Abstract
Purpose
We sought to identify the genetic defect in a large, five-generation Chinese family with autosomal dominant progressive polymorphic congenital coronary cataracts and to examine the clinical features in detail.
Methods
Clinical and ophthalmologic examinations were conducted on family members. All members were genotyped with microsatellite markers at loci previously associated with cataracts. Two-point LOD scores were calculated using a linkage package after genotyping. A mutation was detected by direct sequencing and verified by denaturing high-performance liquid chromatography (DHPLC).
Results
Clinical observations showed that all affected family members had progressive polymorphic coronary cataracts. Linkage analysis was obtained at markers, D22S303 (LOD score [Z]=2.11, recombination fraction [θ]=0.0) and D22S1167 (Z=1.20, θ=0.0). Haplotype analysis indicated that the cataract gene was closely linked with these two markers. Sequencing the βB-crystallin gene (CRYBB2) revealed a C → T transition in exon 6, which changed a codon from Gln to a stop codon (P.Q155X). This mutation cosegregated with all affected individuals and was not observed in any unaffected family member or 100 normal, unrelated individuals.
Conclusions
This study identified a mutation in CRYBB2 in a large Chinese family with autosomal dominant progressive polymorphic congenital coronary cataracts. These results provide evidence that CRYBB2 is a pathogenic gene for congenital cataracts; at the same time, congenital cataracts are a clinically and genetically heterogeneous lens condition.
Introduction
Cataracts are an opacity of the lens that leads to loss of vision, and even blindness, and can be congenital or acquired, unilateral or bilateral [1]. Idiopathic, hereditary syndromes (Down syndrome and Rubinstein-Taybi syndrome), and intrauterine infections (congenital measles) can cause congenital cataracts, and traumatic, metabolism (high blood pressure), and some substances (alcohol and smoking) can cause acquired cataracts [2-4].
Congenital cataracts are a clinically and genetically heterogeneous lens condition responsible for a significant proportion of childhood visual impairment and blindness [5,6]. They can occur in an isolated fashion or as a component of a multi-system disorder. Non-syndromic congenital cataracts have an estimated incidence of 1–6 per 10,000 live births [7-10]. Although congenital cataracts are much less common than age-related cataracts, they are still responsible for approximately 10% of childhood blindness worldwide [11].
Since the first description of the cosegregation of inherited cataracts with the Duffy blood group locus, more than 30 loci have been mapped through linkage analysis and 17 genes have been characterized [12,13]. These genes can be considered in five groups, ten genes encoding crystallins (CRYAA, CRYAB, CRYBA1/A3, CRYBA, CRYBB1, CRYBB2, CRYBB3, CRYGC, CRYGD, and the CRYGs), three encoding membrane transport proteins (MIP, GJA3, and GJA8), one encoding cytoskeletal proteins (BSFP2), three encoding transcription factors (HSF4, MAF, and PITX3), and one encoding a lens intrinsic membrane protein [14-16].
Len crystallins are subdivided into α, β, and γ crystallins and constitute more than 80%–90% of the water-soluble structural proteins present in the vertebrate crystallin lens [17]. α-Crystallins are heat shock proteins that function as molecular chaperones while γ- and β-crystallins are members of a superfamily of microbial stress proteins, which share a common structural feature of four ‘Greek key’ motifs, two (1 and 2) in the NH2- and two (3 and 4) in the COOH-terminal domain [18]. Modifications of crystallins may disrupt their normal structure in the lens and cause cataracts [19].
Here, we report a large, five-generation Chinese family with autosomal dominant progressive polymorphic congenital coronary cataracts. Linkage analysis mapped the disease gene to 22q11.2–12.2, and a nonsense mutation (475C → T) in CRYBB2 was identified in this family, resulting in the substitution of a codon for the conserved amino acid, Gln, with a stop codon. Clinical and ophthalmologic examinations were conducted on family members in detail; all affected members show different clinical features.
Methods
Clinical evaluation and DNA specimens
A large, five-generation family with non-syndromic progressive polymorphic congenital coronary cataracts was recruited at the Beijing Tongren Eye Center, Capital Medical University, Beijing, China. Informed consent was obtained from each participant, consistent with the Declaration of Helsinki. The phenotype was documented by slit-lamp photography. Genomic DNA was extracted from peripheral blood leukocytes using standard protocols.
Genotyping
Polymerase chain reactions (PCRs) were performed with microsatellite markers close to candidate loci associated with autosomal congenital cataracts. PCR products from each DNA sample were separated on a 6% polyacrylamide gel and analyzed. Pedigree and haplotype data were managed using the Cyrillic software (version 2.1). Exclusion analysis was performed by allele sharing in affected individuals [20].
Linkage analysis
A two-point linkage was calculated with the LINKAGE package (version 5.1). Autosomal dominant cataracts were analyzed with full penetrance and a gene frequency of 0.001. The allele frequencies for each marker were assumed to be equal in both genders. The marker order and distances between the markers were taken from the NCBI and GDB databases.
DNA sequencing
Individual exons of the β-crystallin gene cluster were amplified by PCR using primer pairs [21]. PCR products were sequenced using an ABI3730 Automated Sequencer (PE Biosystems, Foster City, CA).
Denaturing high-performance liquid chromatography
Denaturing high-performance liquid chromatography (DHPLC) was used to screen the mutation identified in affected patients, other family members, and 100 normal control subjects in exon 6 of CRYBB2 using a commercial system (Wave DHPLC; Transgenomic, San Jose, CA).
Results
Clinical data
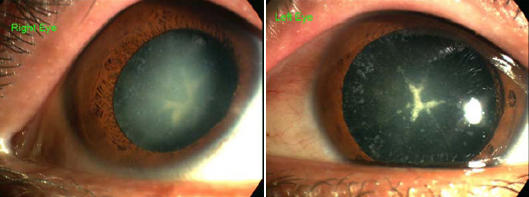
The proband was a 33-year-old male (III: 23) who had bilateral cataracts. From the age of 12 or 13, he had light apprehension and ambiguous visual clinical features. The condition became serious at the age of 25. Slit-lamp examination (III: 23) showed grayish/bluish punctate opacification in the cortex. A large number of spindle-shaped and oval punctate opacities were directed radially in the periphery, just like coronal cataracts. The clinical features of the left and right lenses showed some differences. No systemic or other ocular anomalies were observed in the patient.
This five-generation family included 17 affected individuals with congenital special-type coronary cataracts (Figure 1) and 34 unaffected individuals. The diagnosis was confirmed by ophthalmologists. The clinical diagnosis of the family was progressive polymorphic coronary cataracts with punctate, asteroidal, and nuclear opacities. Each of the affected individuals showed a somewhat different phenotype; in some affected subjects, star-like opacification was present in the upper side of the posterior pole (Table 1). There was no history of other ocular or systemic abnormalities in the family.
Figure 1.
Slit lamp photographs of an affected individual (III:23). The photographs of the affected individual III:23 showed that the opacities were coronary cataracts with punctate, asteroidal, and nuclear opacities. There were pulverulent opacities in the perinuclear areas of the right lens while pulverulent opacities in the left lens were lighter. They were also more coronal in the left eye.
Table 1. Clinical features of affected family members.
|
Number |
Age |
Gender |
Onset age |
Surgery age |
Clinical features |
| II1 |
73 |
Female |
20 |
30 |
Aphakia, after cataract surgery |
| II3 |
61 |
Male |
14–15 |
30 |
Aphakia, after cataract surgery |
| III1 |
53 |
Male |
30 |
Bilateral coronary,punctate, asteroidal (above the posterior pole) cataracts |
|
| III2 |
50 |
Male |
20 |
Bilateral coronary, punctate cataracts; gray opacity in partial coronary area |
|
| III3 |
42 |
Female |
15 |
25 |
Aphakia, after cataract surgery |
| III4 |
47 |
Female |
16–17 |
32 |
Right coronary, punctate, asteroidal (posterior pole) cataracts; left after cataract surgery |
| III5 |
36 |
Female |
14–15 |
27 |
Aphakia, after cataract surgery |
| III20 |
40 |
Female |
13 |
22 |
After bilateral phacoemulsification and intraocular lens implantation |
| III21 |
37 |
Female |
10 |
29 |
After bialateral phacoemulsification and intraocular lens implantation |
| III22 |
35 |
Male |
12 |
Bilateral punctate, asteroidal (above the posterior pole) cataracts |
|
| III23 |
33 |
Male |
12 |
33 |
Proband |
| IV1 |
25 |
Male |
12 |
Bilateral punctate, asteroidal (above the posterior pole) cataracts |
|
| IV4 |
22 |
Female |
12 |
Bilateral punctate, asteroidal (above the posterior pole) cataracts |
|
| IV8 |
19 |
Male |
10 |
Bilateral coronary, punctate cataracts |
|
| IV10 |
24 |
Female |
15 |
Bilateral coronary, inverted T-shaped (posterior pole) cataracts |
|
| IV15 |
13 |
Male |
Bilateral sparse punctate, asteroidal (above the posterior pole) cataracts |
||
| IV16 | 6 | Male | Sparse punctate opacities |
Before the age of 10, no clinical features were manifest (IV16); punctate opacification was primarily scattered in the lens perimeter, causing almost no influence on the life of the affected individuals. In adolescence, affected individuals showed ambiguous visual clinical features. At about the age of 30, the clinical features became serious. Two affected family members (III4, III23) had different clinical features between their two lenses; III1, III2, III22, and IV10 had different clinical features from each other. The clinical features of III22, IV1, and IV4 were similar.
Linkage and haplotype analysis
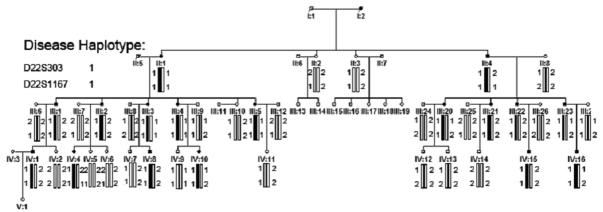
The CRYBB2 gene on chromosome 22 was linked to this family’s disease while other candidate genes were excluded by allele sharing and linkage analysis. Significant linkage was found with markers, D22S303 and D22S1167; the maximum LOD score was 2.11 (at θ=0). Haplotype analysis showed that the phenotype was localized at chromosome 22q11.2–12.2 flanked by markers, D22S303 and D22S1167 (Figure 2, Table 2).
Figure 2.
Pedigree and haplotype of the cataract family. Five-generation pedigree segregates autosomal dominant progressive polymorphic coronary cataracts. Haplotyping showed segregation with two microsatellite markers on 22q. Squares and circles indicate males and females, respectively. Blackened symbols and bars denote affected status.
Table 2. Two-point LOD scores for linkage between cataract locus and chromosome 22 markers.
|
Marker |
LOD scores by recombination fraction (θ) |
||||||
| 0 |
0.04 |
0.09 |
0.14 |
0.19 |
0.24 |
0.29 |
|
| D22S303 |
2.11 |
1.98 |
1.82 |
1.65 |
1.47 |
1.27 |
1.07 |
| D22S1167 | 1.2 | 1.13 | 1.04 | 0.94 | 0.84 | 0.73 | 0.61 |
The highest observed LOD score was 2.11 (θ=0) for marker D22S303.
Mutation analysis for CRYBB1, CRYBB2, CRYBB3, and CRYBA4
Direct cycle sequencing of the amplified fragments of CRYBB2 in two affected individuals identified a single base alteration, C.C475T (Figure 3A), in exon 6 of CRYBB2 (NM_000496), resulting in a substitution of a Gln codon with a stop codon (P.Q155X). The remainder of the coding sequence showed no other change.
Figure 3.
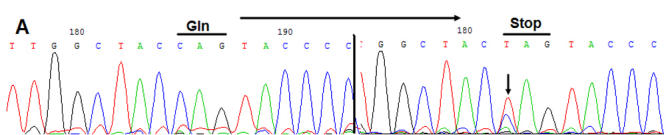
DNA sequence chromatograms of the P.Q155Χ mutation in CRYBB2. Forward sequence analysis of the normal and affected sequence of exon 6 of the CRYBB2 gene in this family is shown. The C→T transition at position 475 resulted in the P.Q155Χ mutation.
Denaturing HPLC
Denaturing HPLC analysis confirmed this mutation (Figure 4), which cosegregated with all affected individuals in the family. Further, this mutation was not observed in any of the unaffected family members or the 100 normal controls.
Figure 4.
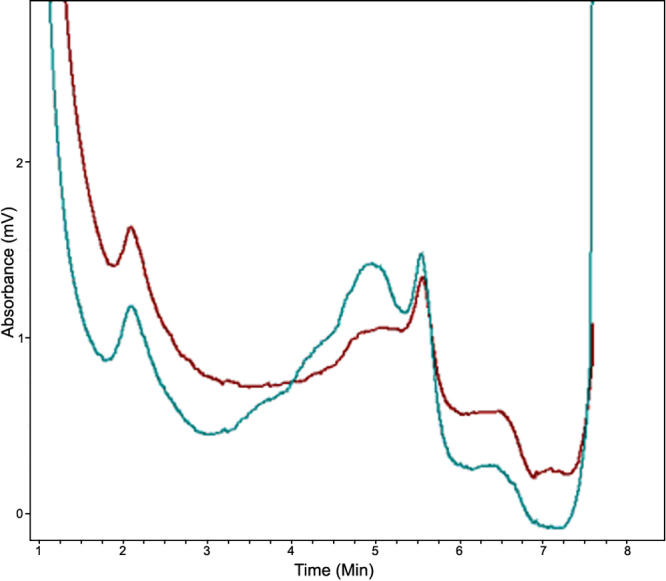
Denaturing high-performance liquid chromatography results of wild type and mutated CRYBB2. DHPLC results show variant traces for CRYBB2 compared with the wild type (WT) trace. The profile in blue is the mutant protein; the profile in red is the wild type protein.
Discussion
We identified a mutation, P.Q155X, in CRYBB2 in a large, five-generation Chinese family with autosomal dominant progressive polymorphic congenital coronary cataracts. The disease gene was linked to 22q11.2–12.2 with a maximum LOD score of 2.11 spanning the β-crystallin gene cluster, which includes CRYBB1, CRYBB2, CRYBB3, and CRYBA4. Mutation analysis of the candidate gene detected a mutation, P.Q155X, in CRYBB2 that cosegregated with the disease phenotype in all affected individuals but was not present in the unaffected family members or the 100 normal control subjects. Clinical and ophthalmologic examinations were conducted on family members in detail; all affected members show different clinical features, and some affected members show different clinical features between the two lenses.
Development of the lens is intrinsically linked to the development of the anterior segment; a transcriptional cascade is involved in early lens development, through Pax6 expression followed by expression of Mafs, Soxs, and Prox1, resulting in the initiation of lens cell differentiation and crystallin expression [22-27]. The β-crystallins are major constituents of the mammalian lens where they associate into dimers, tetramers, and higher oligomers. The appropriate association of crystallins into higher-order complexes is critical to the maintenance of lens transparency and a high refractive index [28]. Opacification in the lens can hinder light from focusing on the retina during key stages of development and lead to permanent visual impairment [29].
CRYBB2 consists of six exons encoding a predicted 205 amino acid protein. The first exon is not translated, the second exon encodes the NH2-terminal extension, and the subsequent four exons are responsible for one ‘Greek key’ motif each [30]. The α-, β-, and γ-crystallins form heterogeneous oligomers in the lens and have molecular weights ranging from 40 to 200 kDa [31]. So far, there have been eight reports in the literature of CRYBB2 gene mutations causing congenital cataracts [32-39]. In addition to Pauli et al. [32], who reported a German family with a mutation in exon5 (p.D128V) which caused a nuclear cataract with an additional ring-shaped cortical opacity and Yao et al. [35] who reported a Chinese family with a mutation in exon 6 (p.Q155 X) which caused highly variable white opacities distributed in the nucleus and cortex, which included pulverulent, dot, strip, star-like and sheet shapes, the clinical features of the other reports were relatively simple (include sutural cataracts, cerulean cataracts and Coppock-like cataracts). This is the sixth reported case of a C.C475T mutation causing congenital cataracts, providing further evidence that CRYBB2 is a pathogenic gene for congenital cataracts and that this site is a hot spot for CRYBB2 mutation.
The clinical diagnosis in the family was coronary cataracts with punctate, asteroidal, and nuclear opacities. Opacification of the lens in affected individuals was bilateral. Each of the affected individuals showed a somewhat different phenotype. In some affected subjects, the star-like opacifications were present at different sites in the posterior pole. Some affected like the proband (pulverulent opacities in the perinuclear areas of the right lens while pulverulent opacities in the left lens were lighter, and in the left lens, they were more coronal) show different clinical features between the two lenses.
Before the age of 10, no clinical features were manifest in the affected individuals, although there was punctate opacification scattered mainly in the lens perimeter, but this had almost no effect on their lives. In adolescence, affected individuals showed ambiguous visual clinical features, and at about the age of 30, the clinical features became serious. Each of the affected individuals showed a somewhat different phenotype. In some affected subjects, the star-like opacifications were present in the upper side of the posterior pole. The opacification style in each affected individual was not alike; some were star-like and some were of an inverted T-shape. These observations provide evidence that congenital cataracts are a clinically heterogeneous lens condition.
Acknowledgments
The authors thank the family for their participation in this project and Dr. Siquan Zhu and Dr. Shuzhen Wang (Beijing Tongren Hospital, Capital University of Medical Sciences, Beijing, China) for phenotype identification. This study was supported by the National Basic Research Program of China (No. 2007CB511905), the National Infrastructure Program of Chinese Genetic Resources (No. 2006DKA21301), and the National Natural Science Foundation of China (No. 30471864).
References
- 1.Gralek M, Kanigowska K, Seroczynska M. Cataract in children - not only an ophthalmological problem. Med Wieku Rozwoj. 2007;XI:227–30. [PubMed] [Google Scholar]
- 2.Yap YC, Woo WW, Kathirgamanathan T, Kosmin A, Faye B, Kodati S.Variation of blood pressure during topical phacoemulsification. Eye 2007[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Perucho-Martínez S, De-la-Cruz-Bertolo J, Tejada-Palacios P. Pediatric cataracts: epidemiology and diagnosis. Retrospective review of 79 cases. Arch Soc Esp Oftalmol. 2007;82:37–42. doi: 10.4321/s0365-66912007000100007. [DOI] [PubMed] [Google Scholar]
- 4.Cumming RG, Mitchell P. Alcohol, smoking, and cataracts: the Blue Mountains Eye Study. Arch Ophthalmol. 1997;115:1296–303. doi: 10.1001/archopht.1997.01100160466015. [DOI] [PubMed] [Google Scholar]
- 5.Scott MH, Hejtmancik JF, Wozencraft LA, Reuter LM, Parks MM, Kaiser-Kupfer MI. Autosomal dominant congenital cataract. Interocular phenotypic variability. Ophthalmology. 1994;101:866–71. doi: 10.1016/s0161-6420(94)31246-2. [DOI] [PubMed] [Google Scholar]
- 6.Hejtmancik JF. The genetics of cataract: our vision becomes clearer. Am J Hum Genet. 1998;62:520–5. doi: 10.1086/301774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert SR, Drack AV. Infantile cataracts. Surv Ophthalmol. 1996;40:427–58. doi: 10.1016/s0039-6257(96)82011-x. [DOI] [PubMed] [Google Scholar]
- 8.Francis PJ, Berry V, Bhattacharya SS, Moore AT. The genetics of childhood cataract. J Med Genet. 2000;37:481–8. doi: 10.1136/jmg.37.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renwick JH, Lawler SD. Probable linkage between a congenital cataract locus and the Duffy blood group locus. Ann Hum Genet. 1963;27:67–84. doi: 10.1111/j.1469-1809.1963.tb00782.x. [DOI] [PubMed] [Google Scholar]
- 10.Reddy MA, Francis PJ, Berry V, Bhattacharya SS, Moore AT. Molecular genetic basis of inherited cataract and associated phenotypes. Surv Ophthalmol. 2004;49:300–15. doi: 10.1016/j.survophthal.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert CE, Canovas R, Hagan M, Rao S, Foster A. Causes of childhood blindness: results from west Africa, south India and Chile. Eye. 1993;7:184–8. doi: 10.1038/eye.1993.39. [DOI] [PubMed] [Google Scholar]
- 12.Litt M, Kramer P, LaMorticella DM, Murphey W, Lovrien EW, Weleber RG. Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum Mol Genet. 1998;7:471–4. doi: 10.1093/hmg/7.3.471. [DOI] [PubMed] [Google Scholar]
- 13.Mackay DS, Andley UP, Shiels A. Cell death triggered by a novel mutation in the alphaA-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genet. 2003;11:784–93. doi: 10.1038/sj.ejhg.5201046. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Ma X, Gu F, Liu NP, Hao XL, Wang KJ, Wang NL, Zhu SQ. A missense mutation S228P in the CRYBB1 gene causes autosomal dominant congenital cataract. Chin Med J (Engl) 2007;120:820–4. [PubMed] [Google Scholar]
- 15.Steele EC, Jr, Kerscher S, Lyon MF, Glenister PH, Favor J, Wang J, Church RL. Identification of a mutation in the MP19 gene, Lim2, in the cataractous mouse mutant To3. Mol Vis. 1997;3:5. [PubMed] [Google Scholar]
- 16.Pras E, Levy-Nissenbaum E, Bakhan T, Lahat H, Assia E, Geffen-Carmi N, Frydman M, Goldman B, Pras E. A missense mutation in the LIM2 gene is associated with autosomal recessive presenile cataract in an inbred Iraqi Jewish family. Am J Hum Genet. 2002;70:1363–7. doi: 10.1086/340318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billingsley G, Santhiya ST, Paterson AD, Ogata K, Wodak S, Hosseini SM, Manisastry SM, Vijayalakshmi P, Gopinath PM, Graw J, Heon E. CRYBA4, a Novel Human Cataract Gene, Is Also Involved in Microphthalmia. Am J Hum Genet. 2006;79:702–9. doi: 10.1086/507712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumaraswamy VS, Lindley PF, Slingsby C, Glover ID. An eye lens protein-water structure: 1.2 A resolution structure of gammaB-crystallin at 150 K. Acta Crystallogr D Biol Crystallogr. 1996;52:611–22. doi: 10.1107/S0907444995014302. [DOI] [PubMed] [Google Scholar]
- 19.Van Rens GL, Driessen HP, Nalini V, Slingsby C, de Jong WW, Bloemendal H. Isolation and characterization of cDNAs encoding beta A2- and beta A4-crystallins: heterologous interactions in the predicted beta A4-beta B2 heterodimer. Gene. 1991;102:179–88. doi: 10.1016/0378-1119(91)90076-n. [DOI] [PubMed] [Google Scholar]
- 20.Fay D. Genetic mapping and manipulation: chapter 2–Two-point mapping with genetic markers. WormBook. 2006:1–6. doi: 10.1895/wormbook.1.91.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litt M, Carrero-Valenzuela R, LaMorticella DM, Schultz DW, Mitchell TN, Kramer P, Maumenee IH. Autosomal dominant cerulean cataract is associated with a chain termination mutation in the human beta-crystallin gene CRYBB2. Hum Mol Genet. 1997;6:665–8. doi: 10.1093/hmg/6.5.665. [DOI] [PubMed] [Google Scholar]
- 22.Ogino H, Yasuda K. Sequential activation of transcription factors in lens induction. Dev Growth Differ. 2000;42:437–48. doi: 10.1046/j.1440-169x.2000.00532.x. [DOI] [PubMed] [Google Scholar]
- 23.Cui W, Tomarev SI, Piatigorsky J, Chepelinsky AB, Duncan MK. Mafs, Prox1, and Pax6 can regulate chicken betaB1-crystallin gene expression. J Biol Chem. 2004;279:11088–95. doi: 10.1074/jbc.M312414200. [DOI] [PubMed] [Google Scholar]
- 24.Kawauchi S, Takahashi S, Nakajima O, Ogino H, Morita M, Nishizawa M, Yasuda K, Yamamoto M. Regulation of lens fiber cell differentiation by transcription factor c-Maf. J Biol Chem. 1999;274:19254–60. doi: 10.1074/jbc.274.27.19254. [DOI] [PubMed] [Google Scholar]
- 25.Reza HM, Ogino H, Yasuda K. L-Maf, a downstream target of Pax6, is essential for chick lens development. Mech Dev. 2002;116:61–73. doi: 10.1016/s0925-4773(02)00137-5. [DOI] [PubMed] [Google Scholar]
- 26.Kondoh H. Transcription factors for lens development assessed in vivo. Curr Opin Genet Dev. 1999;9:301–8. doi: 10.1016/s0959-437x(99)80045-8. [DOI] [PubMed] [Google Scholar]
- 27.Nishiguchi S, Wood H, Kondoh H, Lovell-Badge R, Episkopou V. Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes Dev. 1998;12:776–81. doi: 10.1101/gad.12.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Billingsley G, Santhiya ST, Paterson AD, Ogata K, Wodak S, Hosseini SM, Manisastry SM, Vijayalakshmi P, Gopinath PM, Graw J, Heon E. CRYBA4, a Novel Human Cataract Gene, Is Also Involved in Microphthalmia. J Hum Genet. 2006;79:702–9. doi: 10.1086/507712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ke Yao Xiajing Tang, Xingchao Shentu, Kaijun Wang, Huiying Rao, Kun Xia. Progressive polymorphic congenital cataract caused by a CRYBB2 mutation in a Chinese family. Mol Vis. 2005;11:758–63. [PubMed] [Google Scholar]
- 30.Graw J, Loster J, Soewarto D, Fuchs H, Reis A, Wolf E, Balling R, Hrabe de Angelis M. Aey2, a new mutation in the betaB2-crystallin-encoding gene of the mouse. Invest Ophthalmol Vis Sci. 2001;42:1574–80. [PubMed] [Google Scholar]
- 31.Wang X, Garcia CM, Shui YB, Beebe DC. Expression and regulation of alpha-, beta-, and gamma-crystallins in mammalian lens epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:3608–19. doi: 10.1167/iovs.04-0423. [DOI] [PubMed] [Google Scholar]
- 32.Pauli S, Söker T, Klopp N, Illig T, Engel W, Graw J. Mutation analysis in a German family identified a new cataract-causing allele in the CRYBB2 gene. Mol Vis. 2007;13:962–7. [PMC free article] [PubMed] [Google Scholar]
- 33.Vanita, Sarhardi V, Reis A, Jung M, Singh D, Sperling K, Singh JR, Burger J. A unique form of autosomal dominant cataract explained by gene conversion between β-crystallin B2 and its pseudogene. J Med Genet. 2001;38:392–6. doi: 10.1136/jmg.38.6.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bateman JB, von-Bischhoffshaunsen FR, Richter L, Flodman P, Burch D, Spence MA. Gene conversion mutation in crystallin, beta-B2 (CRYBB2) in a Chilean family with autosomal dominant cataract. Ophthalmology. 2007;114:425–32. doi: 10.1016/j.ophtha.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Yao K, Tang X, Shentu X, Wang K, Rao H, Xia K. Progressive polymorphic congenital cataract caused by a CRYBB2 mutation in a Chinese family. Mol Vis. 2005;11:758–63. [PubMed] [Google Scholar]
- 36.Santhiya ST, Manisastry SM, Rawlley D, Malathi R, Anishetty S, Gopinath PM, Vijayalakshmi P, Namperumalsamy P, Adamski J, Graw J. Mutation analysis of congenital cataracts in Indian families: identification of SNPS and a new causative allele in CRYBB2 gene. Invest Ophthalmol Vis Sci. 2004;45:3599–607. doi: 10.1167/iovs.04-0207. [DOI] [PubMed] [Google Scholar]
- 37.Gill D, Klose R, Munier FL, McFadden M, Priston M, Billingsley G, Ducrey N, Schorderet DF, Héon E. Genetic heterogeneity of the Coppock-like cataract: a mutation in CRYBB2 on chromosome 22q11.2. Invest Ophthalmol Vis Sci. 2000;41:159–65. [PubMed] [Google Scholar]
- 38.Litt M, Carrero-Valenzuela R, LaMorticella DM, Schultz DW, Mitchell TN, Kramer P, Maumenee IH. Autosomal dominant cerulean cataract is associated with a chain termination mutation in the human beta-crystallin gene CRYBB2. Hum Mol Genet. 1997;6:665–8. doi: 10.1093/hmg/6.5.665. [DOI] [PubMed] [Google Scholar]
- 39.Kramer P, Yount J, Mitchell T, LaMorticella D, Carrero-Valenzuela R, Lovrien E, Maumenee I, Litt M. A second gene for cerulean cataracts maps to the beta crystallin region on chromosome 22. Genomics. 1996;35:539–42. doi: 10.1006/geno.1996.0395. [DOI] [PubMed] [Google Scholar]