hexagonal c axis, owing to the very high anisotropy of the diffraction and the consequent lack of completeness the resolution of the data had to be limited to 7 Å. Based on the molecular weight of the protein (30 244 Da including the N-terminal His6 tag) and the volume of the asymmetric unit, a Matthews parameter (Matthews, 1968 ▶) of 3.02 Å3 Da−1 for two molecules of Rv0765c in the asymmetric unit can be calculated. Given the oligomeric state of Rv0765c and the presence of a noncrystallographic twofold axis, the most likely number of molecules in the asymmetric unit of the hexagonal form is two. This corresponds to a solvent content of 59%. The self-rotation function calculated from the data (Fig. 2 ▶ a) only shows the crystallographic symmetry elements. Thus, if two molecules are present in the asymmetric unit and if these are related by a noncrystallographic twofold axis, this axis can only be parallel to one of the crystallographic sixfold or twofold axes.
Figure 2.
(a) Self-rotation function for the hexagonal crystal form calculated using the program MOLREP (Vagin & Teplyakov, 1997 ▶) based on data from 40.0 to 12.0 Å. The labelling of the x and y axes is according to the orthogonal coordinate system defined by MOLREP. (b) Self-rotation function for the tetragonal crystal form calculated based on data from 40.0 to 5.0 Å.
Rv0765c could be successfully methylated: all six Lys residues as well as the N-terminus were methylated. This was confirmed by MALDI–TOF mass spectrometry. The mass difference between the native and methylated Rv0765c was around 200 ± 4, indicating the presence of 14 additional methyl groups attached to the protein. An X-ray diffraction data set for the methylated Rv0765c crystals was collected to 3.2 Å resolution. The crystals belong to the tetragonal crystal system, space group P42212, with unit-cell parameters a = b = 106.6, c = 104.3 Å. The Matthews parameter is 2.45 Å3 Da−1 (Matthews, 1968 ▶) for two molecules of Rv0765c in the asymmetric unit. This corresponds to a solvent content of 50%. The self-rotation function calculated from this data set reveals the presence of a noncrystallographic twofold axis in the xy plane of the crystal between the twofold axes parallel to x and to the xy diagonal. Methylated Rv0765c can also be crystallized from PEG-containing conditions in the same hexagonal crystal form as native Rv0765c. Unfortunately, the diffraction properties are the same as for the native crystals. However, the new tetragonal crystal form obtained from tartrate solution exhibited significantly improved diffraction properties. Although the diffraction is still highly anisotropic (poor diffraction along the c axis), a complete data set to 3.2 Å resolution could be collected. It is important to note that native Rv0765c cannot be crystallized under the same conditions as methylated Rv0765c, suggesting that the crystallization space for Rv0765c was extended by the reductive methylation. Structure determination by molecular replacement is currently in progress.
Acknowledgments
We would like to thank the X-Mtb consortium (http://www.xmtb.org) for funding through BMBF/PTJ grant No. BIO/0312992A.
References
- Camus, J.-C., Pryor, M. J., Médigue, C. & Cole, S. T. (2002). Microbiology, 148, 2967–2973. [DOI] [PubMed] [Google Scholar]
- Cole, S. T. et al. (1998). Nature (London), 393, 537–544. [Google Scholar]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. [Google Scholar]
- Derewenda, Z. S. & Vekilov, P. G. (2006). Acta Cryst. D62, 116–124. [DOI] [PubMed] [Google Scholar]
- French, G. S. & Wilson, K. S. (1978). Acta Cryst. A34, 517–525. [Google Scholar]
- Garnier, T. et al. (2003). Proc. Natl Acad. Sci. USA, 100, 7877–7882. [Google Scholar]
- Gidley, M. J. & Sanders, J. K. (1982). Biochem. J.203, 331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancarik, J. & Kim, S.-H. (1991). J. Appl. Cryst.24, 409–411. [Google Scholar]
- Kobayashi, M., Kubota, M. & Matsuura, Y. (1999). Acta Cryst. D55, 931–933. [DOI] [PubMed] [Google Scholar]
- Kurinov, I. V., Mao, C., Irvin, J. D. & Uckun, F. M. (2000). Biochem. Biophys. Res. Commun.275, 549–552. [DOI] [PubMed] [Google Scholar]
- MacKenzie, A. K., Kershaw, N. J., Hernandez, H., Robinson, C. V., Schofield, C. J. & Andersson, I. (2007). Biochemistry, 46, 1523–1533. [DOI] [PubMed] [Google Scholar]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Means, G. E. & Feeney, R. E. (1968). Biochemistry, 6, 2192–2201. [DOI] [PubMed]
- Onyebujoh, P. & Rook, G. A. (2004). Nature Rev. Microbiol.2, 930–932. [DOI] [PubMed]
- Ota, T. et al. (2004). Nature Genet.36, 40–45. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Price, A. C., Zhang, Y. M., Rock, C. O. & White, S. W. (2001). Biochemistry, 4, 12772–12781. [DOI] [PubMed]
- Rachman, H., Strong, M., Schaible, U., Schuchhardt, J., Hagens, K., Mollenkopf, H., Eisenberg, D. & Kaufmann, S. H. E. (2006). Microbes Infect.8, 747–757. [DOI] [PubMed] [Google Scholar]
- Rachman, H., Strong, M., Ulrichs, T., Grode, L., Schuchhardt, J., Mollenkopf, H., Kosmiadi, G. A., Eisenberg, D. & Kaufmann, S. H. E. (2006). Infect. Immun.74, 1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment, I., Rypniewski, W. R., Schmidt-Base, K., Smith, R., Tomchick, D. R., Benning, M. M., Winkelmann, D. A., Wesenberg, G. & Holden, H. M. (1993). Science, 261, 50–58. [DOI] [PubMed] [Google Scholar]
- Rypniewski, W. R., Holden, H. M. & Rayment, I. (1993). Biochemistry, 32, 9851–9858. [DOI] [PubMed] [Google Scholar]
- Schubot, F. D. & Waugh, D. S. (2004). Acta Cryst. D60, 1981–1986. [DOI] [PubMed] [Google Scholar]
- Vagin, A. A. & Teplyakov, A. (1997). J. Appl. Cryst.30, 1022–1025. [Google Scholar]
- Vaguine, A. A., Richelle, J. & Wodak, S. J. (1999). Acta Cryst. D55, 191–205. [DOI] [PubMed] [Google Scholar]
- Walter, T. S., Meier, C., Assenberg, R., Au, K. F., Ren, J., Verma, A., Nettleship, J. E., Owens, R. J., Stuart, D. I. & Grimes, J. M. (2006). Structure, 14, 1617–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, M. S. (2001). J. Appl. Cryst.34, 130–135. [Google Scholar]
- World Health Organization (2004). Anti-Tuberculosis Drug Resistance in the World: Report No. 3. Geneva, Switzerland: World Health Organization. http://www.who.int/tb/publications/who_htm_tb_2004_343/en/.
- World Health Organization (2006). Tuberculosis Fact Sheet No. 104. Geneva, Switzerland: World Health Organization. http://www.who.int/mediacentre/factsheets/fs104/en/.