Abstract
Objective
Bone marrow failure is a near-universal occurrence in patients with Fanconi Anemia (FA) and thought to result from exhaustion of the hematopoietic stem cell (HSC) pool. Retrovirus mediated expression of the deficient protein corrects this phenotype and makes FA a candidate disease for HSC-directed gene therapy. However, inherent repopulation deficits and stem cell attrition during conventional transduction culture prevent therapeutic chimerism.
Methods
We previously reported rapid transduction protocols to limit stem cell losses after ex vivo culture. Here we describe a complementary strategy intended to improve repopulation through upregulation of chemokine receptor (CXCR) 4, a principal factor in hematopoietic homing.
Results
Using murine models with transgenic disruption of Fanca, -c, and -d2 we found that c-kit+ and sca-1+ progenitor cells express levels of CXCR4 comparable with their wild-type littermates. Lineage-depleted progenitor populations rapidly upregulated CXCR4 transcript and protein in response to cytokine stimulation or hypoxia, regardless of genotype. Hypoxia conditioning of lineage-depleted Fancc−/− progenitors also reduced oxidative stress, improved in vitro migration and led to improved chimerism in myeloablated recipients after transplantation.
Conclusion
These studies provide evidence that CXCR4 regulation in progenitor cells from transgenic mice representing multiple FA genotypes is intact and that modulation of homing offers a potential strategy to offset the FA HSC repopulation deficiency.
Keywords: CXCR4, Hematopoietic Stem Cells, Fanconi Anemia
Introduction
Fanconi anemia (FA) is a recessively inherited DNA repair disorder with a complex genetic basis and prominent manifestation in the hematopoietic system [1]. Progressive marrow aplasia in FA patients is widely believed to result from a pro-apoptotic cellular phenotype and stem cell loss. This idea is supported by the exaggerated in vitro sensitivity of cells to reactive oxygen species (ROS), reduced progenitor clonogenicity, and the involvement of FA proteins in maintaining DNA integrity [2–5]. Further, overexpression of FANCC protects FA phenotype human and murine cells from apoptosis [6,7]. This suggests a role of FA genes in proliferation and survival in the progenitor compartment and may explain the compromised quality and quantity of FA HSC, as well as the repopulation defects in murine models of Fancc, and hypomorphic –d1 [4,8,9]. To what extent the observed repopulation deficits might involve a role for FA proteins in progenitor cell homing and migration has not been studied to date. Taken together, efficient gene transfer to FA HSC and their subsequent engraftment under such constraints have been a challenge and transduction culture itself can compromise repopulating ability and genomic stability [10–13]. We and others recently reported that lentivirus vectors enable the transduction of murine HSC in simplified ex vivo protocols, thereby providing one model for how to limit in vitro differentiation and stem cell loss [14–16]. The current studies were designed to investigate complementary strategies to improve the subsequent homing of hematopoietic target cells after ex vivo transduction culture.
Hematopoietic stem cells are capable of tissue-specific homing and redistribute to the stem cell niche within 15 hours after intravenous injection in mice [17,18]. Homing requires the coordinated interaction of cells, endothelium, and the supportive microenvironment in the marrow through cell surface molecules and their ligands [18,19]. Chemokine receptor (CXCR) 4 and its ligand, stromal derived factor (SDF) -1α, play a prominent role in this process [20,21]. Stable overexpression, or transient upregulation, of CXCR4 in hematopoietic cells improve homing to the marrow and resultant chimerism in murine transplantation experiments [22,23]. Conversely, downregulation of CXCR4 activity, or disruption of CXCR4-SDF1α binding, diminishes homing to the marrow after intravenous injection [22,24]. Homing of intravenously injected stem/progenitor cells is enhanced after brief culture in the presence of cytokines including stem cell factor (SCF), or in response to tissue hypoxia, each involving CXCR4 signaling [25–33]. Homing in general, and the role of CXCR4 expression and regulation in particular, have not previously been investigated in FA.
Results presented here in mice with transgenic disruption of Fanca, -c, or -d2 genes indicate that CXCR4 regulation of FA hematopoietic progenitors is intact and that hypoxia conditioning can upregulate CXCR4 and improve chemotactic migration while limiting oxidative stress during ex vivo culture. We propose that hypoxia-induced CXCR4 upregulation provides a potential strategy to improve the homing of FA phenotype HSC and offset the repopulation disadvantage, especially in the context of ex vivo gene transfer protocols.
Material and Methods
Cell culture and retroviral transduction
293T human kidney fibroblast cells were propagated in DMEM medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/1% streptomycin (Pen/Strep). L1210 cells, a murine hematopoietic cell line, was kindly provided by R. Storms and cultured in RPMI supplemented with 10% FBS and 1% Pen/Strep. Murine whole bone marrow (WBM) and lineage-depleted (lin-) cells were grown in Iscove’s media supplemented with 10% FBS, 10% horse serum 1% Pen/Strep, 50 ng/mL murine Stem Cell Factor (mSCF), and murine interleukin (IL)-3 (Peprotech, Rocky Hill, NJ). Experiments in low oxygen environment were conducted in a dedicated chamber at 1% O2, 5% CO2 at 37°C. All other cell culture occurred at ambient O2 levels.
Flow-cytometry
Cellular CXCR4 expression was analyzed at serial time points using a FACS-Calibur instrument (BD Biosciences) and data was processed using FlowJo software (Tree Star, Ashland, OR. USA). Samples were stained with murine antibodies directed against surface epitopes: CXCR4 (FITC), sca-1 (PE), c-kit (APC), and IgG (FITC, as background-staining control) (all BD Biosciences, San Jose, CA). Following antibody staining according to manufacturer’s recommendation, samples were washed in PBS containing 2% FBS and resuspended in 2% FBS-PBS containing 1 μg/ml propidium iodide solution to exclude dead cells from analysis. For clarity, IgG background values are not shown on all histogram overlays, but were subtracted from each sample to calculate numeric gains. For follow-up studies after transplantation, harvested WBM and peripheral blood underwent hemolysis, leukocytes were stained with anti-CD45.2 (PE) antibody at 4°C for 30-minutes, washed twice in 2%FBS-PBS, and analyzed. Leukocyte subset analysis was based on forward- versus side-scatter gates to distinguish lymphocytes, monocytes and granulocytes, as previously described [14,34,35]
Quantitative real-time rt-PCR assay
Total RNA was extracted from samples using an RNeasy Mini Kit, according to the manufacturer’s protocol (Qiagen Inc., Valencia, CA. USA). Reverse transcription was performed with Oligo(dT)12–18 and SuperScriptTM II RT (Invitrogen), according to manufacturer’s protocol. Complementary DNA expression was assayed via quantitative real time PCR, using CXCR4 primer-probe mix (sense: 5′-GAC CGC CTT TAC CCC GAT AG -3′) and (anti-sense: 5′-GTC CAC CCC GCT TTC CTT TG -3′), purchased from ABI (Perkin-Elmer Applied Biosystems, Foster City, CA. USA) [36] and 18S endogenous control 20x primer-probe set, according to the manufacturer’s instructions (Perkin-Elmer Applied Biosystems, Foster City, CA. USA). DNA from L1210 murine cells was used as a positive control, as this cell line has relatively high, stable endogenous CXCR4 expression. All PCR reactions were set up in a MicroAmp Optical 96-well Reaction Plate (Applied Biosystems), and samples were run in duplicate. All threshold cycle (Ct) values of CXCR4 were normalized to Ct values of an 18S ribosomal RNA internal control. Reactions were run using the ABI Taqman Universal PCR Mastermix (Applied Biosystems) on the ABI prism 7300 sequence detection system (Applied Biosystems) using the following thermal cycling conditions: 50°C for 2min, 95°C for 10min, followed by 40 cycles of 95°C for 15s and 60°C for 1min. The spectrum was then analyzed using ABI Sequence detector v.1.3.
Amplex Red Assay
To compare relative ROS released from Fancc−/− or wt lineage-depleted bone marrow cells and as a general measure of oxidative stress during culture, the Amplex Red Assay (Invitrogen, Carlsbad, CA) was performed according to manufacturer’s protocol. The Amplex Red reagent (10-acetyl-3,7-dihydroxyphenoxazine) reacts with H2O2 in a 1:1 stoichiometry, emitting a red-fluorescent oxidation product. Aliquots were normalized for cell number and cultured in media containing Iscove’s Modified Dulbecco’s Media (IMDM), 10% horse serum (Gibco), 10% fetal bovine serum (Gibco), 1% Penicillin/Streptomycin, 50 ng/ml mSCF (PeproTech, Inc.), and 50 ng/ml IL-3 (PeproTech, Inc.) under hypoxic (3.5% O2) or normoxic conditions for 18 hours. Triplicate reactions were carried out in a 96 well microplate (in additional experiments cells were pelleted and 50 μl cell-free media aliquots were also analyzed, yielding similar results). Fluorescence was measured with a fluorescence microplate reader using excitation and emission detection at 530 nm 590 nm, respectively. A hydrogen peroxide standard curve was generated by diluting known concentrations of H2O2 into IMDM.
In vitro transwell migration assay
Progenitor cells were isolated from wild type, Fancc+/−, and Fancc−/− mice, and were cultured in the presence of IL-3 and mSCF, under normoxic or hypoxic conditions, respectively. Aliquots (2.5 x 105 cells) were sampled every 24 hours for ability to migrate across the 8 μm transwell insert (Corning) toward lower chamber containing media with 30 ng/ml SDF-1α (Peprotech). To ascertain CXCR4 specific migration, a control well contained 1 nM CXCR4 antagonist AMD3100 (Sigma). Cells migrated for 2.5 hours, followed by enumerating migrated cells in lower chamber on a hemacytometer. Each sample was counted 5 times. To calculate statistical differences a 2-tailed, unpaired homoscedastic student t-test was performed. Results were confirmed with a one way ANOVA and Kruskal-Wallis Test (to accommodate small sample size). Because all genotypes were statistically comparable at each time point based on 3 statistical analyses, values were grouped into normoxia- versus hypoxia-conditioned samples for further statistical analysis.
Animal husbandry and transplantation
Mice (C57BL/6 and Boy J -B6.SJL-) were group-housed and allowed ad libitum access to standard chow pellets (Purina Laboratory Rodent Diet 5001, Ralston Purina Co., St. Louis, MO). Mice with transgenic disruption of Fancc (C57BL/6 strain, CD45.2 genotype) were kindly provided by M. Buchwald [37]. Mice with transgenic disruption of Fanca and Fancd2 were described previously [38,39]. Whole bone marrow (WBM) cells were collected by flushing femurs and tibias from 8 to 12 week-old Boy J mice (CD45.1) and CD45.2/Fancc−/− mice with Iscove’s Modified Dulbecco’s Media. Samples were depleted of red cells by hemolysis and lineage-depleted using an Easy Sep® Mouse Hematopoietic Progenitor Cell Enrichment kit according to the manufacturer’s instructions (StemCell Technologies Inc., Vancouver, Canada). Following overnight culture in hypoxic or normoxic conditions, 2.5 x105 lineage-depleted CD45.2/Fancc−/− cells were washed twice in PBS, mixed with 2.5 x 105 CD45.1 lineage-depleted competitor cells, resuspended in 200 μl Hanks Balanced Salt Solution (HBSS) and injected intravenously into myeloablated (950cGy) recipients (CD45.2/Fancc+/− or CD45.2/Fancc+/+). Additional cohorts were transplanted using a ratio of 1 x 104 CD45.2 lineage depleted/2.5 x 105 CD45.1 whole bone marrow competitor cells. Following transplantation, retro-orbital eye bleeds were performed at intervals, and white blood cells were analyzed for CD45.2 expression by flow-cytometry.
Statistical analysis
Numerical results are expressed as average plus or minus standard deviation (SD), except in Figure 6, where median values were used. Data were analyzed using the paired 2-tailed Student t test. P values of less than 0.05 were considered significant. For migration assays, results were confirmed with a one way ANOVA and Kruskal-Wallis Test (to accommodate small sample size).
Results
Mice with transgenic disruption of FA genes present instructive models for the study of qualitative and quantitative defects in FA hematopoietic stem cells [4,8]. We investigated the modulation of CXCR4 cell surface expression in Fanca−/−, Fancc−/−, and Fancd2−/− mice as part of a strategy to improve homing and mitigate FA HSC phenotype repopulation deficits incurred during ex vivo culture necessary for retroviral correction.
Fanca−/−, Fancc−/− and Fancd2−/− mice express similar cell surface levels of CXCR4
Whole bone marrow cells were recovered from animals of all genotypes, Fanca−/−, Fancc−/−, Fancd2−/− and wild-type (wt), and progenitor cells were isolated by immunomagnetic lineage-depletion. Cells were cultured in the presence of cytokines (mSCF and IL-3) under normoxic conditions. We relied on a flow-cytometric assay to detect surface binding of a FITC labeled, anti-murine CXCR4 antibody and determine changes in cell surface protein expression. FITC labeled, anti-murine IgG antibody was used for gating and background staining was subtracted of each sample. Samples were analyzed immediately after harvest and twice more over the subsequent 2-day period. Progenitor cells from each of the FA genotypes demonstrated systematic gains in fluorescence (1.5 to 2-fold, after correction for IgG background for each sample) compared to equivalent baseline levels (Fig. 1A). The increase in CXCR4 expression observed in Fanca−/−, Fancc−/−, or Fancd2−/− cells was not statistically different from wt cells (student 2 tailed, paired t-test). Upregulation was similar for heterozygous Fancc+/− animals (not shown) and they are used interchangeably with wt as control genotype. L1210 cells, a murine T-cell line, served as a positive control and demonstrated stable CXCR4 expression throughout (data not shown). We next confirmed that these gains in CXCR4 antibody binding (i.e fluorescence shifts) by murine progenitors reflect increased CXCR4 message by real-time rt-PCR determination. Results show that cytokine induction (mSCF + mIL-3) led to substantial increases of mRNA species (predominantly by 24 hours with minor additional gains by 48 hours) in samples from Fanca−/−, Fancc−/− and Fancd2−/− progenitor cells (Fig. 1B). With concordant in vitro results for all three murine FA genotypes (p > 0.05, student 2 tailed t-test), we focused further experiments on mice with biallelic Fancc disruption.
Figure 1.
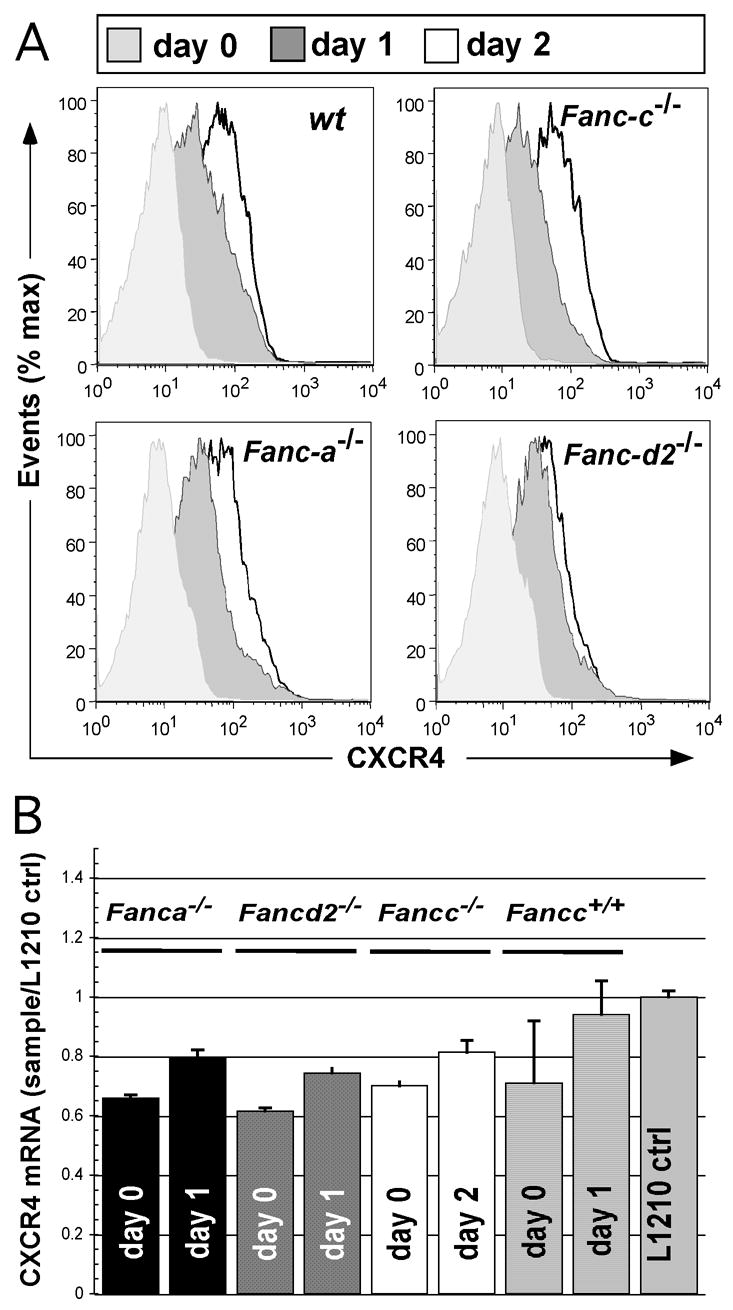
CXCR4 upregulation on the cell surface of whole bone marrow cells from wt, Fancc−/−, Fanca−/− and Fancd2−/− mice. (A) Cells were harvested from one animal per genotype, immunomagnetically depleted of lineage-committed cells and cultured in the presence mIL-3 and mSCF under normoxic conditions. Aliquots were removed from culture at indicated intervals (0h, 24h, 48h) and stained with anti-IgG and anti-CXCR4-FITC labeled antibody. During flow-cytometric analysis, events (>10,000) were gated to exclude dead cells and analyzed for CXCR4 expression. The experiment was performed twice with similar trends observed. (B) CXCR4 mRNA levels during cytokine supplemented culture. Lineage-depleted wt, Fancc−/−, Fanca−/− and Fancd2−/− whole bone marrow cells were cultured in the presence mIL-3 and mSCF. Aliquots were removed from culture at indicated intervals (0h, 24h, or 48h) and total RNA was extracted. Following RNA extraction and subsequent cDNA production, quantitative real-time (Q)-PCR analysis was used to determine the changes in the level of CXCR4 mRNA transcripts over the incubation period. Complementary DNA generated from the L1210 cell line, maintained in expansion culture, was used as a positive control for CXCR4 expression. Real-time-PCR samples were prepared in triplicate. Bars depict averages and standard errors.
Leukocyte subset composition and CXCR4 expression among defined progenitor sets
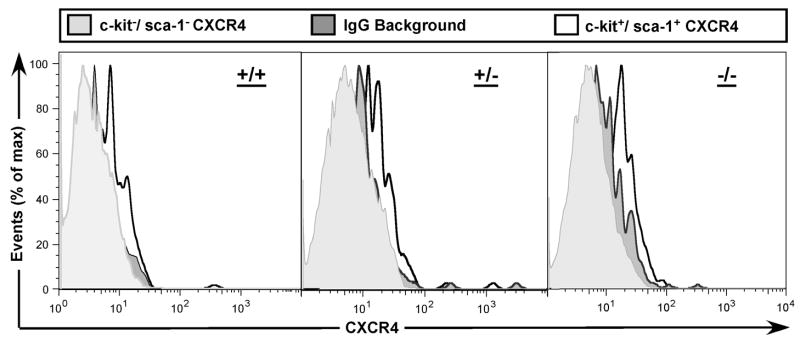
Murine stem and immature progenitor subsets expressing cell surface epitopes sca-1 and c-kit contribute to long-term hematopoietic reconstitution after transplantation. The progenitor yield after depletion of lineage-committed cells was 4.5 +/− 2.6% with similar recovery from Fancc+/− and Fancc−/− animals (data not shown). In order to test CXCR4 expression on leukocyte subsets relevant to hematopoietic repopulation in animals, we next ascertained similar subset distribution among leukocyte progenitors after immunomagnetic depletion of lineage-committed cells with slightly higher percentages of stem and progenitor cells in heterozygous compared to knock-out mice. Changes in leukocyte subset composition during in vitro culture demonstrated similar kinetics in both genotypes (Fig. 2AB). Cell proliferation based on serial cell counts over 72 hours from culture initiation showed comparable contraction in whole bone marrow cells of both genotypes and subsequent modest expansion of lineage depleted progenitor cells in mSCF/mIL-3 supplemented culture of both genotypes (Fig. 2C). In evaluating the differential expression of CXCR4 on progenitor subsets, we found consistently increased levels of cell surface protein by sca-1 and c-kit -positive cells as compared to sca-1, c-kit -negative events (Fig. 3).
Figure 2.
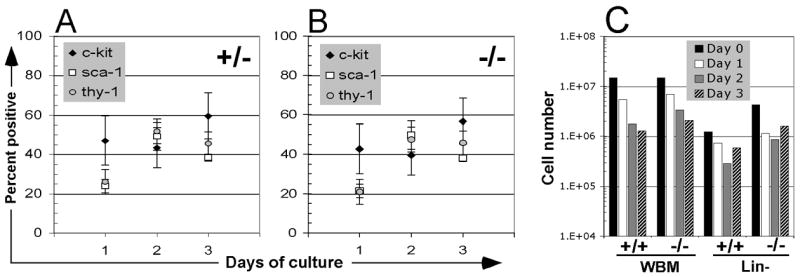
Bone marrow leukocyte progenitor composition and kinetics during in vitro culture. (A) Bone marrow cells from Fancc+/− and (B) Fancc−/− littermates were immunomagnetically depleted of lineage committed cells and cultured in the presence mIL-3 and mSCF. Aliquots were removed from culture at indicated intervals (24h, 48h, 72h) and stained with antibodies against c-kit (open diamond), sca-1 (open square). Events (>10,000) were gated to exclude dead cells and analyzed for epitope expression. Results represent averages from 3 independent experiments and error bars depict the resultant standard deviation. (C) The absolute number of Fancc+/+ and Fancc−/− cells in WBM or lineage-depleted populations over time (day 0 to day 3) is also shown.
Figure 3.
Increased CXCR4 expression on progenitors expressing c-kit and sca-1 epitopes. Bone marrow cells from Fancc−/− (left hand panels) and Fancc+/− (right hand panels) littermates were lineage-depleted, cultured for 24 hours and stained separately with antibodies against c-kit, sca-1 and CXCR4, respectively. Events were collected and analyzed to exclude dead cells and analyze CXCR4 expression on c-kit and sca-1 positive or negative cells.
Hypoxia culture induces increased CXCR4 expression
Culture of FA cells in hypoxia appears to improve their survival and engraftment in xenogenic murine models [10]. At the same time, hypoxia inducible factor (HIF) –1α upregulates CXCR4 expression in hematopoietic cells [30,33]. With CXCR4 regulation unaffected by germline murine FA gene disruption we hypothesized that transient culture in hypoxia can be exploited to upregulate CXCR4 and improve hematopoietic homing while simultaneously limiting oxidative stress-mediated stem and progenitor cell losses in vitro. Our initial studies confirmed upregulation of CXCR4 cell surface expression and mRNA levels in whole bone marrow cells after overnight hypoxia culture (not shown). Further analysis confirmed that CXCR4 was also upregulated on lineage-depleted bone marrow progenitor subsets and double positive sca-1+/c-kit+ cells following overnight hypoxia (1% O2) in mSCF/mIL-3 supplemented culture, without substantial differences among Fancc+/− and Fancc−/− genotypes (Fig. 4AB).
Figure 4.
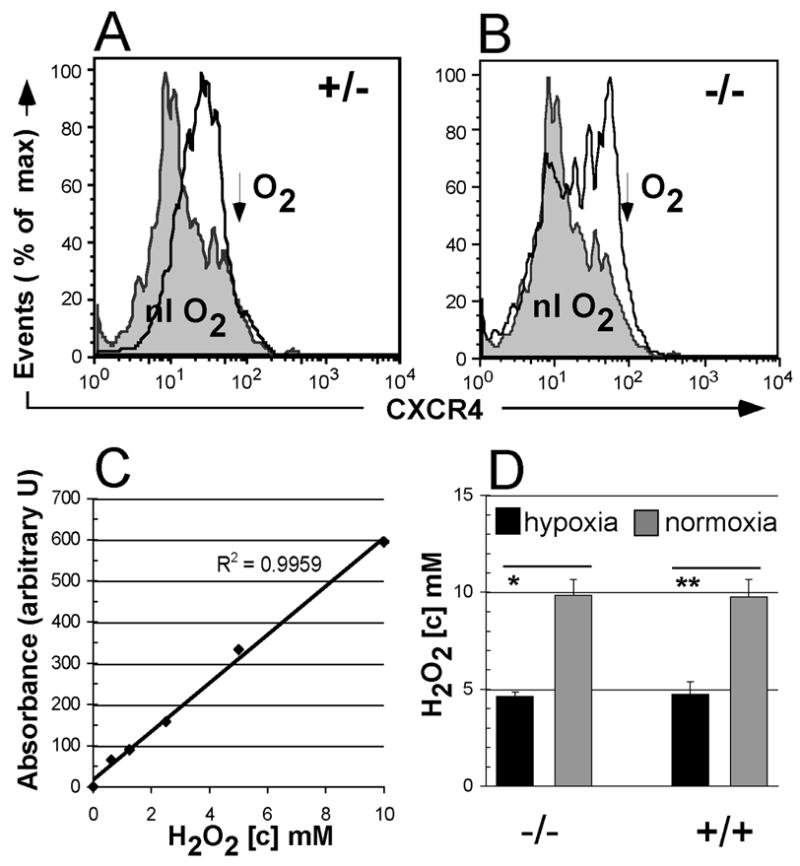
CXCR4 expression and ROS production in murine hematopoietic progenitors. Increased CXCR4 expression on hypoxia-conditioned sca-1+/c-kit+ double-positive cells from Fancc+/− (A) and Fancc−/− (B) animals. Lineage-depleted marrow progenitor cells were cultured for 24 hours under normoxic or hypoxic conditions and analyzed by flow-cytometry for CXCR4 expression. Grey histogram, normoxia; open line histogram, hypoxia. (C) Standard curve from Amplex Red assay used to determine H2O2 concentrations. (D) H2O2 generation by lineage-depleted progenitor cells from Fancc−/− and Fancc+/+ animals. Reactions were performed in triplicate. Error bars denote standard deviation. Asterisk denotes significant (*, p<0.05) or highly significant (**, p<0.01) difference between hypoxia- and normoxia- cell populations (2 tailed, paired T-test).
Generation of reactive oxygen species by murine progenitors after overnight hypoxia culture
Responses to oxidative challenge play a prominent role in the pathophysiology of FA hematopoiesis and are presumably involved in the pro apoptotic stem cell phenotype [3,40]. Having demonstrated hypoxia-induction of CXCR4, we next compared the in vitro generation of reactive oxygen species (ROS) by progenitors from Fancc+/+ and Fancc−/− animals. Results from multiple repeat experiments demonstrate that overnight hypoxia conditions produce a concomitant significant (p< 0.05) decrease in the generation of (ROS) in both genotypes (Fig. 4CD). These hypoxia conditions do not adversely affect lentiviral gene transfer to target cells (data not shown).
Hypoxia conditioning of lineage depleted progenitor cells improves in vitro transwell migration
To confirm that up-regulation of CXCR4 following hypoxia reflected chemotactic activity of cells toward a SDF-1α gradient, we performed an in vitro transwell migration assay, previously used by others [41,42]. Comparing SDF-1α-mediated migration of progenitor cells cultured under hypoxic versus normoxic conditions we found no statistically significant differences between wild-type, Fancc+/−, Fancc−/− genotypes at any of the time points examined (Fig. 5). But, the percentage of cells that migrated across the transwell membrane increased steadily with time from culture initiation in a statistically significant manner (p <0.01 for non-conditioned samples compared to day 2 normoxia or hypoxia conditioned samples). Next, in pooling data from all genotypes (in the absence of statistically significant differences between them) we found a significant statistical difference in migration between normoxia and hypoxia-conditioned progenitor cells (p < 0.001, student 2-tailed t test). To demonstrate that the migration observed was specifically due to up-regulation of CXCR4, we tested the ability of AMD 3100 (a CXCR4 antagonist) to impede migration. This antagonist inhibited migration of cells cultured under normoxic or hypoxic conditions in all genotypes. The reduction in cell migration in the presence of AMD 3100 is statistically significant (p-value for each genotype is <0.01).
Figure 5.
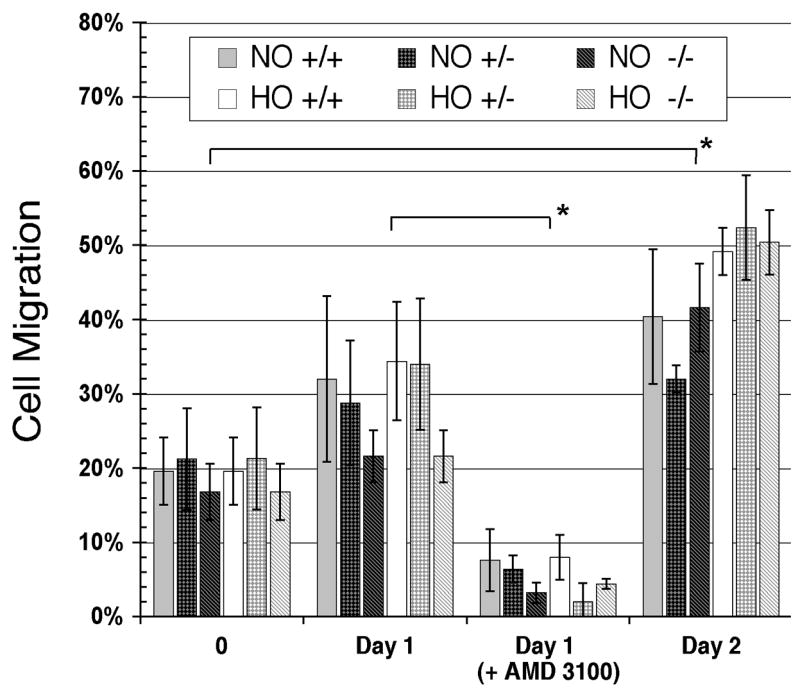
In vitro transwell murine protenitor cell migration assay. Lineage depleted progenitor cells from Fancc+/+, Fancc+/−, Fancc−/− were divided for normoxia (NO) and hypoxia (HO) conditioning in the presence of mSCF/IL-3. Samples at the 0-hour time point are duplicates preceeding conditioning. Every 24 hours an aliquot of 2.5 x 105 cells was added to a transwell and tested for the ability to migrate toward SDF-1 containing media. Error bars are standard deviations of 5 counts taken per sample. AMD 3100 (1 nM) was added to the lower chamber of transwells on day 1 to block CXCR4 from binding SDF-1α. Asterisks denote significant (p<0.01) differences between day 0 and day 2 populations; and between day 1 and day 1 + AMD 3100 populations.
Transient hypoxia effects on chimerism after transplantation
Based on our in vitro data showing hypoxia-induced upregulation of CXCR4 and improved migration in hematopoietic progenitors, we decided to test the feasibility of this strategy in improving the Fancc−/− HSC repopulation in murine recipients. We studied cohorts of myeloablated recipients that received grafts of immunomagnetically lineage-depleted cells, composed of 2.5 x 105 CD45.2/Fancc−/−cells cultured over night at normal versus reduced (1%) O2 concentration pooled with 2.5 x 105 CD45.1 competitor cells (also lineage-depleted and normoxia cultured), respectively. Following hematopoietic reconstitution, we evaluated the CD45.2 (i.e. Fancc−/−) chimerism contributions in animals at serial time points. Results demonstrate increasing chimerism (p= 0.055 at 17 weeks) in animals that received hypoxia-conditioned CD45.2/Fancc−/− cells as compared to the cohort receiving normoxia-cultured cells (Fig. 6). To amplify the potential hypoxia on Fancc−/− progenitor repopulation we next transplanted additional cohorts of animals with reduced numbers of conditioned cells (1 x104) and unselected whole bone marrow (2.5 x105) competitor populations. In these animals leukocyte specific chimerism differences at 4 and 12 weeks was improved similarly in lymphocyte, granulocyte and monocyte subsets for animals that received hypoxia conditioned cells, but did not reach statistical significance (Table 1). While we did not observe a substantial difference in cell survival during the brief culture period in hypoxia versus normoxia, this does not preclude more subtle changes in viability among the different genotypes. Taken together, this data complements our in vitro studies on hypoxia upregulation of CXCR4, but falls short of demonstrating significant advantages for the hypoxia conditions explored. Precise conditions for graft composition, oxygen concentration and hypoxia duration may yield more distinct results.
Figure 6.
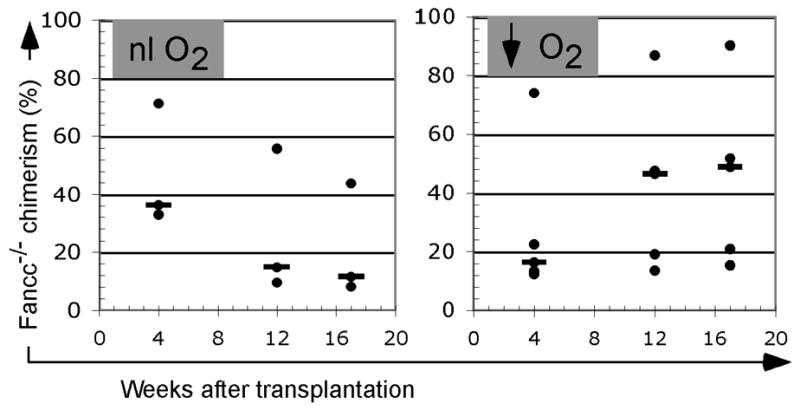
Chimerism from CD45.2/Fancc−/− donor cells after transplantation. (A) Myeloablated recipients received 2.5 x105 CD45.2/Fancc−/− cells cultured over night in normal O2 environment. (B) Comparison cohort received the identical number of Fancc−/− cells cultured a 1% O2. Animals in both cohorts (n=3–5/cohort) also received 2.5 x105 CD45.1/Fancc+/+ competitor cells (normoxia cultured) at the same time. Blood was obtained at indicated time-points and the percentage donor isotype was determined by staining with antibody against CD45.2. Bars illustrate median values.
Table 1.
Post transplantation chimerism at 4 and 12 weeks in leukocyte subsets from animals receiving hypoxia- or normoxia-conditioned, lineage-depleted Fancc−/− progenitor cells. CD45.2 chimerism in leukocyte subsets of myeloablated recipients of cells conditioned over night in hypoxia (1% O2) vs. normoxia. Animals received 950 cGy irradiation followed 24 hours later by 1 x104 lineagedepleted Fancc−/−, conditioned progenitors mixed with 5 x105 CD45.1 (normoxia-cultured) whole bone marrow cells. The table shows the percentage of CD 45.2 (Fancc−/−) events in leukocyte subsets at 4 and 12 weeks after transplantation. Subpopulations were gated based on forward and side-scatter (FSC – SSC) profile.
| Leukocyte subset | Granulocytes | Lymphocytes | Monocytes | Total Leukocytes | ||||
|---|---|---|---|---|---|---|---|---|
| Weeks after transplantation | 4 | 12 | 4 | 12 | 4 | 12 | 4 | 12 |
| 13.5 | 10.8 | 46.4 | 10.9 | 28.9 | 4.4 | 25.6 | 11.0 | |
| 33.5 | 4.4 | 39.3 | 21.7 | 38.6 | 5.7 | 40.4 | 20.8 | |
| Hypoxia | 17.0 | 58.7 | 19.6 | 34.4 | 18.7 | 13.2 | 20.2 | 35.5 |
| 2.6 | 5.2 | 27.4 | 5.9 | 3.2 | 20.0 | 14.5 | 6.73 | |
| 17.3 | 21.5 | 27.4 | 11.8 | 5.1 | 7.3 | 26.6 | 12.5 | |
| 4.6 | 84.8 | 88.7 | 97.0 | ND | 98.4 | 82.6 | 96.8 | |
|
| ||||||||
| Average | 14.8 | 30.9 | 40.2 | 30.3 | 18.9 | 24.8 | 35.0 | 30.6 |
|
| ||||||||
| 13.3 | 12.3 | 24.8 | 34.7 | 21.2 | 12.4 | 21.3 | 33.0 | |
| 2.7 | 43.0 | 13.7 | 26.4 | ND | 10.1 | 12.5 | 27.6 | |
| Normoxia | 15.6 | 13.5 | 37.8 | 51.1 | 15.3 | 26.5 | 29.6 | 47.8 |
| 26.3 | 0.65 | 41.0 | 28.1 | 34.8 | 10.3 | 35.4 | 25.2 | |
| 14.9 | 29.6 | 34.8 | 35.1 | 39.6 | 14.6 | 27.7 | 19.8 | |
| 3.8 | 15.2 | 36.7 | 7.3 | 24.4 | 5.9 | 17.0 | 8.9 | |
| Average | 12.8 | 19.0 | 31.5 | 30.4 | 27.1 | 13.3 | 23.9 | 27.1 |
Discussion
Hematopoietic stem and progenitor cells from FA patients and murine models of FA display a complex phenotype characterized in part by the impaired in vitro clonogenicity and poor stem cell mobilization [1,43,44]. However, attempts to rescue the FA phenotype by retroviral transduction of HSC entail their ex vivo culture and result in engraftment and repopulation defects, in sum undermining the therapeutic efficacy of gene therapy in FA [13,45–47]. Studies in Fancc−/− mice illustrate the FA HSC repopulating defect which can be overcome by increasing the cell inoculum to increase the number of engrafting cells [4,48,49]. This is not an option for FA patients who mobilize autologous HSC targets poorly, but it suggests that inherent FA phenotype repopulation deficits could be overcome by enhancing homing mechanisms, thereby increasing numbers of HSC and progenitor cells relocating to the microenvironment [44,49]. The current study is the first to investigate CXCR4 expression and homing mechanisms in the FA HSC and progenitor compartment.
Initial experiments investigated CXCR4 expression on progenitor cells from three murine models of FA, representing complementation groups A, -C, and -D2. Flow-cytometric and confirmatory real-time rt-PCR studies demonstrated that cytokine mediated regulation of CXCR4 was intact in lineage-depleted progenitor cells from all three genotypes. These experiments show systematic upregulation of CXCR4 over 48 hours of culture and levels comparable with those on wild-type cells. Similar starting percentages of c-kit+ and sca-1+ progenitor subsets and proliferation kinetics between the different genotypes minimized any confounding bias from differential expansion during the observation period and allowed the direct comparison of CXCR4 expression on progenitor subsets. The subsequent direct comparison of CXCR4 levels on lineage-depleted progenitor cells from Fancc−/− and Fancc+/− animals revealed increased cell surface protein levels on c-kit+ and sca-1+ cells, again without substantial differences between genotypes.
CXCR4 is known to be upregulated in response to HIF-1α induction [31]. We hypothesized that CXCR4 surface expression after transient hypoxia might not only improve stem and progenitor cell homing following injection, but simultaneously minimize exaggerated FA-phenotype apoptosis by reducing ROS. Results from our experiments confirm that CXCR4 can be efficiently upregulated during incubation in hypoxia culture with comparable resulting CXCR4 levels in lineage-depleted progenitors from Fancc−/− and Fancc+/− animals. Further, our studies specifically show that sca-1+/c-kit+ double-positive cells (which encompass murine hematopoietic repopulating cells) express higher levels of CXCR4 after overnight hypoxia culture than those negative for these markers, regardless of Fancc genotype. Importantly, we demonstrate that the de novo generation of ROS, H2O2 specifically, is significantly reduced under hypoxia conditions.
Others have shown that hypoxia improves the survival and replicative potential of hematopoietic progenitor cells [10,50]. To demonstrate that hypoxia conditioning of progenitor cells directly improves progenitor cell homing we conducted an in vitro transwell migration assay. SDF-1α-directed migration of cells was significantly improved when cells were cultured under hypoxic versus normoxic conditions. The increase in cellular migration peaked at 48 hours and followed the kinetics of CXCR4 upregulation in our experiments. Migration was abrogated in the presence of AMD 3100 confirming the direct role of CXCR4 in cell migration. These results demonstrate that hypoxia-mediated CXCR4-upregulation leads to increased progenitor cell homing. This is also the first demonstration that CXCR4 regulation and migration capacity are conserved in FA phenotype murine progenitor cells.
With intact CXCR4 regulation and migratory responses, our subsequent transplantation experiments focused on exploiting CXCR4 upregulation as part of a homing based strategy to offset repopulation deficits in this model of FA. They also highlight the unanticipated complexity of our strategy with changes in graft phenotype, cell number and degree of hypoxia all likely to contribute to the varying outcomes. In the initial cohorts, we performed a transplantation study using hypoxia-, or normoxia conditioned CD45.2 isotype hematopoietic progenitors competed against identical numbers of CD45.1 competitors for injection in CD45.2 lethally irradiated recipients. Remarkably, the increasing chimerism in the Fancc−/− hypoxia cohort developed in the face of substantial numbers of lineage-depleted Fanccwt CD45.1 competitor cells. Additional cohorts of animals received grafts with decreased numbers of hypoxia-conditioned cells and unseparated whole bone marrow competitors, to reduce competitive engraftment. In these animals we analyzed engraftment in different leukocyte subsets and showed non-significant advantages resulting from hypoxia conditioning. Others have described similar hypoxia culture conditions (5% O2) as conducive to engraftment of genetically modified human FA HSC in a xenogenic FA transplantation model [10]. We suggest that our studies, in directly demonstrating hypoxia effects on ROS generation, homing receptor expression in murine cells and cell migration, may provide the mechanistic basis for the improvements in cell survival and engraftment reported by these investigators.
In sum, we show that a principal homing mechanism for hematopoietic cells is conserved after genetic disruption of murine loci for FA complementation groups A, C, and D2. This finding would argue that the previously reported repopulation deficiency in Fancc−/− animals is based on a replicative deficiency, rather than an engraftment defect, at least one based on CXCR4 expression [4]. With a prior report that extended culture of Fancc−/− progenitors in vitro amplifies their myeloproliferative potential (precluding ex vivo cell expansion strategies) and repopulation disadvantage, as well as the growing implication of ROS in the pathophysiology of the FA HSC phenotype [13,40], CXCR4 upregulation and hypoxia culture should be further explored in preclinical models of FA.
Acknowledgments
Studies were supported in part by: NIH- HL077231 and the Friends of Doernbecher. We wish to thank Yung-Wei Pan for his contributions to these experiments. We acknowledge the assistance of Tammy T. Luoh and Daniel Kwon. We wish to thank Dr. Steve Back for use of his hypoxia-chamber and Dr. Daniel Marks for use of his real-time thermocycler. We also thank Paul Lees, M.S. for performing statistical computations. Aspects of this work were presented at the 48th annual meeting of the American Society of Hematology, Orlando, FL 2006 and the 19th annual scientific meeting of the Fanconi Anemia Research Fund in Chicago, IL 2007.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bagby GC, Alter BP. Fanconi anemia. Semin Hematol. 2006;43:147–56. doi: 10.1053/j.seminhematol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi T, D'Andrea AD. Molecular pathogenesis of Fanconi anemia: recent progress. Blood. 2006;107:4223–4233. doi: 10.1182/blood-2005-10-4240. [DOI] [PubMed] [Google Scholar]
- 3.Cumming RC, Lightfoot J, Beard K, Youssoufian H, O'Brien PJ, Buchwald M. Fanconi anemia group C protein prevents apoptosis in hematopoietic cells through redox regulation of GSTP1. Nat Med. 2001;7:814–820. doi: 10.1038/89937. [DOI] [PubMed] [Google Scholar]
- 4.Haneline LS, Gobbett TA, Ramani R, et al. Loss of FancC function results in decreased hematopoietic stem cell repopulating ability. Blood. 1999;94:1–8. [PubMed] [Google Scholar]
- 5.Daneshbod-Skibba G, Martin J, Shahidi NT. Myeloid and erythroid colony growth in non-anaemic patients with Fanconi's anaemia. Br J Haematol. 1980;44:33–38. doi: 10.1111/j.1365-2141.1980.tb01181.x. [DOI] [PubMed] [Google Scholar]
- 6.Whitney MA, Royle G, Low MJ, et al. Germ cell defects and hematopoietic hypersensitivity to gamma-interferon in mice with a targeted disruption of the Fanconi anemia C gene. Blood. 1996;88:49–58. [PubMed] [Google Scholar]
- 7.Rathbun RK, Christianson TA, Faulkner GR, et al. Interferon-gamma-induced apoptotic responses of Fanconi anemia group C hematopoietic progenitor cells involve caspase 8-dependent activation of caspase 3 family members. Blood. 2000;96:4204–4211. [PubMed] [Google Scholar]
- 8.Navarro S, Meza NW, Quintana-Bustamante O, et al. Hematopoietic dysfunction in a mouse model for Fanconi anemia group D1. Mol Ther. 2006;14:525–535. doi: 10.1016/j.ymthe.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Williams DA, Croop J, Kelly P. Gene therapy in the treatment of Fanconi anemia, a progressive bone marrow failure syndrome. Curr Opin Mol Ther. 2005;7:461–466. [PubMed] [Google Scholar]
- 10.Cohen-Haguenauer O, Peault B, Bauche C, et al. In vivo repopulation ability of genetically corrected bone marrow cells from Fanconi anemia patients. Proc Natl Acad Sci U S A. 2006;103:2340–2345. doi: 10.1073/pnas.0510613103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galimi F, Noll M, Kanazawa Y, et al. Gene therapy of Fanconi anemia: preclinical efficacy using lentiviral vectors. Blood. 2002;100:2732–2736. doi: 10.1182/blood-2002-04-1245. [DOI] [PubMed] [Google Scholar]
- 12.Hall KM, Horvath TL, Abonour R, Cornetta K, Srour EF. Decreased homing of retrovirally transduced human bone marrow CD34+ cells in the NOD/SCID mouse model. Exp Hematol. 2006;34:433–442. doi: 10.1016/j.exphem.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Le Beau MM, Ciccone S, et al. Ex vivo culture of Fancc−/− stem/progenitor cells predisposes cells to undergo apoptosis, and surviving stem/progenitor cells display cytogenetic abnormalities and an increased risk of malignancy. Blood. 2005;105:3465–3471. doi: 10.1182/blood-2004-06-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurre P, Anandakumar P, Kiem HP. Rapid 1-hour transduction of whole bone marrow leads to long-term repopulation of murine recipients with lentivirus-modified hematopoietic stem cells. Gene Ther. 2006;13:369–373. doi: 10.1038/sj.gt.3302659. [DOI] [PubMed] [Google Scholar]
- 15.Kurre P, Anandakumar P, Harkey MA, Thomasson B, Kiem HP. Efficient marking of murine long-term repopulating stem cells targeting unseparated marrow cells at low lentiviral vector particle concentration. Mol Ther. 2004;9:914–922. doi: 10.1016/j.ymthe.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Mostoslavsky G, Kotton DN, Fabian AJ, Gray JT, Lee JS, Mulligan RC. Efficiency of transduction of highly purified murine hematopoietic stem cells by lentiviral and oncoretroviral vectors under conditions of minimal in vitro manipulation. Mol Ther. 2005;11:932–940. doi: 10.1016/j.ymthe.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Quesenberry PJ, Colvin G, Abedi M. Perspective: fundamental and clinical concepts on stem cell homing and engraftment: a journey to niches and beyond. Exp Hematol. 2005;33:9–19. doi: 10.1016/j.exphem.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson SK, Simmons PJ, Bertoncello I. Hemopoietic stem cell engraftment. Exp Hematol. 2006;34:123–129. doi: 10.1016/j.exphem.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- 20.Broxmeyer HE, Kim CH, Cooper SH, Hangoc G, Hromas R, Pelus LM. Effects of CC, CXC, C, and CX3C chemokines on proliferation of myeloid progenitor cells, and insights into SDF-1-induced chemotaxis of progenitors. Ann N Y Acad Sci. 1999;872:142–162. doi: 10.1111/j.1749-6632.1999.tb08460.x. [DOI] [PubMed] [Google Scholar]
- 21.Papayannopoulou T. Bone marrow homing: the players, the playfield, and their evolving roles. Curr Opin Hematol. 2003;10:214–219. doi: 10.1097/00062752-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Christopherson KW, 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 23.Brenner S, Whiting-Theobald N, Kawai T, et al. CXCR4-transgene expression significantly improves marrow engraftment of cultured hematopoietic stem cells. Stem Cells. 2004;22:1128–1133. doi: 10.1634/stemcells.2003-0196. [DOI] [PubMed] [Google Scholar]
- 24.Christopherson KW, 2nd, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169:7000–7008. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- 25.Solanilla A, Grosset C, Duchez P, et al. Flt3-ligand induces adhesion of haematopoietic progenitor cells via a very late antigen (VLA)-4- and VLA-5-dependent mechanism. Br J Haematol. 2003;120:782–786. doi: 10.1046/j.1365-2141.2003.04155.x. [DOI] [PubMed] [Google Scholar]
- 26.Zheng Y, Watanabe N, Nagamura-Inoue T, et al. Ex vivo manipulation of umbilical cord blood-derived hematopoietic stem/progenitor cells with recombinant human stem cell factor can up-regulate levels of homing-essential molecules to increase their transmigratory potential. Exp Hematol. 2003;31:1237–1246. doi: 10.1016/j.exphem.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Ponomaryov T, Peled A, Petit I, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 29.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 30.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 31.Pituch-Noworolska A, Majka M, Janowska-Wieczorek A, et al. Circulating CXCR4-positive stem/progenitor cells compete for SDF-1-positive niches in bone marrow, muscle and neural tissues: an alternative hypothesis to stem cell plasticity. Folia Histochem Cytobiol. 2003;41:13–21. [PubMed] [Google Scholar]
- 32.Zhong JF, Zhan Y, Anderson WF, Zhao Y. Murine hematopoietic stem cell distribution and proliferation in ablated and nonablated bone marrow transplantation. Blood. 2002;100:3521–3526. doi: 10.1182/blood-2002-04-1256. [DOI] [PubMed] [Google Scholar]
- 33.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 34.Kurre P, Morris J, Andrews RG, Kohn DB, Kiem HP. Kinetics of fluorescence expression in nonhuman primates transplanted with GFP retrovirus-modified CD34 cells. Mol Ther. 2002;6:83–90. doi: 10.1006/mthe.2002.0623. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson JK, Hubbard M, Jones BM. Use of CD45 fluorescence and side-scatter characteristics for gating lymphocytes when using the whole blood lysis procedure and flow cytometry. Cytometry. 1996;26:16–21. doi: 10.1002/(SICI)1097-0320(19960315)26:1<16::AID-CYTO3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 36.Pan D, Gunther R, Duan W, et al. Biodistribution and toxicity studies of VSVG-pseudotyped lentiviral vector after intravenous administration in mice with the observation of in vivo transduction of bone marrow. Mol Ther. 2002;6:19–29. doi: 10.1006/mthe.2002.0630. [DOI] [PubMed] [Google Scholar]
- 37.Chen M, Tomkins DJ, Auerbach W, et al. Inactivation of Fac in mice produces inducible chromosomal instability and reduced fertility reminiscent of Fanconi anaemia. Nat Genet. 1996;12:448–451. doi: 10.1038/ng0496-448. [DOI] [PubMed] [Google Scholar]
- 38.Houghtaling S, Timmers C, Noll M, et al. Epithelial cancer in Fanconi anemia complementation group D2 (Fancd2) knockout mice. Genes Dev. 2003;17:2021–2035. doi: 10.1101/gad.1103403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noll M, Battaile KP, Bateman R, et al. Fanconi anemia group A and C double-mutant mice: functional evidence for a multi-protein Fanconi anemia complex. Exp Hematol. 2002;30:679–688. doi: 10.1016/s0301-472x(02)00838-x. [DOI] [PubMed] [Google Scholar]
- 40.Sejas DP, Rani R, Qiu Y, et al. Inflammatory reactive oxygen species-mediated hemopoietic suppression in Fancc-deficient mice. J Immunol. 2007;178:5277–5287. doi: 10.4049/jimmunol.178.8.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kollet O, Spiegel A, Peled A, et al. Rapid and efficient homing of human CD34(+)CD38(-/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood. 2001;97:3283–3291. doi: 10.1182/blood.v97.10.3283. [DOI] [PubMed] [Google Scholar]
- 42.Voermans C, Anthony EC, Mul E, van der Schoot E, Hordijk P. SDF-1-induced actin polymerization and migration in human hematopoietic progenitor cells. Exp Hematol. 2001;29:1456–1464. doi: 10.1016/s0301-472x(01)00740-8. [DOI] [PubMed] [Google Scholar]
- 43.Haneline LS, Li X, Ciccone SL, et al. Retroviral-mediated expression of recombinant Fancc enhances the repopulating ability of Fancc−/− hematopoietic stem cells and decreases the risk of clonal evolution. Blood. 2003;101:1299–1307. doi: 10.1182/blood-2002-08-2404. [DOI] [PubMed] [Google Scholar]
- 44.Croop JM, Cooper R, Fernandez C, et al. Mobilization and collection of peripheral blood CD34+ cells from patients with Fanconi anemia. Blood. 2001;98:2917–2921. doi: 10.1182/blood.v98.10.2917. [DOI] [PubMed] [Google Scholar]
- 45.Kittler EL, Peters SO, Crittenden RB, et al. Cytokine-facilitated transduction leads to low-level engraftment in nonablated hosts. Blood. 1997;90:865–872. [PubMed] [Google Scholar]
- 46.Mazurier F, Gan OI, McKenzie JL, Doedens M, Dick JE. Lentivector-mediated clonal tracking reveals intrinsic heterogeneity in the human hematopoietic stem cell compartment and culture-induced stem cell impairment. Blood. 2004;103:545–552. doi: 10.1182/blood-2003-05-1558. [DOI] [PubMed] [Google Scholar]
- 47.Takatoku M, Sellers S, Agricola BA, et al. Avoidance of stimulation improves engraftment of cultured and retrovirally transduced hematopoietic cells in primates. J Clin Invest. 2001;108:447–455. doi: 10.1172/JCI12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu JM, Kim S, Read EJ, et al. Engraftment of hematopoietic progenitor cells transduced with the Fanconi anemia group C gene (FANCC) Hum Gene Ther. 1999;10:2337–2346. doi: 10.1089/10430349950016988. [DOI] [PubMed] [Google Scholar]
- 49.Kelly PF, Radtke S, Kalle C, et al. Stem cell collection and gene transfer in fanconi anemia. Mol Ther. 2007;15:211–219. doi: 10.1038/sj.mt.6300033. [DOI] [PubMed] [Google Scholar]
- 50.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]