An acridone-producing novel type III polyketide synthase from H. serrata has been overexpressed in E. coli, purified and crystallized. Diffraction data have been collected to 2.0 Å.
Keywords: acridone, Huperzia serrata, polyketide synthases
Abstract
Polyketide synthase 1 (PKS1) from Huperzia serrata is a plant-specific type III polyketide synthase that shows an unusually versatile catalytic potential, producing various aromatic tetraketides, including chalcones, benzophenones, phlorogulucinols and acridones. Recombinant H. serrata PKS1 expressed in Escherichia coli was crystallized using the hanging-drop vapour-diffusion method. The crystals belonged to space group I222 or I212121, with unit-cell parameters a = 73.3, b = 85.0, c = 137.7 Å, α = β = γ = 90.0°. Diffraction data were collected to 2.0 Å resolution using synchrotron radiation at BL24XU of SPring-8.
1. Introduction
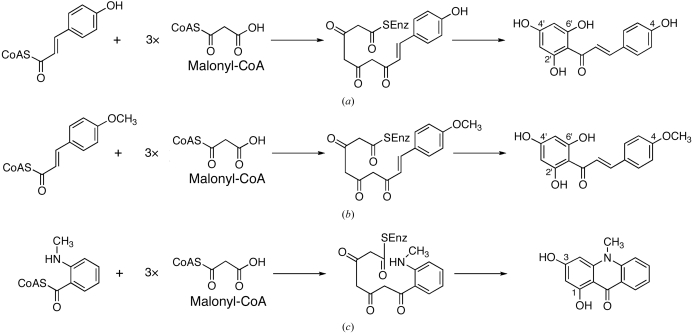
Polyketide synthase 1 (PKS1) from the primitive club moss Huperzia serrata is a novel type III polyketide synthase that shows unusually broad catalytic promiscuity, producing various aromatic tetraketides, including chalcones, benzophenones, phlorogulucinols and acridones (Wanibuchi et al., 2007 ▶). The amino-acid sequence of H. serrata PKS1 shares 44–66% identity with those of other plant enzymes from the chalcone synthase (CHS) superfamily; it has 66% identity with that of CHS from Medicago sativa and 58% identity with that of acridone synthase (ACS) from Ruta graveolens. Recent crystallographic and site-directed mutagenesis studies have revealed that a small modification of the active-site architecture generates the remarkable functional diversity of the type III PKSs (Ferrer et al., 1999 ▶; Jez et al., 2000 ▶; Austin, Bowman et al., 2004 ▶; Sankaranarayanan et al., 2004 ▶; Austin, Izumikawa et al., 2004 ▶; Morita et al., 2007 ▶). Sequence comparisons revealed that H. serrata PKS1 retains most of the CHS active-site residues, including Met137, Gly211, Phe215, Gly216, Phe265 and Pro375, as well as the Cys-His-Asn catalytic triad (numbering according to M. sativa CHS). Moreover, the active-site residues Thr197, Gly256 and Ser338, which are the important residues for starter-substrate selectivity and product chain-length control, are also conserved in H. serrata PKS1. As expected from the sequence similarity, H. serrata PKS1 expressed in Escherichia coli efficiently produces naringenin chalcone from 4-coumaroyl-CoA after three condensations with maloyl-CoA (Fig. 1 ▶ a). One of the most characteristic features is that unlike regular CHS, H. serrata PKS1 catalyzes the formation of 4-methoxy-2′,4′,6′-trihydroxychalcone from a bulky starter substrate, 4-methoxycinnamoyl-CoA (Fig. 1 ▶ b). Furthermore, H. serrata PKS1 readily accepts N-methylanthraniloyl-CoA to produce 1,3-dihydroxy-N-methylacridone (Fig. 1 ▶ c), which had previously only been reported for R. graveolens ACS, even though H. serratia PKS1 does not conserve the active-site residues proposed to convey this specificity to R. graveolens ACS: Ser132, Ala133 and Val265 are replaced with Thr132, Thr133 and Phe265, respectively, in H. serrata PKS1 (Springob et al., 2000 ▶; Lukacin et al., 2001 ▶, 2005 ▶). It is quite remarkable that despite the apparent structural differences in the active site, H. serrata PKS1 still displays the unusual and efficient acridone-producing activity. To further clarify the intimate three-dimensional structural details of the enzyme-catalyzed processes and to elucidate the structure–function relationship of the type III PKS enzymes, we now report the crystallization and preliminary crystallographic analysis of H. serrata PKS1.
Figure 1.
Proposed mechanism for the formation of (a) naringenin chalcone from 4-coumaroyl-CoA catalyzed by regular CHS and H. serrata PKS1, (b) 4-methoxy-2′,4′,6′-trihydroxychalcone from 4-methoxycinnamoyl-CoA catalyzed by H. serrata PKS1 and (c) 1,3-dihydroxy-N-methylacridone from N-methylanthraniloyl-CoA catalyzed by R. graveolens ACS and H. serrata PKS1.
2. Experimental
2.1. Expression and purification
The pQE81L vector, encoding full-length H. serrata PKS1 (Wanibuchi et al., 2007 ▶), was used as a template to amplify the H. serrata PKS1 gene by PCR, using 5′-GCGCCCGGGAATGACAATCAAGGGATCAGGGTCA-3′ as a sense primer, which introduces a SmaI restriction site, and 5′-GCGGTCGACTCAAATGTTGATACTTCTCAGCAA-3′ as an antisense primer, which introduces a SalI restriction site. The amplified DNA fragment was digested with SmaI/SalI and was cloned into the SmaI/SalI sites of a modified pET22b(+) expression vector (Novagen) for expression with a glutathione S-transferase (GST) fusion protein at the N-terminus. A PreScission Protease (GE Healthcare) cleavage site (LEVLFQGP) was introduced between GST and H. serrata PKS1. After confirmation of the sequence, the resultant expression plasmid was transformed into E. coli BL21(DE3) pLysS. The cells harbouring the plasmid were cultured to an OD600 of 0.6 in LB medium containing 50 µg ml−1 carbenicillin at 303 K. 0.4 mM isopropyl β-d-thiogalactopyranoside was then added to induce protein expression and the culture was incubated at 303 K for a further 16 h.
All the following procedures were performed at 277 K. The E. coli cells were harvested by centrifugation at 5000g and resuspended in 50 mM Tris–HCl buffer pH 8.0 containing 0.2 M NaCl, 5% glycerol and 2 mM DTT. The cells were disrupted by sonication and the lysate was centrifuged at 12 000g for 30 min. The supernatant was loaded onto a glutathione Sepharose 4B affinity column (GE Healthcare) equilibrated with Tris–HCl buffer and the column was then washed with Tris–HCl buffer. The GST tag was cleaved on the column by PreScission Protease overnight and the target protein was eluted with Tris–HCl buffer. The resultant H. serrata PKS1 protein thus contains three additional residues (GPG) at the N-terminal flanking region derived from the PreScission Protease recognition sequence. After fivefold dilution of the recombinant H. serrata PKS1 protein solution with 50 mM Tris–HCl buffer pH 8.0 containing 5% glycerol and 2 mM DTT, the protein solution was applied onto a Resource-Q column (GE Healthcare). The column was washed with Tris–HCl buffer containing 50 mM NaCl and the protein was subsequently eluted using a linear gradient of 50–150 mM NaCl. The protein solution was further purified to homogeneity by chromatography on a Superdex 200HR (10/100 GL) column (GE Healthcare) and was concentrated to 23 mg ml−1 in 20 mM HEPES–NaOH pH 7.5 buffer containing 100 mM NaCl and 2 mM DTT. The typical yield of protein was about 0.5 mg per litre of culture.
2.2. Crystallization and X-ray data collection
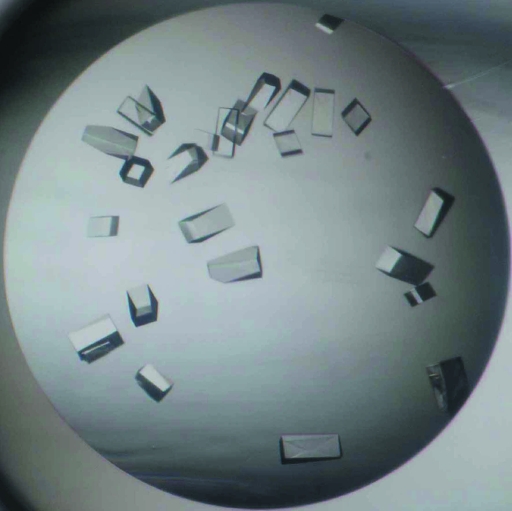
Initial crystallization attempts were carried out at 293 K by the sitting-drop vapour-diffusion method using a 96-condition crystallization screen originally designed by Mitsubishi Chemical Corporation. Although crystals were not observed under any of the conditions, several crystallization conditions using ammonium sulfate (Hampton Research) as a precipitant showed clear drops after two weeks, while all other conditions formed protein aggregates within a day. Further crystallization attempts thus proceeded using Additive Screens (Hampton Research) at various pH values using 1785 mM ammonium sulfate as a precipitant with the sitting-drop vapour-diffusion method. Crystals grew in 100 mM MES–NaOH buffer pH 6.5 (Hampton Research) containing 1785 mM ammonium sulfate and 4%(v/v) 1,1,1,3,3,3-hexafluoro-2-propanol (Hampton Research) and the crystallization conditions were further optimized. Finally, diffraction-quality crystals were obtained at 293 K in 100 mM MES–NaOH buffer pH 6.5 containing 1925 mM ammonium sulfate and 0.5%(v/v) 1,1,1,3,3,3-hexafluoro-2-propanol using the hanging-drop vapour-diffusion method. The crystallization drops were prepared by mixing 0.5 µl protein solution and an equal volume of reservoir solution and were equilibrated against 500 µl reservoir solution. The crystals appeared reproducibly within 2 d and grew to average dimensions of approximately 0.15 × 0.05 × 0.05 mm (Fig. 2 ▶).
Figure 2.
Crystals of H. serrata PKS1 grown by the hanging-drop method. The dimensions of the crystals were approximately 0.15 × 0.05 × 0.05 mm.
The crystals were transferred to a cryoprotectant containing 100 mM MES–NaOH pH 6.5, 1300 mM ammonium sulfate, 0.5%(v/v) 1,1,1,3,3,3-hexafluoro-2-propanol, 12%(v/v) glycerol and 14%(w/v) PEG 400, picked up in a nylon loop and then flash-cooled at 100 K in a nitrogen-gas stream. X-ray diffraction data were collected from a single crystal at SPring-8 beamline BL24XU using a Rigaku R-AXIS V imaging-plate area detector. The wavelength of the synchrotron radiation was 0.82656 Å and the distance between the crystal and the detector was 400 mm. 180 frames were recorded with 1° oscillation and 60 s exposure time. The data were indexed, integrated and scaled using the HKL-2000 program package (Otwinowski & Minor, 1997 ▶).
3. Results and discussion
Recombinant H. serrata PKS1 was heterologously expressed in E. coli as a fusion protein with GST at the N-terminus. After cleavage of the GST tag, the purified enzyme migrated as a single band with a molecular weight of 44 kDa on SDS–PAGE, which is in good agreement with the calculated value of 43 575 Da. In contast, a gel-filtration experiment gave a molecular weight of 88 kDa, suggesting that H. serrata PKS1 is a homodimeric enzyme, as is the case for other known type III PKSs (Austin & Noel, 2003 ▶).
A complete data set was collected to 2.0 Å resolution. From the diffraction data collection, the space group was determined to be either I222 or I212121, with unit-cell parameters a = 73.3, b = 85.0, c = 137.7 Å, α = β = γ = 90.0°. Detailed data-processing statistics are shown in Table 1 ▶. It should be noted that the data were only processed to the edges of the square detector. Thus, higher resolution data may be obtained in the future. Although H. serrata PKS1 functions as a homodimeric enzyme in aqueous solution, as confirmed by the gel-filtration experiment, the crystallographic asymmetric unit contains one monomer of H. serrata PKS1, with a Matthews volume (V M; Matthews, 1968 ▶) of 2.5 Å3 Da−1 and a solvent content of 48.8%. The calculated value is in the range normally observed for protein crystals, thus suggesting that H. serrata PKS1 forms a symmetric dimer with a crystallographical twofold axis. Further determination of phases using the molecular-replacement method with the crystal structure of M. sativa CHS (PDB code 1bq6; Ferrer et al., 1999 ▶) as a search model and structural analyses are in progress. Simultaneously, we are also attempting to crystallize H. serrata PKS1 complexed with substrate and product analogues. These structural analyses will provide valuable insights into the polyketide-formation reaction of the functionally diverse type III PKSs.
Table 1. Data-collection statistics.
Values in parentheses are for the highest resolution shell.
| Space group | I222 or I212121 |
| Unit-cell parameters | |
| a (Å) | 73.3 |
| b (Å) | 85.0 |
| c (Å) | 137.7 |
| α = β = γ (°) | 90.0 |
| Resolution (Å) | 30.0–2.0 (2.07–2.00) |
| Unique reflections | 29050 |
| Redundancy | 7.1 (7.1) |
| Completeness (%) | 100.0 (100.0) |
| 〈I/σ(I)〉 | 49.2 (10.8) |
| Rsym† (%) | 5.6 (28.4) |
R
sym = 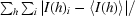
 , where I(h) is the intensity of reflection h,
, where I(h) is the intensity of reflection h,  is the sum over all reflections and
is the sum over all reflections and 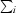 is the sum over i measurements of reflection h.
is the sum over i measurements of reflection h.
Acknowledgments
This work was supported in part by a grant from the National Project on Protein Structural and Functional Analyses.
References
- Austin, M. B., Bowman, M. E., Ferrer, J. L., Schröder, J. & Noel, J. P. (2004). Chem. Biol.11, 1179–1194. [DOI] [PubMed] [Google Scholar]
- Austin, M. B., Izumikawa, M., Bowman, M. E., Udwary, D. W., Ferrer, J. L., Moore, B. S. & Noel, J. P. (2004). J. Biol. Chem.279, 45162–45174. [DOI] [PubMed] [Google Scholar]
- Austin, M. B. & Noel, J. P. (2003). Nat. Prod. Rep.20, 79–110. [DOI] [PubMed] [Google Scholar]
- Ferrer, J. L., Jez, J. M., Bowman, M. E., Dixon, R. A. & Noel, J. P. (1999). Nature Struct. Biol.6, 775–784. [DOI] [PubMed] [Google Scholar]
- Jez, J. M., Austin, M. B., Ferrer, J., Bowman, M. E., Schroder, J. & Noel, J. P. (2000). Chem. Biol.7, 919–930. [DOI] [PubMed] [Google Scholar]
- Lukacin, R., Schreiner, S. & Matern, U. (2001). FEBS Lett.508, 413–417. [DOI] [PubMed] [Google Scholar]
- Lukacin, R., Schreiner, S., Silber, K. & Matern, U. (2005). Phytochemistry, 66, 277–284. [DOI] [PubMed] [Google Scholar]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Morita, H., Kondo, S., Oguro, S., Noguchi, H., Sugio, S., Abe, I. & Kohno, T. (2007). Chem. Biol.14, 359–369. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Sankaranarayanan, R., Saxena, P., Marathe, U. B., Gokhale, R. S., Shanmugam, V. M. & Rukmini, R. (2004). Nature Struct. Mol. Biol.11, 894–900. [DOI] [PubMed]
- Springob, K., Lukacin, R., Ernwein, C., Groning, I. & Matern, U. (2000). Eur. J. Biochem.267, 6552–6559. [DOI] [PubMed] [Google Scholar]
- Wanibuchi, K., Zhang, P., Abe, T., Morita, H., Kohno, T., Chen, G., Noguchi, H. & Abe, I. (2007). FEBS J.274, 1073–1082. [DOI] [PubMed] [Google Scholar]