A crystal structure of NADP+-bound T. thermophilus Δ1-pyrroline-5-carboxylate dehydrogenase refined to 1.55 Å resolution is reported. The structure provides structural insights into the mechanism of preference for coenzymes and enzyme activity.
Keywords: Δ1-pyrroline-5-carboxylate dehydrogenase, coenzyme binding mode, NAD(H), NADP(H), proline metabolic pathway
Abstract
Δ1-Pyrroline-5-carboxylate dehydrogenase (P5CDh) is known to preferentially use NAD+ as a coenzyme. The k cat value of Thermus thermophilus P5CDh (TtP5CDh) is four times lower for NADP+ than for NAD+. The crystal structure of NADP+-bound TtP5CDh was solved in order to study the structure–activity relationships for the coenzymes. The binding mode of NADP+ is essentially identical to that in the previously solved NAD+-bound form, except for the regions around the additional 2′-phosphate group of NADP+. The coenzyme-binding site can only accommodate this group by the rotation of a glutamate residue and subtle shifts in the main chain. The 2′-phosphate of NADP+ increases the number of hydrogen bonds between TtP5CDh and NADP+ compared with that between TtP5CDh and NAD+. Furthermore, the phosphate of the bound NADP+ would restrict the ‘bending’ of the coenzyme because of steric hindrance. Such bending is important for dissociation of the coenzymes. These results provide a plausible explanation of the lower turnover rate of NADP+ compared with NAD+.
1. Introduction
The proline metabolic pathway provides substrates for protein synthesis. However, it also often performs special functions; for example, defense against osmotic challenge in prokaryotes (Csonka, 1981 ▶) and plants (Hu et al., 1992 ▶). It has been proposed that proline and its interconversions function in a mechanism for redox balance and as a mediator of redox-dependent mechanisms (Phang et al., 2001 ▶). In addition, it has been suggested that the proline metabolic pathway plays an important role in p53-induced apoptosis in human cancer cells (Yoon et al., 2004 ▶). Deficiencies of enzymes in the proline metabolic system cause inherited disorders such as hyperprolinemia, hypoprolinemia and hyperornithinemia with gyrate atropy (Phang et al., 2001 ▶).
Δ1-Pyrroline-5-carboxylate dehydrogenase (P5CDh; EC 1.5.1.12) is one of the key enzymes in the proline metabolic pathway. P5CDh irreversibly catalyzes the oxidation of glutamate-γ-semialdehyde (GSA) to glutamate, with the reduction of NAD+ to NADH. In aqueous solution, GSA is in spontaneous equilibrium with its cyclized tautomer Δ1-pyrroline-5-carboxylate (P5C). P5C is a precursor of proline as well as its degradation product (Phang et al., 2001 ▶). P5CDh plays the primary role in the degradation of P5C; that is, the primary outflow of P5C and proline from the proline metabolic pathway. A deficiency of P5CDh causes an inherited disorder, type II hyperprolinemia (MIM 239510). Proline accumulation has been shown to induce oxidative stress in vivo and in vitro, which may be linked to the brain dysfunction observed in hyperprolinemic patients (Delwing et al., 2003 ▶).
P5CDh utilizes NAD+ as a coenzyme in preference to NADP+. In the case of Thermus thermophilus P5CDh (TtP5CDh), we found that the k cat value for NADP+ was fourfold lower than that for NAD+, while the K m values were comparable (Inagaki et al., 2006 ▶). However, the structural basis for the coenzyme preference was not clear. In order to understand the structural basis for the coenzyme preference, we solved the crystal structure of TtP5CDh in the NADP+-bound form (TtP5CDh–NADP) and compared it with the crystal structure of TtP5CDh in the NAD+-bound form (TtP5CDh–NAD) that we have reported previously (Inagaki et al., 2006 ▶). The structures revealed differences in the binding mode between NADP+ and NAD+ and provided a structural basis for the coenzyme preference as well as further insights into the catalytic activity of the enzyme.
2. Materials and methods
2.1. Crystallization and preparation of the NADP+-bound form
The methods for the expression, purification and crystallization of TtP5CDh and the preparation of complex crystals have been described previously (Inagaki et al., 2005 ▶). The crystals used in this study were obtained from reservoir solution containing 28–40% MPD and 50 mM sodium citrate buffer pH 5.2. Crystals of the NADP+-bound form were prepared by a 15 min soak in solution containing 30% MPD, 5 mM NADP+ and 50 mM sodium acetate buffer pH 5.2.
2.2. Data collection
A complete diffraction data set was collected at 100 K using synchrotron radiation at SPring-8 beamline BL44B2 (Hyogo, Japan). The intensity data were indexed, integrated and scaled using DENZO and SCALEPACK as implemented in the HKL-2000 program package (Otwinowski, 1993 ▶; Otwinowski & Minor, 1997 ▶). The crystal parameters and data-processing statistics are summarized in Table 1 ▶. Estimation of the twinning fraction (Yeates, 1997 ▶) using DETWIN (Taylor & Leslie, 1998 ▶) from the CCP4 suite (Collaborative Computational Project, Number 4, 1994 ▶) revealed the presence of hemihedral twinning (28.3%) and the data were detwinned using DETWIN.
Table 1. Summary of crystal data and data-collection and refinement statistics.
Values in parentheses are for the highest resolution shell.
| Crystal data | |
| Space group | R3 |
| Unit-cell parameters (Å) | a = 102.43, c = 278.84 |
| No. of molecules in unit cell (Z) | 18 |
| Solvent content (%) | 50.2 |
| Data collection | |
| Source | BL44B2 |
| Detector type | ADSC Q210 |
| Wavelength (Å) | 1.0 |
| Temperature (K) | 100 |
| Resolution range (Å) | 25–1.55 (1.59–1.55) |
| No. of unique reflections | 158273 (10482) |
| Redundancy | 5.7 (5.6) |
| Completeness (%) | 100.0 (100.0) |
| Rmerge | 0.060 (0.435) |
| 〈I/σ(I)〉 | 30.9 (4.2) |
| Refinement statistics | |
| Resolution range (Å) | 25–1.55 |
| Rwork | 0.158 |
| Rfree (5% of reflections) | 0.180 |
| Overall average B factor excluding solvent (Å2) | 12.4 |
| No. of atoms refined | |
| Protein atoms | 8204 |
| Ligand atoms | 170 |
| Solvent atoms | 1060 |
| No. of reflections used in refinement | 145352 |
| No. of reflections in test set for Rfree | 7695 |
| R.m.s. deviations from target values | |
| Bond lengths (Å) | 0.009 |
| Bond angles (°) | 1.2 |
| Ramachandran plot analysis†, residues in | |
| Most favoured regions (%) | 92.7 |
| Additionally allowed regions (%) | 7.1 |
| Generously allowed regions (%) | 0.2 |
| Disallowed regions (%) | 0.0 |
Calculated using PROCHECK (Laskowski et al., 1993 ▶).
2.3. Structure determination and refinement
The structure of the TtP5CDh–NAD dimer (Inagaki et al., 2006 ▶; PDB code 2bhp) was used as an initial model for refinement using REFMAC5 (Murshudov et al., 1997 ▶) from the CCP4 suite. Model rebuilding and subsequent manual adjustments were performed using Coot (Emsley & Cowtan, 2004 ▶). The positions of the bound NADP+ molecules were determined from the 2F o − F c and F o − F c Fourier maps. Clear electron density was apparent for NADP+ in the binding site. After refinement, additional electron density indicated an alternative conformer of the nicotinamide mononucleotide part (NMN) of NADP+ extending into a solvent region. However, except for the pyrophosphate linkage part, the conformer could not be defined because of poor electron density. The disorder of NADP+ was confirmed by the alternative conformations of Thr289 and Gly290, including flipping of the peptide bond between them, as found in TtP5CDh–NAD; one conformer corresponds to the NMN-bound conformer, forming a hydrogen bond to the Thr289 O atom, and the other to the unbound conformer (Inagaki et al., 2006 ▶).
In the proximity of Cys322 Sγ in TtP5CDh–NADP, electron density was present that indicated a possible water molecule forming hydrogen bonds to Cys322 N and Asn184 Nδ. However, the presence of Cys322 as an oxidized S-hydroxyl form cannot be ruled out. The final refinement statistics and assessment of the model quality are summarized in Table 1 ▶. Figures were generated using PyMOL (DeLano, 2002 ▶).
3. Results and discussions
3.1. NADP+ binding
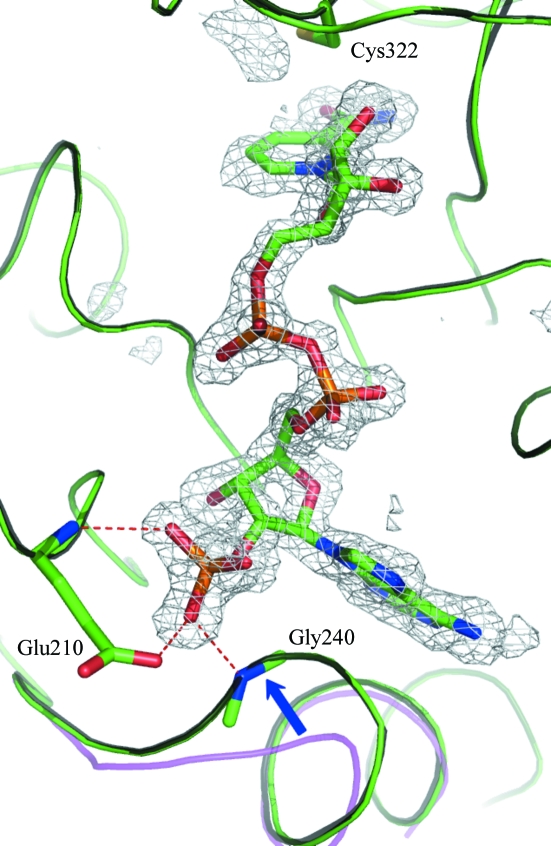
OMIT electron-density maps clearly showed bound NADP+ (Fig. 1 ▶). There are no large-scale conformational changes such as domain movements elicited by NADP+ binding. The r.m.s.d. values between equivalent Cα atoms of TtP5CDh–NADP and the ligand-free form are 0.29 Å for 514 residues (excluding two disordered N-terminal residues). A surface loop and part of the following helix (residues 238–246) undergo a large shift of up to 4.5 Å for the Cα atoms, which results in the closure of the adenine-binding cleft to a certain extent as shown in TtP5CDh–NAD (Inagaki et al., 2006 ▶; Fig. 1 ▶).
Figure 1.
The binding mode of NADP+. F o − F c OMIT map of bound NADP+. The catalytic cysteine (Cys322) and the Glu210 and Gly240 residues that anchor the 2′-phosphate group are shown in stick representation. The difference Fourier electron density is contoured at 3σ. The backbones of TtP5CDh–NADP, TtP5CDh–NAD and ligand-free TtP5CDh are colored green, grey and magenta, respectively. TtP5CDh–NAD and ligand-free TtP5CDh were superimposed on TtP5CDh–NADP.
3.2. The differences in the binding modes of the coenzymes
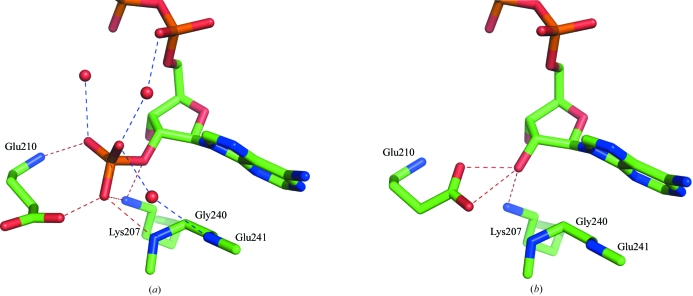
The enzyme structure of TtP5CDh–NADP is nearly identical to that of TtP5CDh–NAD. The r.m.s.d. value between equivalent Cα atoms of TtP5CDh–NADP and TtP5CDh–NAD is 0.08 Å for 514 residues (excluding two disordered N-terminal residues). The bound NADP+ adopts the same binding mode as NAD+, except for the additional 2′-phosphate group of the bound NADP+, which is sandwiched between Glu210 and Gly240 (Figs. 1 ▶ and 2 ▶).
Figure 2.
The differences in the binding modes between NADP+ and NAD+. Hydrogen bonds are drawn as dotted lines. The hydrogen bonds to the bound NADP+ of TtP5CDh–NADP (a) and NAD+ of TtP5CDh–NAD (b) are shown.
With NADP+, the Glu210 side chain is rotated away, with the O∊ and N atoms forming hydrogen bonds to the phosphate group (Fig. 1 ▶); residues 240–243 undergo subtle shifts of 0.23–0.37 Å, with Gly240 N forming a hydrogen bond. These structural modifications result in the formation of a shallow pocket which can accommodate the phosphate group in the binding site. Furthermore, the phosphate group is also stabilized by a hydrogen bond to Lys207 N∊ and a water-mediated hydrogen-bonding network with the residues in the binding site and other polar groups of the coenzyme (Fig. 2 ▶ a).
3.3. Inspection of the coenzyme-binding modes for enzyme-activity study
In our previous work (Inagaki et al., 2006 ▶), we proposed a catalytic mechanism for TtP5CDh that would include two steps: (i) nucleophilic attack by the catalytic cysteine on the aldehyde C atom of a substrate, followed by hydride transfer to a coenzyme with production of a thioacylenzyme, and (ii) hydrolysis of the thioacylenzyme. Before step (ii), the reduced nicotinamide group of NMN would need to be displaced from the catalytic center in order to place a water molecule at the proper position of NAD+ for hydrolysis. In addition, we also reported that the k cat for NADP+ is fourfold lower than that for NAD+, while their K m values were comparable.
In this study, it has been identified that the binding modes of NADP+ and NAD+ are essentially identical except for the regions around the ribose 2′-phosphoryl and 2′-hydroxyl groups of the adenylate parts of the coenzymes (adenosine 5′-phosphate and 2′,5′-diphosphate parts; APs), respectively. Therefore, the difference in the activity should be attributable to the differences between them. As mentioned above, the number of hydrogen bonds involving the 2′-group of NADP+ is larger than that for NAD+ (five for NADP+ and three for NAD+). In addition, the 2′-phosphate of NADP+ is stabilized by three water-mediated hydrogen bonds, while the 2′-hydroxyl group is not. These interactions could retain NADP+ more strongly in the binding site than NAD+ and would therefore cause a decrease in the dissociation rate of reduced coenzyme (NADPH). As a result of this, the turnover rate of NADP(H) would be decreased.
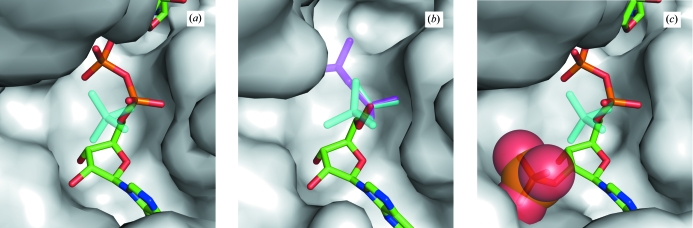
In our previous study, the NMNs of NAD+ in TtP5CDh–NAD and NADH in the NADH-bound form (TtP5CDh–NADH) revealed two types of conformers: one is the clearly identified ‘binding’ conformer in the NMN-binding site and the other is the poorly identified ‘excluded’ conformer extending into the solvent region from the pyrophosphate linkage (Figs. 3 ▶ a and 3 ▶ b; Inagaki et al., 2006 ▶). The excluded conformers could not be defined except for the pyrophosphate linkage, indicating that they are highly disordered (Figs. 3 ▶ a and 3 ▶ b). Meanwhile, the APs of the coenzymes can apparently be defined without disorder. These results indicate that the APs of NAD+ and NADH can remain in the binding site despite the exclusion of the NMNs. Hence, the NMNs are expected to be displaced earlier than the APs during the enzymatic process (Inagaki et al., 2006 ▶) and the exclusion of the NMNs requires a ‘bend’ at the pyrophosphate linkage. This indicates that this ‘bending’ is an important determinant of coenzyme dissociation. However, in TtP5CDh–NADP, steric hindrance between the 2′-phosphate group of AP and the pyrophosphate group restrains the ‘bending’ of NADP+. Consequently, bound NADP+ cannot adopt a conformation similar to the NAD+ conformation with the ‘excluded’ conformer (Fig. 3 ▶ c).
Figure 3.
The coenzyme-binding pockets with ‘excluded’ and ‘binding’ conformers of bound cofactors. The NMN parts of ‘excluded’ conformers cannot be defined except for the bridging pyrophosphate groups. (a) TtP5CDh–NAD with bound NAD+: the ‘excluded’ conformer is colored cyan. (b) TtP5CDh–NADH with bound NADH. It does not have a ‘binding’ conformer, only ‘excluded’ conformers (colored cyan and magenta). (c) TtP5CDh–NADP with bound NADP+: the ‘excluded’ conformer in TtP5CDh–NAD is superimposed (colored by cyan) and the 2′-phosphate group (except for the bridging O atom) of NADP+ is shown as sticks and VdW spheres.
3.4. Conclusions
A comparative analysis of the TtP5CDh–NADP and TtP5CDh–NAD structures suggests that (i) NADP+ binds more tightly to the protein than NAD+ and (ii) the bound NADP(H) would encounter more structural restraints than NAD(H) in the course of dissociation from the binding site. These two factors contribute to the decreased rate of NADPH dissociation compared with NADH following the enzymatic reaction, which is consistent with the results of kinetic studies for NADP+ and NAD+, i.e. a fourfold lower k cat for NADP+ than that for NAD+.
Supplementary Material
PDB reference: Δ1-pyrroline-5-carboxylate dehydrogenase, 2ehq, r2ehqsf
Acknowledgments
This work was supported by the RIKEN Structural Genomics/Proteomics Initiative (RSGI), the National Project on Protein Structural and Functional Analyses, Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. [Google Scholar]
- Csonka, L. N. (1981). Mol. Gen. Genet.182, 82–86. [DOI] [PubMed] [Google Scholar]
- DeLano, W. L. (2002). The PyMOL Molecular Graphics System. http://www.pymol.org.
- Delwing, D., Bavaresco, C. S., Chiarani, F., Wannmacher, C. M., Wajner, M., Dutra-Filho, C. S. & de Souza Wyse, A. T. (2003). Brain Res.991, 180–186. [DOI] [PubMed] [Google Scholar]
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed] [Google Scholar]
- Hu, C. A, Delauney, A. J. & Verma, D. P. (1992). Proc. Natl Acad. Sci. USA, 89, 9354–9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki, E., Ohshima, N., Takahashi, H., Kuroishi, C., Yokoyama, S. & Tahirov, T. H. (2006). J. Mol. Biol.362, 490–501. [DOI] [PubMed] [Google Scholar]
- Inagaki, E., Takahashi, H., Kuroishi, C. & Tahirov, T. H. (2005). Acta Cryst. F61, 609–611. [DOI] [PMC free article] [PubMed]
- Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. (1993). J. Appl. Cryst.26, 283–291. [Google Scholar]
- Murshudov, G. N., Vagin, A. A. & Dodson, E. J. (1997). Acta Cryst. D53, 240–255. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z. (1993). Proceedings of the CCP4 Study Weekend. Data Collection and Processing, edited by L. Sawyer, N. Isaacs & S. Bailey, pp. 56–62. Warrington: Daresbury Laboratory.
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Phang, J. M., Hu, C. A. & Valle, D. (2001). The Metabolic and Molecular Bases of Inherited Disease, 8th ed., edited by C. R. Scriver, A. R. Beaudet, W. Sly & D. Valle, pp. 1821–1838. New York: McGraw–Hill.
- Taylor, H. O. & Leslie, A. G. W. (1998). CCP4 Newsl.35, 9.
- Yeates, T. O. (1997). Methods Enzymol.276, 344–358. [PubMed] [Google Scholar]
- Yoon, K.-A., Nakamura, Y. & Arakawa, H. (2004). J. Hum. Genet.49, 134–140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: Δ1-pyrroline-5-carboxylate dehydrogenase, 2ehq, r2ehqsf