In order to gain new insights into the protein structure and its possible interaction with a metal ion or effector ligand, BigR from X. fastidiosa was crystallized in native and selenomethionine (SeMet) labelled forms using the hanging-drop vapour-diffusion method.
Keywords: BigR, transcription repressors, biofilm formation, Xylella fastidiosa
Abstract
BigR (biofilm growth-associated repressor) is a novel repressor protein that regulates the transcription of an operon implicated in biofilm growth in both Xylella fastidiosa and Agrobacterium tumefaciens. This protein binds to a palindromic TA-rich element located in the promoter of the BigR operon and strongly represses transcription of the operon. BigR contains a helix–turn–helix (HTH) domain that is found in some members of the ArsR/SmtB family of metal sensors, which control metal resistance in bacteria. Although functional studies have suggested that BigR does not act as a metal sensor, the presence of two cysteines and a methionine in its primary structure raised the possibility of BigR being a metal-ligand protein. In order to gain new insights into the protein structure and its possible interaction with a metal ion or effector ligand, BigR from X. fastidiosa was crystallized in native and selenomethionine (SeMet) labelled forms using the hanging-drop vapour-diffusion method. X-ray diffraction data were collected from native and SeMet crystals to resolutions of 1.95 and 2.2 Å, respectively. Both crystals belong to space group P321 and contain one molecule per asymmetric unit.
1. Introduction
Xylella fastidiosa is a plant pathogen that causes economically important diseases in citrus plants, grapevines and coffee (Davis et al., 1978 ▶; Chang et al., 1993 ▶; De Lima et al., 1998 ▶). The bacterium, which is transmitted from plant to plant by specific insect vectors, colonizes the xylem vessels, leading to water and nutrient stress (Brlansky et al., 1983 ▶). The development of the symptoms of the disease is currently thought to be caused by vascular occlusion of the xylem vessels arising from bacterial biofilm formation (Newman et al., 2004 ▶). The process of biofilm formation in the Xylella–plant interaction is not very well understood, but recent studies have shown that an operon that is conserved in several plant bacterial species is activated during bacterial biofilm growth (Barbosa & Benedetti, unpublished work). This operon encodes proteins of unknown function and BigR, a transcriptional factor that represses transcription of the operon through binding to an operator sequence located at the −10 region of the operon promoter (Barbosa & Benedetti, unpublished work).
BigR contains a central HTH domain that is commonly found in members of the ArsR/SmtB protein family, which control metal tolerance in bacteria (Busenlehner et al., 2003 ▶). These transcriptional repressors have conserved metal-binding sites and function as metal sensors. The transcriptional control of metal-resistant genes by this class of repressors depends on the repressor metal-binding status. Although functional studies have suggested that BigR does not act as a metal sensor, the presence of two cysteines and a methionine that are conserved in various BigR homologues raised the possibility of BigR being a metal-ligand protein. To gain insight into the three-dimensional structure of BigR and to investigate the possibility of this protein interacting with a metal ion or a nonmetal effector ligand, we have crystallized the X. fastidiosa repressor for X-ray diffraction and structure determination.
2. Materials and methods
2.1. Cloning, expression and purification
The XF0767 gene (NP_298057) was amplified from X. fastidiosa genomic DNA with primers 5′-CCATGGTGAACGAAATGCGAG-3′ and 5′-CTCGAGTCACGCCTGTTTCTC-3′. PCR products were cloned into pGEMt (Promega), sequenced and subcloned into pET28a (Novagen) for expression of native BigR. To obtain the 12-amino-acid N-terminal deletion (ΔBigR), pET28a-BigR was cut with NdeI/XhoI and the corresponding BigR fragments were inserted into pET29a (Novagen).
Recombinant BigR was produced in Escherichia coli BL21 (DE3) cells transformed with pET28a and pET29a (Novagen) containing the BigR and ΔBigR sequence, respectively. Cells were grown at 310 K under agitation and proteins were produced by the addition of IPTG (0.4 mM) after the optical density of the culture reached 0.6 at 600 nm. After 3 h induction in LB medium, cells were disrupted by sonication. The selenomethionine-substituted protein (SeMet-ΔBigR) was produced in the same E. coli strain BL21 (DE3) as used for nonlabelled protein expression. Cells were grown in 1.5 l M9 minimum media (Sambrook & Russell, 2001 ▶) containing 50 µM FeCl3, 10 µM MnCl2, 10 µM ZnSO4, 2 µM CoCl2 and 2 µM NiSO4 at 303 K under agitation until the optical density of the culture reached 0.6 at 600 nm. The culture was then supplemented with 150 mg lysine, 150 mg phenylalanine, 150 mg threonine, 75 mg isoleucine, 75 mg valine and 90 mg selenomethionine 15 min before induction with 0.4 mM IPTG for 7 h at 303 K and 200 rev min−1.
Cell extracts containing BigR were loaded onto a Q-Sepharose FF column (Amersham Biosciences) pre-equilibrated with 20 mM Tris–HCl pH 7.0 and 0.5 mM DTT. The protein was eluted with a linear gradient of 0–1 M NaCl in the same buffer. Ammonium sulfate was added to the protein fraction to a final concentration of 1 M and the protein fraction was then loaded onto a Phenyl-Sepharose HP column (Amersham Biosciences) pre-equilibrated with 20 mM Tris–HCl pH 7.0, 0.5 mM DTT and 1 M ammonium sulfate. The protein was eluted with a linear gradient of 1–0 M ammonium sulfate in the same buffer. ΔBigR and ΔBigR-SeMet were purified using the same protocol as described above, except that the pH of the buffer was 7.7 and an additional gel-filtration step was performed after the hydrophobic adsorption chromatography. In this step, ΔBigR and ΔBigR-SeMet were loaded onto a Superdex G75-16/60 column (Amersham Biosciences) pre-equilibrated with 20 mM Tris–HCl pH 7.5, 0.5 mM DTT and 50 mM ammonium sulfate. All proteins were concentrated and dialyzed against the same buffer containing 10 mM ammonium sulfate. Selenomethionine incorporation was confirmed by mass spectrometry, which indicated that the three methionines of ΔBigR were replaced by selenomethionines.
2.2. Crystallization
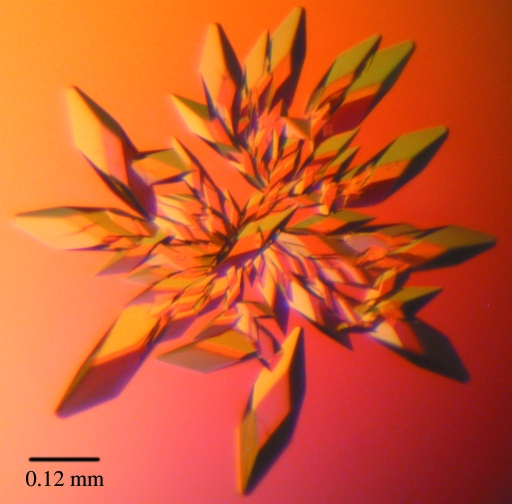
Initial crystallization trials were performed with native BigR protein at 10 mg ml−1 using screens from Hampton Research (Crystal Screens I and II), Emerald BioStructures (Wizard I and II) and Jena Bioscience (JB I and II). Crystals were obtained using the hanging-drop vapour-diffusion method at 293 K, but despite extensive optimization trials they only diffracted to low resolution (∼6 Å). In an attempt to improve the crystal quality, ΔBigR protein was submitted to crystallization trials using the same screens. Crystals were observed 24 h after mixing 2 µl protein solution (7 mg ml−1) with 2 µl reservoir buffer containing 15% ethanol and 0.1 M CHES pH 9.5. Crystals were optimized by varying the ethanol concentration and the buffer pH around the initial condition. The best ΔBigR crystals were obtained in 18% ethanol and 0.1 M Tris–HCl pH 9.5. ΔBigR-SeMet protein was submitted to the same refinement screen and the best crystals were grown in 17% ethanol and 0.1 M Tris–HCl pH 8.5. ΔBigR crystals reached dimensions of approximately 0.3 × 0.1 × 0.1 mm after one week (Fig. 1 ▶).
Figure 1.
BigR crystals from X. fastidiosa obtained by hanging-drop vapour diffusion. The maximum crystal dimensions are approximately 0.3 × 0.1 × 0.1 mm.
2.3. Data collection and processing
X-ray diffraction data from a native crystal were collected at the D03B-MX1 beamline of the Brazilian Synchrotron Light Laboratory (LNLS), Campinas, Brazil. Crystals were cryoprotected with 15% 2-methyl-2,4-pentanediol prior to flash-cooling in a 100 K nitrogen-gas stream. A complete data set was collected using a MAR CCD detector and an oscillation width of 0.8° per image. The beamline wavelength was set to 1.43 Å. Data from a ΔBigR-SeMet crystal were collected at the W01A-MX2 beamline of the LNLS at various wavelengths (peak, inflection, remote) derived from a scan of the Se K absorption edge. Diffraction data were processed with MOSFLM (Leslie, 1992 ▶) and SCALA (Kabsch, 1988 ▶; Blessing, 1995 ▶) from the CCP4 package (Collaborative Computational Project, Number 4, 1994 ▶).
3. Results and discussion
BigR crystallization trials using the complete protein resulted in crystals of poor diffraction quality. In order to obtain better crystals, an N-terminally truncated form of the protein beginning at residue 13 (ΔBigR) was produced. ΔBigR (11.5 kDa) crystals diffracted to 1.95 Å resolution and belong to space group P321, with unit-cell parameters a = b = 33.68, c = 139.07 Å. Matthews coefficient calculation (Matthews, 1968 ▶) indicated the presence of one monomer in the asymmetric unit and a solvent content of 35.8%. Table 1 ▶ summarizes the data-collection statistics.
Table 1. Data-collection statistics.
Values in parentheses are for the outer resolution shell.
| SeMet | ||||
|---|---|---|---|---|
| Data set | Native | Peak | Inflection | Remote |
| Wavelength (Å) | 1.43 | 0.9795 | 0.9797 | 0.9282 |
| Space group | P321 | P321 | P321 | P321 |
| Unit-cell parameters (Å) | a = b = 33.68, c = 139.07 | a = b = 34.23, c = 140.78 | a = b = 34.23, c = 140.81 | a = b = 34.23, c = 140.78 |
| Resolution limits (Å) | 29.17–1.95 (2.06–1.95) | 29.64–2.20 (2.32–2.20) | 29.64–2.25 (2.37–2.25) | 29.64–2.25 (2.37–2.25) |
| Completeness (%) | 93.0 (93.0) | 98.2 (98.2) | 98.4 (98.4) | 98.4 (98.4) |
| Multiplicity | 17.8 (18.4) | 11.2 (11.7) | 11.2 (11.8) | 11.2 (11.8) |
| Rsym (%) | 5.6 (37.5) | 5.9 (33.3) | 6.8 (39.4) | 7.2 (42.1) |
| Mean I/σ(I) | 9.5 (2.0) | 34.9 (7.3) | 31.7 (6.4) | 30.4 (6.3) |
| Anomalous completeness (%) | — | 98.9 (98.5) | 99.0 (98.3) | 99.1 (98.5) |
| Anomalous multiplicity | — | 6.3 (6.2) | 6.3 (6.2) | 6.3 (6.2) |
| CCanom (overall) | — | 0.626 | 0.499 | 0.494 |
| Resolution limit of anomalous signal† (Å) | — | 2.6 | 2.8 | 3.0 |
Resolution to which the correlation of the anomalous differences is larger than 0.4.
Extensive attempts to solve the ΔBigR structure by molecular-replacement methods using HTH protein models deposited in the Protein Data Bank were unsuccessful. The sequence identity to the search models varied from 15% to 25% and the models were used intact or trimmed to account for insertions and deletions. Polyalanine models were also used without success.
As an alternative to the molecular-replacement approach, selenomethionine-labelled ΔBigR was produced. Mass spectrometry showed that all three methionines of ΔBigR were substituted by selenomethionines, thus allowing the use of the MAD technique. SeMet crystals diffracted to 2.20 Å and belong to space group P321, with unit-cell parameters that are very similar to those of the native crystal (Table 1 ▶). Two selenium sites were found using the program SHELXD (Schneider & Sheldrick, 2002 ▶) and initial phase calculation was carried out with autoSHARP (Vonrhein et al., 2006 ▶). Electron-density map analysis and model building are currently in progress.
In conclusion, we have crystallized both native and SeMet-labelled X. fastidiosa BigR protein and expect that the three-dimensional structure of BigR will provide insight into its possible interaction with metal ions or other effector ligands.
Acknowledgments
This work was supported by FAPESP grants (03/08316-5, Smolbnet 00/10266-8 and Cepid 98/14138-2). RLB and FCR received PhD fellowships from FAPESP (02/12329-2 and 03/12875-0). CEB received a fellowship from CnPq.
References
- Blessing, R. H. (1995). Acta Cryst. A51, 33–38. [DOI] [PubMed] [Google Scholar]
- Brlansky, R. H., Timmer, I. W., French, W. J. & McCoy, R. E. (1983). Phytopathology, 73, 530–535.
- Busenlehner, L. S., Pennella, M. A. & Giedroc, D. P. (2003). FEMS Microbiol. Rev.27, 131–143. [DOI] [PubMed] [Google Scholar]
- Chang, C. J., Garnier, M., Zreik, L., Rossetti, V. & Bové, J. M. (1993). Curr. Microbiol.27, 137–142. [DOI] [PubMed]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. [Google Scholar]
- Davis, M. J., Purcell, A. H. & Thomson, S. V. (1978). Science, 199, 75–77. [DOI] [PubMed]
- De Lima, J. E. O., Miranda, V. S., Hartung, J. S., Brlansky, R. H., Coutinho, A., Roberto, S. R. & Carlos, E. F. (1998). Plant Dis.82, 94–97. [DOI] [PubMed]
- Kabsch, W. (1988). J. Appl. Cryst.21, 916–924. [Google Scholar]
- Leslie, A. G. W. (1992). Jnt CCP4/ESF–EACBM Newsl. Protein Crystallogr.26
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Newman, K. L., Almeida, R. P., Purcell, A. H. & Lindow, S. E. (2004). Proc. Natl Acad. Sci. USA, 101, 1737–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J. & Russell, D. W. (2001). Molecular Cloning: A Laboratory Manual, 3rd ed. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press.
- Schneider, T. R. & Sheldrick, G. M. (2002). Acta Cryst. D58, 1772–1779. [DOI] [PubMed] [Google Scholar]
- Vonrhein, C., Blanc, E., Roversi, P. & Bricogne, G. (2006). Macromolecular Crystallography Protocols, edited by S. Doublié, Vol. 2, pp. 215–230. Totowa, NJ, USA: Humana Press.