Abstract
Conserved semaphorin–plexin signaling systems govern various aspects of animal development, including axonal guidance in vertebrates and epidermal morphogenesis in Caenorhabditis elegans. Here we provide in vivo evidence that stimulation of mRNA translation via eukaryotic initiation factor 2α (eIF2α) is an essential downstream event of semaphorin signaling in C. elegans. In semaphorin/plexin mutants, a marked elevation in the phosphorylation of eIF2α is observed, which causes translation repression and is causally related to the morphological epidermal phenotype in the mutants. Conversely, removal of constraints on translation by genetically reducing the eIF2α phosphorylation largely bypasses requirement for the semaphorin signal in epidermal morphogenesis. We also identify an actin-depolymerizing factor/cofilin, whose expression in the mutants is predominantly repressed, as a major translational target of semaphorin signaling. Thus, our results reveal a physiological significance for translation of mRNAs for cytoskeletal regulators, linking environmental cues to cytoskeletal rearrangement during cellular morphogenesis in vivo.
[Keywords: C. elegans, cofilin, eIF2, epidermal morphogenesis, mRNA translation, semaphorin]
Ligand binding to receptors at the surface of individual cells triggers changes in gene expressions and cytoskeletal organization, which underlies dynamic cellular morphogenesis. The signaling system consisting of semaphorins and their receptor plexins, both in vertebrates and in invertebrates, is involved in the regulation of diverse developmental events including axonal extension and cell migration in the nervous system, growth of endothelial cells, and cardiac development (Kruger et al. 2005).
Elucidation of semaphorin–plexin signaling cascades is paramount for understanding these morphogenetic events. For instance, Sema3A, a secreted semaphorin in vertebrates, triggers collapse or repulsive turning of growth cones as a consequence of asymmetrical alteration in F-actin structure (Fan and Raper 1995). Cytoskeletal regulators, such as Rho family small GTPases (Jin and Strittmatter 1997; Kuhn et al. 1999; Rohm et al. 2000; Zanata et al. 2002; Oinuma et al. 2004; Turner et al. 2004), collapsin response mediator proteins (CRMPs) (Goshima et al. 1995), and an actin-depolymerizing factor (ADF)/cofilin (Aizawa et al. 2001), mediate the growth cone responses by acting as molecular relays to transduce the Sema3A’s repulsive signal. In parallel with these demonstrations, great interest has been focused on local mRNA translation occurring in Sema3A-induced axonal events since the discovery by Campbell and Holt (2001).
mRNA translation initiation is the rate-limiting step in the process of protein synthesis, in which eukaryotic initiation factor 2 (eIF2) complex and eIF4F complex formations are the major events. eIF2 forms a ternary complex with GTP and initiator methionyl-tRNA (Met-tRNAiMet) and recruits Met-tRNAiMet to the ribosome (Hershey and Merrick 2000). Phosphorylation of the α subunit of eIF2 (eIF2α) inhibits global translation, while it also increases translation of a few selected mRNAs (Holcik and Sonenberg 2005). eIF4F complex, composed of eIF4A (RNA helicase), eIF4E (cap-binding protein), and eIF4G (scaffold protein), serves to initiate 5′ cap-dependent translation by recruiting mRNA to the ribosome (Gingras et al. 1999; Hershey and Merrick 2000). Sema3A accelerates protein synthesis locally in growth cones as marked by rapid rises in the phosphorylation of translational regulators eIF4E and 4E-BP1, and pharmacological translation inhibition blocks the Sema3A-induced responses of isolated growth cones (Campbell and Holt 2001), suggesting that Sema3A exerts its navigatory influence through the translational regulation at axonal terminals. Recently, mRNAs for RhoA (Wu et al. 2005), ADF/cofilin (Piper et al. 2006), and β-actin (Leung et al. 2006) have been identified as targets to be translated in response to Sema3A and other guidance cues such as Slit2 and Netrin-1. Thus, cytoskeletal reorganization mediated by synthesis of cytoskeletal regulators and/or components is suggested to be a fundamental mechanism for cue-induced axonal navigation. In spite of these understandings, however, the functional significance of translational regulation in semaphorin signaling in vivo has not been addressed. In order to gain insight into semaphorin signaling in vivo, we used the genetic analysis in nematode Caenorhabditis elegans, where two membrane-bound semaphorins interact with an A-type plexin to regulate morphogenesis of epidermal tissues including the “rays” in the male tail (Fujii et al. 2002; Ginzburg et al. 2002; Dalpe et al. 2004).
In this study, we present several lines of in vivo evidence that the semaphorin–plexin signal regulates the phosphorylation level of eIF2α and stimulates translation. Furthermore, we identify cofilin as a key functional target of translation in response to semaphorin. Our results reveal a physiological significance for regulated translation of mRNA species, especially those relevant to cytoskeletal regulation, which provides some clues to the mechanism that links environmental cues to cytoskeletal reorganization underlying alteration of cell morphology in vivo.
Results
A role for semaphorin–plexin signaling in the epidermal ray morphogenesis
We have shown previously that in C. elegans membrane-bound semaphorins (SMP-1 and SMP-2; SMPs) interact with an A-type plexin (PLX-1) to regulate the morphogenesis of epidermal tissues including the rays: nine bilateral pairs of sensilla enveloped in a cuticular fan in the male tail (Fujii et al. 2002; Ginzburg et al. 2002; Dalpe et al. 2004).
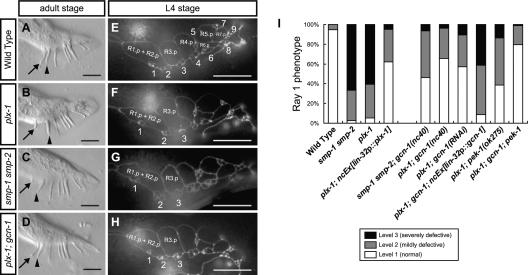
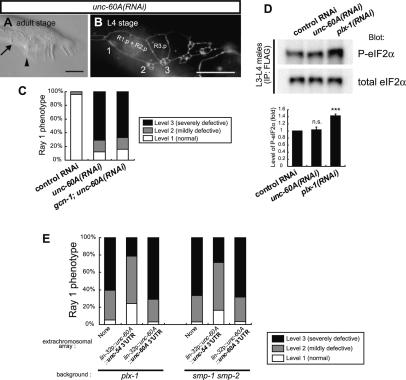
In wild-type adults, ray 1, the anterior-most ray, is mostly found juxtaposed to its neighboring ray 2 (“Level 1” phenotype) (Fig. 1A). In contrast, in plx-1(nc37, ev724) single and smp-1(ev715) smp-2(ev709) double mutant adults, ray 1 is frequently displaced anteriorly (Fig. 1B,C). The displaced ray 1 is often found outside of a fan (“Level 3” phenotype), and in some cases ray 1 is separate from ray 2 within a fan (“Level 2” phenotype) (see Supplemental Fig. S1 for examples of the three levels).
Figure 1.
Ray configurations in adults (A–D) and in larvae at the L4 stage (E–H). Anterior is left, and dorsal is top. Bars, 10 μm. Ray 1 (arrow), which is normally located juxtaposed to its neighboring ray 2 (arrowhead) in wild-type adults (A), is found anteriorly in plx-1 (B) and smp-1 smp-2 (C) mutants. (D) gcn-1(nc40) was isolated as a mutation that suppresses the ray 1 phenotype in plx-1. (E–H) Configurations of epidermal ray precursor cells visualized with ajm-1∷gfp at the L4 stage, when R1.p and R2.p have already fused. Numbers indicate ray precursor cluster (n), where n = 1–9. (E) In wild type, ray precursor cluster 1 is associated with the vicinal cluster 2. In contrast, in plx-1 (F) and smp-1 smp-2 (G), cluster 1 shifts anteriorly. (H) In plx-1; gcn-1, cluster 1 is located close to cluster 2 as in wild type. (I) Quantitative evaluation of the ray 1 phenotype in adult males of the indicated genotypes.
Previous studies showed that the SMPs–PLX-1 system determines the position of ray 1 by regulating the shape and arrangement of epidermal ray precursor cells (Fujii et al. 2002; Ginzburg et al. 2002; Dalpe et al. 2004). On each side of a larval male tail are nine epidermal cell-derived R(n) cells, each of which divides to give rise to Rn.a and Rn.p (n = 1–9). Through successive divisions, each Rn.a becomes a ray precursor cluster comprising three cells that later develops into a mature ray (Sulston et al. 1980; Emmons 2005). Examination of ray precursor cells with the adherens junction marker ajm-1∷gfp (Baird et al. 1991; Mohler et al. 1998) revealed that the ray 1 phenotype in the mutant adults results from the abnormal shaping of R1.p, which consequently affects the arrangement of ray precursor cluster 1 (Fig. 1E–G; Fujii et al. 2002). The ray 1 phenotype in plx-1 adults is remarkably rescued by driving expression of plx-1 under the lin-32 promoter (lin-32p) (Fig. 1I; Nakao et al. 2007). Since in larval males the lin-32p drives gene expressions predominantly in nine R(n) cells and their descendants (Supplemental Fig. S2; Portman and Emmons 2000), where expressions of SMPs and PLX-1 overlap (Fujii et al. 2002; Ginzburg et al. 2002), it is likely that the SMPs–PLX-1 signal in epidermal ray precursor cells is responsible for their morphogenesis.
Loss of a GCN1 homolog function suppresses the ray 1 phenotype
To gain insight into SMPs signaling, we conducted a screen for mutations that suppress the ray 1 phenotype in plx-1 mutants. One isolated mutation, nc40, suppressed the ray defect in plx-1 as well as that in smp-1 smp-2 mutants (Fig. 1D,H,I). nc40 single mutants displayed normal ray configuration (Supplemental Tables S1, S2).
nc40 was mapped to the clone Y48G9A on the left arm of linkage group III. Sequencing revealed that nc40 is a 6880-base-pair (bp) deletion between nucleotides 90,212 and 97,091 on Y48G9A, including four microRNA genes and part of the GCN1 homolog-encoding gene (termed “gcn-1”) (Supplemental Fig. S3A). We cloned the gcn-1 cDNA and found the gene to be a composite of Y48G9A.1, Y48G9A.2, and Y48G9A.3, which had been registered as three separate genes in WormBase (http://www.wormbase.org; Supplemental Fig. S3A). RNAi against gcn-1 in plx-1 mutants suppressed the ray 1 phenotype, and lin-32p-driven gcn-1 expression markedly reversed the suppression by nc40 (Fig. 1I), confirming that loss of gcn-1 function was responsible for suppressing the ray defect in plx-1/smp-1 smp-2 mutants. The sequence corresponding to the 12th and the 13th exons are entirely deleted in the nc40-type gcn-1 transcript, which is presumably translated into a truncated form of GCN-1 protein. The deleted region, between amino acids 895 and 1138, is reportedly necessary for GCN1 function in yeast (Sattlegger and Hinnebusch 2000). In addition, nc40 in trans to a deficiency (nc40/tDf9) suppressed the ray 1 phenotype in plx-1 adults equally to the nc40 homozygotes (Supplemental Table S1), indicating that nc40 acts genetically as a null allele of gcn-1.
GCN1, conserved among eukaryotes (Supplemental Fig. S3B), is known to function as a negative regulator for the translation initiation of global mRNAs (Marton et al. 1993). A trimeric GTPase eIF2, composed of α, β, and γ subunits, forms a ternary complex with GTP and Met-tRNAiMet and participates in the initiation of translation by recruiting Met-tRNAiMet to the 40S ribosomal subunit (Hershey and Merrick 2000). In yeast and mammalian cells, GCN1 activates the serine/threonine kinase GCN2 in response to nutrient limitation, which in turn phosphorylates the specific serine residue of eIF2α. On the phosphorylation of eIF2α, eIF2 forms a stable complex with eIF2B, a guanine nucleotide exchange factor, to prevent the recycling between a GDP-bound and a GTP-bound state, and thereby global translation initiation is inhibited (Hinnebusch 2000). Paradoxically, however, the eIF2α phosphorylation also increases the rate of translation of a few selected mRNAs (Holcik and Sonenberg 2005).
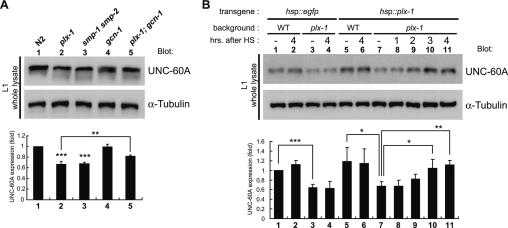
The 49th serine residue of a C. elegans eIF2α homolog, which is encoded by the Y37E3.10 gene (Rhoads et al. 2006), is the putative phosphorylation site, as predicted by the complete identity of the surrounding residues among eukaryotes (Supplemental Fig. S4), suggesting the conserved regulatory mechanism of the eIF2α phosphorylation. We carried out Western blot analysis to detect the level of eIF2α phosphorylation (P-eIF2α) in wild-type and gcn-1 mutant larvae at stage 1 (L1). In gcn-1 mutants, a reduction in P-eIF2α to 28% of wild type was observed (Fig. 2A), indicating that GCN-1 does participate in the eIF2α phosphorylation.
Figure 2.
The SMPs signal lowers P-eIF2α, which is required for the proper ray morphogenesis. (A) Western blot showing the level of P-eIF2α in L1 whole animals of the indicated genotypes. The intensity of P-eIF2α normalized by total eIF2α is shown in the graph below. Shown are the means ± SEM of at least three independent experiments. gcn-1 (P < 0.001, t-test) and pek-1 (P < 0.001) reduce P-eIF2α, whereas plx-1 (P < 0.001) and smp-1 smp-2 (P < 0.005) increase it. plx-1 slightly increases P-eIF2α in gcn-1 (P < 0.15), pek-1 (P < 0.16), or gcn-1; pek-1 (P < 0.15). (B) Transient SMPs signaling acutely reduces P-eIF2α. Wild-type or plx-1 animals at the L1 stage carrying either hsp∷egfp (lanes 1–4), hsp∷plx-1 (lanes 5–8), or hsp∷smp-1 and hsp∷plx-1 (lanes 9,10) were examined for the level of P-eIF2α. Heat-shocked samples (2) were collected 2 h after HS. Shown in the graph are the means of normalized P-eIF2α ± SEM of four independent experiments. (C) P-eIF2α is highly elevated in ray precursor cells. L3–L4 males carrying either lin-32p∷eIF2α∷Flag (lanes 1–5) or unc-54p∷eIF2α∷Flag (lanes 6–10) were collected, and Flag-tagged eIF2α proteins were immunoprecipitated and subjected to Western blot analysis. Shown in the graph are the means of normalized P-eIF2α ± SEM of three independent experiments (black bars represent P-eIF2α in lin-32-expressing cells; gray bars represent P-eIF2α in unc-54-expressing cells). (D,E) Forced expression of the phosphomimetic form of eIF2α, S49D, in ray precursor cells causes the ray defect similarly to plx-1/smp-1 smp-2. (F) Quantitative ray 1 phenotype in adult males carrying the indicated transgenes. Note that expression of the nonphosphorylatable form of eIF2α, S49A, partially suppresses the ray 1 phenotype in plx-1. (*) P < 0.05; (**) P < 0.005; (***) P < 0.001.
Loss-of-function mutations in a PERK gene suppress the ray defect
In metazoans, a kinase PERK, which is activated by unfolded protein response, phosphorylates eIF2α at the same residue as GCN1 signaling (Ron and Harding 2000). Although less pronounced than gcn-1, loss-of-function mutations in a C. elegans PERK homolog, pek-1 (Shen et al. 2001), also suppressed the ray defect in plx-1/smp-1 smp-2 mutants (Fig. 1I; Supplemental Table S2) and reduced P-eIF2α (Fig. 2A). In addition, the gcn-1; pek-1 double mutation suppressed the ray 1 phenotype in plx-1 adults and reduced P-eIF2α more strongly than either single mutation (Figs. 1I, 2A).
The semaphorin signal lowers the eIF2α phosphorylation
The finding that mutations that diminish P-eIF2α suppressed the ray defect in plx-1/smp-1 smp-2 mutants prompted us to examine whether the SMPs signal regulates the ray morphogenesis by controlling P-eIF2α. We first compared P-eIF2α in wild type with that in plx-1/smp-1 smp-2 mutants at the L1 stage, when the proportion of cells expressing SMPs/PLX-1 in the whole body is large. In the mutants, an ∼1.6-fold increase in P-eIF2α was observed (Fig. 2A), indicating that the SMPs signal is necessary to keep P-eIF2α low. Next, we prepared a plx-1 mutant line expressing plx-1 transcripts under a heat-shock promoter (hsp), and compared P-eIF2α before and after heat shock (HS). Induced expression of PLX-1 reduced P-eIF2α to a wild-type level by 2 h after HS (Fig. 2B). In addition, we analyzed P-eIF2α in a line expressing both smp-1 and plx-1 transcripts under hsp. Induced coexpression of SMP-1 and PLX-1 in the whole body of wild-type animals resulted in a reduction of P-eIF2α to 58% of a control (Fig. 2B). Thus, the reduction in P-eIF2α is indicated to be a direct and acute consequence of SMPs signaling.
Furthermore, the introduction of gcn-1 and/or pek-1 mutations to plx-1 mutants reduced P-eIF2α (Fig. 2A), which correlated with the degree of suppression of the ray 1 phenotype. The presence of the plx-1 mutation slightly increased P-eIF2α in gcn-1, pek-1, or gcn-1; pek-1 mutants (Fig. 2A), indicating that neither the gcn-1 nor pek-1 mutation alone is completely epistatic to the plx-1 mutation in respect to P-eIF2α. This suggests that the SMPs signal may simultaneously inactivate multiple pathways involved in the eIF2α phosphorylation, including GCN-1-dependent and PEK-1-dependent pathways. Alternatively, the signal might activate eIF2α phosphatase(s).
A semaphorin-induced reduction in the eIF2α phosphorylation is essential for the proper epidermal ray morphogenesis
To investigate further the causal relationship between P-eIF2α and the ray phenotype, we used a line expressing Flag-tagged eIF2α under the lin-32p and analyzed P-eIF2α in the Flag immunoprecipitates from transgenic males at the L3–L4 stage. During this period, which is crucial for ray positioning by SMPs, the majority of immunoprecipitated eIF2α is presumably derived from ray precursor cells (Supplemental Fig. S2). In plx-1/smp-1 smp-2 mutants, P-eIF2α in the immunoprecipitates was elevated by ∼2.5-fold (Fig. 2C), thus correlating elevated P-eIF2α and the ray defect in the mutants. P-eIF2α in the supernatant was comparable in wild-type and plx-1/smp-1 smp-2 mutants (Supplemental Fig. S5), consistent with the limited proportion of SMPs/PLX-1-expressing cells in the whole body during this period. We also prepared another line expressing eIF2α∷Flag under the promoter of unc-54, a gene specific to muscles, where PLX-1 expression is absent. P-eIF2α in the Flag immunoprecipitates, representing P-eIF2α in PLX-1-absent cells, was comparably high in both wild type and plx-1/smp-1 smp-2 mutants at the L3–L4 stage (Fig. 2C). Thus, the reduction in P-eIF2α in wild-type ray precursor cells is likely to depend on SMPs signaling.
Next, we examined the phenotype in transgenic males expressing the phosphomimetic eIF2α [eIF2α(S49D)] under the lin-32p. Similarly to plx-1/smp-1 smp-2 mutant males, they often exhibited a ray defect (Fig. 2D–F), suggesting that the distorted ray morphogenesis in the mutants can be attributed, at least partly, to elevated P-eIF2α. Conversely, another transgene expressing the nonphosphorylatable eIF2α [eIF2α(S49A)] partially suppressed the ray 1 phenotype in plx-1 adults (Fig. 2F). Likewise, gcn-1 and/or pek-1 mutations reduced P-eIF2α in the plx-1 mutant background in lin-32-expressing cells (Fig. 2C; Supplemental Fig. S6B), suggesting that suppression of the ray defect in plx-1/smp-1 smp-2 mutants is largely dependent on reduced P-eIF2α in ray precursor cells. These results indicate that the SMPs signal serves to lower P-eIF2α in ray precursor cells, which contributes to the proper positioning of ray 1.
Having characterized the roles for GCN-1 and PEK-1, we reasoned that knockdown of a GCN2 homolog, Y81G3A.3, would likewise suppress the ray 1 phenotype in plx-1/smp-1 smp-2 mutants and reduce P-eIF2α. Y81G3A.3 protein seems to have an eIF2α kinase activity as evidenced by a reduction in P-eIF2α in Y81G3A.3 mutant whole animals (Supplemental Fig. S6B,C). Unexpectedly, however, the Y81G3A.3 mutations neither suppressed the ray 1 phenotype in plx-1 adults (Supplemental Fig. S6A) nor significantly reduced P-eIF2α in lin-32-expressing cells at the L3–L4 stage (Supplemental Fig. S6B). Thus, it is implied that Y81G3A.3 protein plays only a minor role as an eIF2α kinase in ray precursor cells and that GCN-1 signaling relies on yet-unidentified eIF2α kinase(s).
Down-regulated protein synthesis is causally related to the ray defect
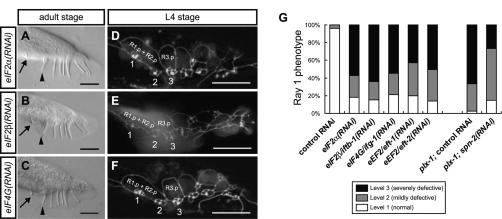
Elevated P-eIF2α is known to mediate global translation inhibition as well as translation stimulation of certain mRNA species (Holcik and Sonenberg 2005), prompting us to examine whether repressed or enhanced translation causes the ray defect. eIF2 and eIF4F complexes and translation elongation factors (eEFs) play indispensable roles in translation, whereas eIF4E-binding protein (4E-BP) represses translation by interfering with eIF4E (Hershey and Merrick 2000). RNAi against eIF2α, eIF2β/iftb-1, eIF4G/ifg-1 (a component of eIF4F), eEF2/eft-1, and eEF2/eft-2 (Rhoads et al. 2006) all caused the growth defect, but animals that survived into the L4 and adult stages exhibited the ray defect similarly to plx-1/smp-1 smp-2 mutants (Fig. 3). In contrast, RNAi against spn-2, a gene-encoding protein that has partial homology with mammalian 4E-BP and might be its counterpart in C. elegans (W. Li and L. Rose, pers. comm.), partially suppressed the ray 1 phenotype in plx-1 mutants (Fig. 3G). Thus, these results argue that down-regulated translation is causally related to the ray defect in plx-1/smp-1 smp-2 mutants.
Figure 3.
Down-regulated translation is causally related to the ray defect. Knockdown of translational machineries including eIF2α (A,D), eIF2β (B,E), and eIF4G (C,F) causes a ray defect. (G) Quantitative ray 1 phenotype in adult males treated with the indicated RNAi. Note that knockdown of eEF2 (eft-1 and eft-2) causes a ray defect, while knockdown of spn-2 partially suppresses the ray 1 phenotype in plx-1.
Semaphorin preferentially stimulates translation of unc-60A/cofilin in a manner dependent on its 3′ untranslated region (UTR)
Having established a role for the SMPs signal in translation stimulation, what are the key translational targets? One particular candidate is ADF/cofilin, whose vertebrate ortholog is suggested to be a downstream component of Sema3A signaling (Aizawa et al. 2001; Piper et al. 2006). A C. elegans cofilin ortholog is encoded by unc-60, which produces two functionally distinct isoforms, UNC-60A and UNC-60B (Ono and Benian 1998). Since UNC-60B is expressed specifically in muscles, we turned to the analysis of ubiquitously expressed UNC-60A.
We compared the expression level of UNC-60A in L1 whole animals. In plx-1/smp-1 smp-2 mutants, UNC-60A expression was decreased to 67% of wild type. The gcn-1 mutation, which did not affect UNC-60A expression alone, restored it to 82% of wild type in the plx-1 mutant background (Fig. 4A), consistent with the notion that reduced P-eIF2α leads to enhanced protein synthesis. The amount of unc-60A transcripts in L1 animals was comparable among wild type and plx-1/smp-1 smp-2 mutants by Northern blot analysis (Supplemental Fig. S7), indicating that UNC-60A expression in the mutants is repressed at the translational or the post-translational level. We also analyzed the time course of SMPs-induced UNC-60A synthesis in plx-1 mutants carrying the hsp∷plx-1 transgene at the L1 stage (Fig. 4B). The UNC-60A expression level did not differ from that of untreated animals at 2 h after HS, the time sufficient to rescue the elevated P-eIF2α phenotype in the mutants. At 3 and 4 h after HS, however, it increased significantly and reached a wild-type level. Thus, it is suggested that the SMPs signal acutely reduces P-eIF2α, which may in turn stimulate de novo translation of mRNAs including unc-60A.
Figure 4.
UNC-60A synthesis is stimulated by the SMPs signal. (A) Western blot showing the level of UNC-60A expression. Samples from L1 whole animals were analyzed with either anti-UNC-60A or anti-α-tubulin antibodies. UNC-60A expression normalized by α-tubulin is shown in the graph below. Shown are the means ± SEM of four independent experiments. (B) Induced expression of PLX-1 results in restoration of UNC-60A expression in plx-1. A similar protocol was used as in Figure 2B. Samples were collected at the indicated times after HS. Shown in the graph are the means of normalized UNC-60A expression level ± SEM of three independent experiments. (*) P < 0.05; (**) P < 0.005; (***) P < 0.001.
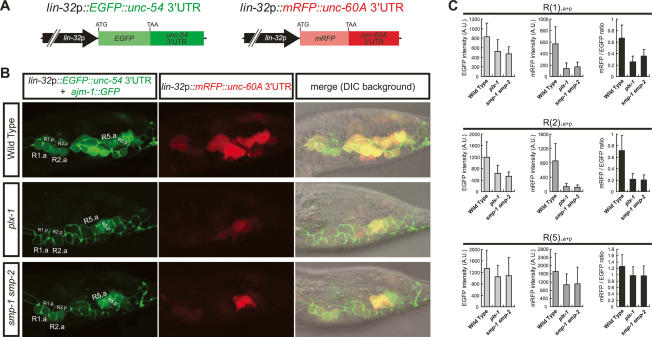
Translational efficiency in a large number of mRNAs can be controlled by a regulatory sequence in their 3′UTRs (Kuersten and Goodwin 2003). To examine whether translation of unc-60A by SMPs signaling is dependent on its 3′UTR, which is highly conserved among nematodes (Supplemental Fig. S8), we used a line harboring chromosomally integrated reporters expressing lin-32p-driven EGFP with unc-54 3′UTR (lin-32p∷EGFP∷unc-54 3′UTR) and lin-32p-driven mRFP with unc-60A 3′UTR (lin-32p∷mRFP∷unc-60A 3′UTR) (Fig. 5A). We quantified the intensities of both fluorescence in a ray precursor unit composed of Rn.aa, Rn.ap, and Rn.p cells [collectively designated as R(n).a + p] at the L3 stage, when their morphologies still appear unaffected in plx-1/smp-1 smp-2 mutants. In wild type, both EGFP and mRFP were intensely detected in every R(n).a + p. However, in plx-1/smp-1 smp-2 mutants, EGFP intensity was decreased in most R(n).a + p (Fig. 5B,C). Since unc-54 expression is absent in ray precursor cells, and thus its 3′UTR is unlikely to confer translational specificity by the SMPs signal, decreased EGFP expression in the mutants indicates globally down-regulated gene expression, consistent with elevated P-eIF2α in the mutants. Intriguingly, R(1).a + p and R(2).a + p units displayed more pronounced decreases in expression of mRFP than those of EGFP in the mutants (Fig. 5B,C), suggesting that 3′UTR of unc-60A mediates its preferential translation by the SMPs signal. In contrast, in R(5).a + p, decreases in expression of mRFP were comparable with those of EGFP in the mutants (Fig. 5B,C). Similar phenotypes were observed in another line expressing mRFP with unc-54 3′UTR and EGFP with unc-60A 3′UTR under the lin-32p (Supplemental Fig. S9). Thus, these results suggest that the SMPs signal stimulates global translation and simultaneously activates unc-60A translation in a target gene- and cell type-specific manner.
Figure 5.
The SMPs signal simultaneously stimulates global translation and preferential translation of unc-60A via its 3′UTR in ray precursor cells. (A) Structures of lin-32p∷EGFP∷unc-54 3′UTR and lin-32p∷mRFP∷unc-60A 3′UTR reporter constructs. (B) Expression profiles of the reporter transgenes. In the left column, lin-32p-driven EGFP expression, together with AJM-1∷GFP expression delineating the epidermal cell boundaries, are shown. In the middle column, lin-32p-driven mRFP expression is shown. Merged images in the DIC background are shown in the right column. (C) The fluorescent intensities of EGFP (light-gray bars) and mRFP (dark-gray bars) in arbitrary units (A.U.) and the ratio of mRFP/EGFP intensities (black bars) within R(1).a + p, R(2).a + p, and R(5).a + p. Shown are the means ± SEM of 20 independent samples.
Translation of unc-60A/cofilin mediates the semaphorin-regulated ray morphogenesis
Predominant repression of unc-60A 3′UTR-dependent protein expression in R(1).a + p and R(2).a + p units appears to correspond with the structural ray phenotype in plx-1/ smp-1 smp-2 mutants, leading us to suspect that unc-60A translation is essential for the SMPs-regulated ray morphogenesis. Consistently, RNAi against unc-60A caused, in addition to the growth defect, a highly penetrant ray defect similarly to the plx-1/smp-1 smp-2 mutations (Fig. 6A–C). unc-60A RNAi in the gcn-1 mutant background caused a ray defect as severely as in the wild-type background (Fig. 6C), suggesting that unc-60A genetically functions downstream from the translational regulation. Despite the similar ray defect as in plx-1/smp-1 smp-2 mutants, unc-60A RNAi did not change P-eIF2α (Fig. 6D), implying that elevated P-eIF2α is a cause, but not a consequence, of the morphological defect in plx-1/smp-1 smp-2 mutants.
Figure 6.
UNC-60A mediates the SMPs-regulated ray morphogenesis. (A,B) Knockdown of unc-60A phenocopies plx-1/smp-1 smp-2. (C) Quantitative ray 1 phenotype in adult males of the indicated genotypes. (D) Knockdown of unc-60A does not elevate the level of P-eIF2α. Samples treated with the indicated RNAi were collected as in Figure 2C, and the Flag immunoprecipitates were subjected to Western blot analysis. Shown in the graph are the means of normalized P-eIF2α ± SEM of three independent experiments. (***) P < 0.001. (E) Quantitative ray 1 phenotype in plx-1/smp-1 smp-2 mutant adults carrying the indicated transgenes. Note that expression of unc-60A transcripts fused with unc-54 3′UTR, but not with unc-60A 3′UTR, partially suppresses the ray 1 phenotype in the mutants.
Furthermore, expression of unc-60A transcripts with unc-54 3′UTR under the lin-32p (lin-32p∷unc-60A∷unc-54 3′UTR) partially suppressed the ray 1 phenotype in plx-1/smp-1 smp-2 adults (Fig. 6E), indicating a functional link between SMPs–PLX-1 and UNC-60A during the ray morphogenesis. In contrast, unc-60A transcripts with unc-60A 3′UTR (lin-32p∷unc-60A∷unc-60A 3′UTR) failed to suppress the ray 1 phenotype in the mutants (Fig. 6E), implicating unsuccessful synthesis of UNC-60A in the absence of the SMPs signal with this transgene, which is insufficient for the proper ray morphogenesis. Taken together, we speculate that, in addition to enhancing global translation mainly by reducing P-eIF2α, the SMPs signal uses 3′UTR of unc-60A to allow its selective translation, and that synthesized UNC-60A regulates the epidermal morphogenesis for the proper ray 1 positioning, probably by accelerating actin cytoskeletal turnover and shaping ray precursor cells.
Discussion
Here, our study on C. elegans provided the in vivo demonstration that translation stimulation is an essential downstream event of the SMPs–PLX-1 signal during the cellular morphogenesis. Our genetic approach enabled us to provide several lines of evidence that the SMPs signal uses eIF2α as a major translational regulator. First, in plx-1/smp-1 smp-2 mutants, P-eIF2α was elevated in ray precursor cells (Fig. 2C), implying down-regulated protein synthesis. Second, expression of the phosphomimetic eIF2α mimicked the ray defect in the mutants (Fig. 2D–F), suggesting that the mutant phenotype is at least partly attributed to elevated P-eIF2α. Third, the ray defect in the mutants was markedly suppressed by genetically reducing P-eIF2α (Figs. 1, 2), suggesting that up-regulated protein synthesis largely bypasses the requirement for the SMPs signal. Thus, our results reveal both requirement and sufficiency of mRNA translation stimulation via eIF2α in the SMPs-regulated epidermal ray morphogenesis.
eIF2α phosphorylation has been hitherto regarded as a stress-induced event, and its involvement in semaphorin signaling is unexpected. Our results, however, indicate a direct effect of the SMPs signal on reducing P-eIF2α. Forced expression of SMP-1 and PLX-1 was sufficient to quickly reduce P-eIF2α in otherwise normal wild-type animals (Fig. 2B), indicating that increase in P-eIF2α in plx-1/smp-1 smp-2 mutants is not a consequence of possible cellular stresses due to morphological defects in the mutants, but is caused by failure to reduce P-eIF2α due to lack of SMPs signaling. This notion is supported by the finding that P-eIF2α in PLX-1-expressing ray precursor cells is lower than that in PLX-1-absent muscle cells (Fig. 2C). In agreement with our proposal, independent studies have revealed that BDNF treatment (Takei et al. 2001), fibroblast adhesion (Gorrini et al. 2005), and L-LTP-inducing protocol (Costa-Mattioli et al. 2005, 2007) enhance translation by reducing P-eIF2α. The regulation of P-eIF2α also has physiological roles in various developmental events (Harding et al. 2001; Shen et al. 2001; Fang et al. 2003), which indicates the involvement of signals unrelated to stress in multiple aspects of eIF2α-mediated biological processes.
Suppression of the ray defect in plx-1/smp-1 smp-2 mutants by gcn-1; pek-1 mutation was highly but not fully penetrant (Fig. 1I), despite the fact that P-eIF2α in the mutants was even lower than that in wild type (Fig. 2C), implicating mechanism(s) other than the eIF2α regulation as another branch of SMPs signaling. The finding of selective translation via unc-60A mRNA 3′UTR by the SMPs signal (Fig. 5; Supplemental Fig. S9) also suggests this possibility (discussed below). Considering our findings that knockdown of eIF4G phenocopied plx-1/smp-1 smp-2 mutants and that knockdown of putative 4E-BP, spn-2, partially suppressed the ray 1 phenotype in plx-1 mutants (Fig. 3), eIF4F complex formation might be another possible target event of SMPs signaling. This speculation agrees with the previous finding that eIF4F complex formation is regulated by Sema3A via mTOR, a well-known eIF4F regulator, in vertebrate neurons (Campbell and Holt 2001). Thus, we hypothesize that the SMPs signal up-regulates translation by reducing P-eIF2α and simultaneously activating eIF4F complex during the ray morphogenesis. Indeed, other studies have shown that eIF2 complex and eIF4F complex formations are sometimes regulated coordinately (Takei et al. 2001; Gorrini et al. 2005), and that there is a cross-talk between eIF2α and TOR (Cherkasova and Hinnebusch 2003; Kubota et al. 2003). Given that both complex formations are the rate-limiting steps in translation (Hershey and Merrick 2000), this hypothesis appears reasonable.
We identified cofilin/UNC-60A as a potent target synthesized in response to the SMPs signal (Fig. 4), which coincides with the recent report on rapid cofilin synthesis by Sema3A application in vertebrate neurons (Piper et al. 2006). Aizawa et al. (2001) reported that Sema3A induces a rapid elevation and a subsequent reduction in the cofilin phosphorylation during the growth cone collapse, indicating the importance of a cycle of cofilin between activation and inactivation. It is not known whether such an activation–inactivation cycle exists for UNC-60A in C. elegans, whose genome lacks any genes for a cofilin kinase LIM-kinase (Arber et al. 1998; Yang et al. 1998) and a phosphatase, Slingshot (Niwa et al. 2002). Nevertheless, UNC-60A is known to play a role in F-actin depolymerization (Ono and Benian 1998), which we consider may be important for the arrangement of ray precursor cells as a downstream event of SMPs signaling. It is documented that each ray precursor cluster is positioned at the junctional site between two adjacent Rn.ps (Baird et al. 1991). Indeed, in plx-1 mutants, the boundary between R1.p and R2.p shifts anteriorly, causing the anterior displacement of ray precursor cluster 1 to its normal position (Fujii et al. 2002). Notably, we found that in R(1).a + p and R(2).a + p units of plx-1/smp-1 smp-2 mutants, prominent repression of unc-60A 3′UTR-dependent translation appeared to precede their defective morphogenesis (Fig. 5; Supplemental Fig. S9), suggesting that down-regulated UNC-60A expression is a major cause of the structural ray phenotype. Expectedly, unc-60A knockdown phenocopied plx-1/smp-1 smp-2 mutants, and forced expression of UNC-60A partially suppressed the ray defect in the mutants (Fig. 6). In contrast to R(1).a + p and R(2).a + p, the SMPs signal appeared to have a relatively minor effect on unc-60A 3′UTR-dependent translation in the R(5).a + p unit (Fig. 5), which could account for the apparent lack of positional defects in ray 5 in plx-1/smp-1 smp-2 mutants. Taken together, we propose that the SMPs signal preferentially stimulates UNC-60A synthesis in R1.p and R2.p, and hence determines the position of ray 1 by posteriorly shifting the boundary between the two cells.
In cultured vertebrate growth cones, guidance cues rapidly activate translation in minutes at the site close to their application (Campbell and Holt 2001). Although our Western blot analysis using the hsp∷plx-1 rescue construct showed that unc-60A translation is initiated by 2–4 h after HS (Figs. 2B, 4B), the time necessary for the production of functional PLX-1 molecules after HS is not known, leaving the likely time scale of the SMPs translation events in the epidermal system undetermined. Considering that R1.p makes contact with R2.p until their fusion, and that PLX-1 expression in ray precursor cells lasts throughout their development (Fujii et al. 2002), the SMPs signal may stimulate protein synthesis, including UNC-60A, continuously, rather than transiently. Future examination of the spatiotemporal pattern of signaling events would provide further clues to the mechanism of cellular morphogenesis regulated by SMPs-induced UNC-60A synthesis.
In cultured vertebrate neurons, several guidance cues activate the common translational regulators (eIF4E and 4E-BP1), but they stimulate synthesis of different kinds of proteins that generate distinct cellular responses, depending on whether they are attractive cues or repulsive ones (Wu et al. 2005; Leung et al. 2006; Piper et al. 2006). mRNAs for cytoskeletal regulators/components like β-actin (Zhang et al. 2001; Leung et al. 2006) and RhoA (Wu et al. 2005), for instance, contain motifs in their 3′UTRs that are implicated in their selective translation in response to these cues. Similarly to such gene-specific regulation, we found preferential stimulation of unc-60A 3′UTR-dependent translation by the SMPs signal. Since in most of the known cases 3′UTR-mediated translational regulation involves the interference with eIF4F assembly at the 5′ cap of mRNAs by 3′UTR-binding translational repressors, the SMPs signal might inhibit the repressors so as to enable the preferential unc-60A mRNA translation. This idea is supported by the presence of a putative cytoplasmic polyadenylation element (CPE) in its 3′UTR (Supplemental Fig. S8), a consensus sequence that, via its binding protein CPEB, can be targeted by such translational repressors (Mendez and Richter 2001). An alternative unprecedented idea is that the SMPs signal might stimulate 3′UTR-dependent unc-60A translation via the regulation of eIF2α. A possible scenario would be that eIF2 ternary complex binding to the 40S ribosomal subunit in the 43S preinitiation complex is destabilized in the presence of the presumptive factors bound to unc-60A 3′UTR, which can be overcome as the SMPs signal lowers eIF2α phosphorylation and increases the level of ternary complex formation.
Only partial suppression of the ray 1 phenotype in plx-1/smp-1 smp-2 mutants by UNC-60A expression (Fig. 6E) implies that a protein other than UNC-60A is also synthesized by and required for SMPs signaling. Other cytoskeletal regulators/components might be possible targets of translational regulation. One candidate is RhoA, whose transcripts display localized distribution to the axonal tips, where they are translated by Sema3A in mammals (Wu et al. 2005). Interestingly, knockdown of C. elegans RhoA, rho-1, was reported to produce a mildly penetrant ray 1 phenotype similarly to plx-1/smp-1 smp-2 mutants (Dalpe et al. 2004).
To summarize, we provided lines of in vivo evidence that the SMPs signal stimulates mRNA translation. The signal reduces P-eIF2α and, as our data infer, may concertedly activate eIF4F complex formation, which leads to global translation combined with selective translation as illustrated with unc-60A in a manner dependent on its 3′UTR. Together with accumulating evidence in the nervous system (Wu et al. 2005; Leung et al. 2006; Piper et al. 2006; Schratt et al. 2006; Lin and Holt 2007), our results imply that translation of mRNA species, especially those relevant to cytoskeleton, is a fundamental mechanism for regulating cell morphology.
Materials and methods
Strains
Standard techniques for C. elegans culture and genetics were used as described by Brenner (1974). For analysis of the male tails, strains carried the him-5 mutation. For Western blot and Northern blot analyses, N2 was used as a wild-type control, unless otherwise noted. The following alleles were used: [LGI] smp-1(ev715), smp-2(ev709); [LGII] rrf-3(pk1426), Y81G3A.3 (tm1267, ok871, ok886), ncIs13[ajm-1∷gfp]; [LGIII] gcn-1(nc40), tDf9; [LGIV] plx-1(nc37, ev724), jcIs1[ajm-1∷gfp; unc-29(+); rol-6(su1006)]; [LGV] him-5(e1490); [LGX] pek-1(ok275, tm629); Bristol N2; Hawaiian CB4856. The linkage groups of ncIs17[hsp∷egfp], ncIs19[hsp∷plx-1; hsp∷egfp; rol-6(su1006)], ncIs32[lin-32p∷mRFP∷unc-54 3′UTR; lin-32p∷EGFP∷unc-60A 3′UTR; rol-6(su1006)], and ncIs33[lin-32p∷EGFP∷unc-54 3′UTR; lin-32p∷mRFP∷unc-60A 3′UTR; rol-6(su1006)] have not been determined.
Isolation of suppressor mutants
Young adult plx-1(nc37); him-5(e1490) mutants were mutagenized with ethylmethane sulfonate (EMS). Males from F3 progeny representing up to 1000 haploid genomes were screened under Nomarski optics (Zeiss Axioplan) to examine whether the ray 1 anterior displacement characteristic of plx-1; him-5 was suppressed. One of the isolated suppressor mutants, plx-1; him-5; nc40, was out-crossed 10 times to plx-1; him-5. nc40 single mutants were fertile and appeared healthy, but the brood size was reduced to half (N2: 358 ± 23; nc40: 178 ± 28 [n = 3 for each]).
Genetic mapping of nc40
Two-factor and three-factor crosses mapped nc40 at 1.4 map units right to dpy-1 on linkage group III. Further mapping was performed by using single nucleotide polymorphisms (SNPs) between N2 and CB4856 strains. F2 progeny from plx-1; him-5; nc40 (N2 background) × plx-1; him-5 (CB4856 background) crosses were isolated, and populations were generated from each isolate. Male tails from each population were tested to determine the genotype of the suppressor gene. Genomic DNA was prepared from each population, which was either wild type or homozygous nc40, and SNPs were scored by PCR amplification followed by restriction enzyme digestion or sequencing. Using genomic DNA from 72 populations, nc40 was mapped between nucleotides 84,618 and 108,185 on Y48G9A.
RNAi
Genomic DNA fragments of gcn-1 (Y48G9A: nucleotides 105,712–106,114), eIF2α (Y37E3: 14,315–14,862), eIF2β/iftb-1 (C54G4: 34,320–K04G2: 287), eIF4G/ifg-1 (M110: 15,908– 16,949), eEF2/eft-1 (ZK328: 13,513–15,136), eEF2/eft-2 (F25H5: 10,535–11,429), spn-2 (F56F3: 21,390–22,631), unc-60A (C38C3: 19–1074), 3′UTR of unc-60A (C38C3: 976–1682), and a cDNA fragment of plx-1 (nucleotides 603–1912) were subcloned into the pPD129.36 vector, and the resulting constructs were transformed into HT115 bacteria to allow for the synthesis of dsRNA in the presence of 1 mM IPTG (Timmons and Fire 1998). Young adult hermaphrodites were fed with the transformed bacteria, and the F1 progeny were analyzed for their phenotypes. Animals carried the rrf-3(pk1426) mutation, which gives hypersensitivity to RNAi (Simmer et al. 2002) but does not affect the morphology of male tails alone. Animals fed with bacteria harboring the empty pPD129.36 vector were used as controls.
Generation of anti-eIF2α antibody
A rabbit polyclonal antibody was raised against a synthetic peptide corresponding to 16 amino acid residues near the C terminus of C. elegans eIF2α (BioGate). The peptide consisted of NH2-VDAEEASRDNRKKAGD-COOH and was coupled to keyhole limpet hemocyanin. The antibody specifically labels C. elegans eIF2α protein, as evidenced by the disappearance of a 39-kDa immunoblot signal in eIF2α(RNAi) animals (data not shown).
Immunoprecipitation
L3 and L4 males were collected in lysis buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1 mM PMSF, and protease inhibitor cocktail (Sigma), and dissolved by brief sonication and incubation with a rotator for 1 h at room temperature. Flag-tagged proteins were immunoprecipitated with anti-Flag M2 affinity gel (Sigma) according to Sigma’s instructions. Both immunoprecipitates and supernatants were subjected to Western blot analysis.
Western blot analysis
Samples (8 or 20 μg of total proteins per lane) were separated by SDS–polyacrylamide gel electrophoresis and transferred to an Immobilon-P PVDF membrane (Millipore). Western blots were probed with anti-eIF2α, anti-phopho-eIF2α (Cell Signaling Technology), anti-α-tubulin (Woods et al. 1989), or anti-UNC-60A (Ono and Benian 1998) primary antibodies. The immunoblot signals were then visualized by incubation with anti-mouse or anti-rabbit IgG antibodies conjugated with horseradish peroxidase (HRP) (Cell Signaling Technology) followed by detection with an Immobilon Western chemiluminescent HRP substrate (Millipore). Images were captured and quantified with Luminescent Image Analyzer LAS-1000 (FujiFilm). One-way ANOVA was used to evaluate differences in the signal intensities.
Confocal laser microscopy
Confocal images were captured and quantified with an Olympus Fluoview300 to analyze the expression profiles of EGFP and mRFP in the male tails of ncIs32 or ncIs33 at the L3 stage, when each ray precursor unit, R(n).a + p, is composed of one Rn.p cell and two Rn.a descendants, Rn.aa and Rn.ap cells. Cell boundaries were delineated with ncIs13[ajm-1∷gfp]. In each observation, the same laser intensity and exposure conditions were used. As a reference, the fluorescent intensity of an AVM cell body was measured. AVM, which does not express plx-1, gave an indistinguishable value in each observation (data not shown).
Acknowledgments
We thank the staff of Fujisawa and Oda Laboratories, Hiromi Hirata, Kunihiro Matsumoto, Naoki Hisamoto, and Jonathan Ewbank for discussion and comments; Mie Chikuma for nc40 mapping; Motoshi Suzuki for constructing ncIs17 and ncIs19; Yuji Kohara for cDNA clones; Andrew Fire for pPD49.26 and other vectors; Shoichiro Ono for anti-UNC-60A antibody; Keith Gull for anti-α-tubulin antibody; Roger Tsien and Hiroshi Kagoshima for mRFP cDNA; Lesilee S. Rose for information on spn-2; and Douglas S. Portman for information on lin-32p. Some strains were provided by the Caenorhabditis Genetic Center, which is funded by the National Institutes for Health National Center for Research Resources, and National Bioresource Project funded by the Ministry of Education, Culture, Sports, Science and Technology of Japan. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to H.F., T.I., Y.O., and S.T.); a grant from CREST (Core Research for Evolutional Science and Technology) of the Japan Science and Technology Corporation (JST) (to H.F.); and a grant from The Sumitomo Foundation (to S.T.).
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1644008.
References
- Aizawa H., Wakatsuki S., Ishii A., Moriyama K., Sasaki Y., Ohashi K., Sekine-Aizawa Y., Sehara-Fujisawa A., Mizuno K., Goshima Y., et al. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat. Neurosci. 2001;4:367–373. doi: 10.1038/86011. [DOI] [PubMed] [Google Scholar]
- Arber S., Barbayannis F.A., Hanser H., Schneider C., Stanyon C.A., Bernard O., Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- Baird S.E., Fitch D.H., Kassem I.A., Emmons S.W. Pattern formation in the nematode epidermis: Determination of the arrangement of peripheral sense organs in the C. elegans male tail. Development. 1991;113:515–526. doi: 10.1242/dev.113.2.515. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D.S., Holt C.E. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- Cherkasova V.A., Hinnebusch A.G. Translational control by TOR and TAP42 through dephosphorylation of eIF2α kinase GCN2. Genes & Dev. 2003;17:859–872. doi: 10.1101/gad.1069003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M., Gobert D., Harding H., Herdy B., Azzi M., Bruno M., Bidinosti M., Ben Mamou C., Marcinkiewicz E., Yoshida M., et al. Translational control of hippocampal synaptic plasticity and memory by the eIF2α kinase GCN2. Nature. 2005;436:1166–1173. doi: 10.1038/nature03897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M., Gobert D., Stern E., Gamache K., Colina R., Cuello C., Sossin W., Kaufman R., Pelletier J., Rosenblum K., et al. eIF2α phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpe G., Zhang L.W., Zheng H., Culotti J.G. Conversion of cell movement responses to Semaphorin-1 and Plexin-1 from attraction to repulsion by lowered levels of specific RAC GTPases in C. elegans. Development. 2004;131:2073–2088. doi: 10.1242/dev.01063. [DOI] [PubMed] [Google Scholar]
- Emmons S.W. Male development. In: The C. elegans Research Community, editor. WormBook. 2005. http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Raper J.A. Localized collapsing cues can steer growth cones without inducing their full collapse. Neuron. 1995;14:263–274. doi: 10.1016/0896-6273(95)90284-8. [DOI] [PubMed] [Google Scholar]
- Fang R., Xiong Y., Singleton C.K. IfkA, a presumptive eIF2 α kinase of Dictyostelium, is required for proper timing of aggregation and regulation of mound size. BMC Dev. Biol. 2003;3:3. doi: 10.1186/1471-213X-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Nakao F., Shibata Y., Shioi G., Kodama E., Fujisawa H., Takagi S. Caenorhabditis elegans PlexinA, PLX-1, interacts with transmembrane semaphorins and regulates epidermal morphogenesis. Development. 2002;129:2053–2063. doi: 10.1242/dev.129.9.2053. [DOI] [PubMed] [Google Scholar]
- Gingras A.C., Raught B., Sonenberg N. eIF4 initiation factors: Effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- Ginzburg V.E., Roy P.J., Culotti J.G. Semaphorin 1a and semaphorin 1b are required for correct epidermal cell positioning and adhesion during morphogenesis in C. elegans. Development. 2002;129:2065–2078. doi: 10.1242/dev.129.9.2065. [DOI] [PubMed] [Google Scholar]
- Gorrini C., Loreni F., Gandin V., Sala L.A., Sonenberg N., Marchisio P.C., Biffo S. Fibronectin controls cap-dependent translation through β1 integrin and eukaryotic initiation factors 4 and 2 coordinated pathways. Proc. Natl. Acad. Sci. 2005;102:9200–9205. doi: 10.1073/pnas.0409513102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima Y., Nakamura F., Strittmatter P., Strittmatter S.M. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature. 1995;376:509–514. doi: 10.1038/376509a0. [DOI] [PubMed] [Google Scholar]
- Harding H.P., Zeng H., Zhang Y., Jungries R., Chung P., Plesken H., Sabatini D.D., Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- Hershey J.W.B., Merrick W.C. Pathway and mechanism of initiation of protein synthesis. In: Sonenberg N, et al., editors. Translational control of gene expression. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2000. pp. 33–88. [Google Scholar]
- Hinnebusch A.G. Mechanism and regulation of initiator methyonyl-tRNA binding to ribosomes. In: Sonenberg N, et al., editors. Translational control of gene expression. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2000. pp. 185–243. [Google Scholar]
- Holcik M., Sonenberg N. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 2005;6:318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- Jin Z., Strittmatter S.M. Rac1 mediates collapsin-1-induced growth cone collapse. J. Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R.P., Aurandt J., Guan K.L. Semaphorins command cells to move. Nat. Rev. Mol. Cell Biol. 2005;6:789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- Kubota H., Obata T., Ota K., Sasaki T., Ito T. Rapamycin-induced translational derepression of GCN4 mRNA involves a novel mechanism for activation of the eIF2 α kinase GCN2. J. Biol. Chem. 2003;278:20457–20460. doi: 10.1074/jbc.C300133200. [DOI] [PubMed] [Google Scholar]
- Kuersten S., Goodwin E.B. The power of the 3′ UTR: Translational control and development. Nat. Rev. Genet. 2003;4:626–637. doi: 10.1038/nrg1125. [DOI] [PubMed] [Google Scholar]
- Kuhn T.B., Brown M.D., Wilcox C.L., Raper J.A., Bamburg J.R. Myelin and collapsin-1 induce motor neuron growth cone collapse through different pathways: Inhibition of collapse by opposing mutants of rac1. J. Neurosci. 1999;19:1965–1975. doi: 10.1523/JNEUROSCI.19-06-01965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K.M., van Horck F.P., Lin A.C., Allison R., Standart N., Holt C.E. Asymmetrical β-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat. Neurosci. 2006;9:1247–1256. doi: 10.1038/nn1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A.C., Holt C.E. Local translation and directional steering in axons. EMBO J. 2007;26:3729–3736. doi: 10.1038/sj.emboj.7601808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton M.J., Crouch D., Hinnebusch A.G. GCN1, a translational activator of GCN4 in Saccharomyces cerevisiae, is required for phosphorylation of eukaryotic translation initiation factor 2 by protein kinase GCN2. Mol. Cell. Biol. 1993;13:3541–3556. doi: 10.1128/mcb.13.6.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez R., Richter J.D. Translational control by CPEB: A means to the end. Nat. Rev. Mol. Cell Biol. 2001;2:521–529. doi: 10.1038/35080081. [DOI] [PubMed] [Google Scholar]
- Mohler W.A., Simske J.S., Williams-Masson E.M., Hardin J.D., White J.G. Dynamics and ultrastructure of developmental cell fusions in the Caenorhabditis elegans hypodermis. Curr. Biol. 1998;8:1087–1090. doi: 10.1016/s0960-9822(98)70447-6. [DOI] [PubMed] [Google Scholar]
- Nakao F., Hudson M.L., Suzuki M., Peckler Z., Kurokawa R., Liu Z., Gengyo-Ando K., Nukazuka A., Fujii T., Suto F., et al. The PLEXIN PLX-2 and the ephrin EFN-4 have distinct roles in MAB-20/Semaphorin 2A signaling in Caenorhabditis elegans morphogenesis. Genetics. 2007;176:1591–1607. doi: 10.1534/genetics.106.067116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa R., Nagata-Ohashi K., Takeichi M., Mizuno K., Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108:233–246. doi: 10.1016/s0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- Oinuma I., Ishikawa Y., Katoh H., Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305:862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- Ono S., Benian G.M. Two Caenorhabditis elegans actin depolymerizing factor/cofilin proteins, encoded by the unc-60 gene, differentially regulate actin filament dynamics. J. Biol. Chem. 1998;273:3778–3783. doi: 10.1074/jbc.273.6.3778. [DOI] [PubMed] [Google Scholar]
- Piper M., Anderson R., Dwivedy A., Weinl C., van Horck F., Leung K.M., Cogill E., Holt C. Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron. 2006;49:215–228. doi: 10.1016/j.neuron.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portman D.S., Emmons S.W. The basic helix–loop–helix transcription factors LIN-32 and HLH-2 function together in multiple steps of a C. elegans neuronal sublineage. Development. 2000;127:5415–5426. doi: 10.1242/dev.127.24.5415. [DOI] [PubMed] [Google Scholar]
- Rhoads R.E., Dinkova T.D., Korneeva N.L. Mechanism and regulation of translation in C. elegans. In: The C. elegans Research Community, editor. WormBook. 2006. http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohm B., Rahim B., Kleiber B., Hovatta I., Puschel A.W. The semaphorin 3A receptor may directly regulate the activity of small GTPases. FEBS Lett. 2000;486:68–72. doi: 10.1016/s0014-5793(00)02240-7. [DOI] [PubMed] [Google Scholar]
- Ron D., Harding H.P. PERK and tranlational control by stress in the endoplasmic reticulum. In: Sonenberg N, et al., editors. Translational control of gene expression. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2000. pp. 547–560. [Google Scholar]
- Sattlegger E., Hinnebusch A.G. Separate domains in GCN1 for binding protein kinase GCN2 and ribosomes are required for GCN2 activation in amino acid-starved cells. EMBO J. 2000;19:6622–6633. doi: 10.1093/emboj/19.23.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt G.M., Tuebing F., Nigh E.A., Kane C.G., Sabatini M.E., Kiebler M., Greenberg M.E. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Shen X., Ellis R.E., Lee K., Liu C.Y., Yang K., Solomon A., Yoshida H., Morimoto R., Kurnit D.M., Mori K., et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893–903. doi: 10.1016/s0092-8674(01)00612-2. [DOI] [PubMed] [Google Scholar]
- Simmer F., Tijsterman M., Parrish S., Koushika S.P., Nonet M.L., Fire A., Ahringer J., Plasterk R.H. Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr. Biol. 2002;12:1317–1319. doi: 10.1016/s0960-9822(02)01041-2. [DOI] [PubMed] [Google Scholar]
- Sulston J.E., Albertson D.G., Thomson J.N. The Caenorhabditis elegans male: Postembryonic development of nongonadal structures. Dev. Biol. 1980;78:542–576. doi: 10.1016/0012-1606(80)90352-8. [DOI] [PubMed] [Google Scholar]
- Takei N., Kawamura M., Hara K., Yonezawa K., Nawa H. Brain-derived neurotrophic factor enhances neuronal translation by activating multiple initiation processes: Comparison with the effects of insulin. J. Biol. Chem. 2001;276:42818–42825. doi: 10.1074/jbc.M103237200. [DOI] [PubMed] [Google Scholar]
- Timmons L., Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Turner L.J., Nicholls S., Hall A. The activity of the plexin-A1 receptor is regulated by Rac. J. Biol. Chem. 2004;279:33199–33205. doi: 10.1074/jbc.M402943200. [DOI] [PubMed] [Google Scholar]
- Woods A., Sherwin T., Sasse R., MacRae T.H., Baines A.J., Gull K. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J. Cell Sci. 1989;93:491–500. doi: 10.1242/jcs.93.3.491. [DOI] [PubMed] [Google Scholar]
- Wu K.Y., Hengst U., Cox L.J., Macosko E.Z., Jeromin A., Urquhart E.R., Jaffrey S.R. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–1024. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Higuchi O., Ohashi K., Nagata K., Wada A., Kangawa K., Nishida E., Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- Zanata S.M., Hovatta I., Rohm B., Puschel A.W. Antagonistic effects of Rnd1 and RhoD GTPases regulate receptor activity in Semaphorin 3A-induced cytoskeletal collapse. J. Neurosci. 2002;22:471–477. doi: 10.1523/JNEUROSCI.22-02-00471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.L., Eom T., Oleynikov Y., Shenoy S.M., Liebelt D.A., Dictenberg J.B., Singer R.H., Bassell G.J. Neurotrophin-induced transport of a β-actin mRNP complex increases β-actin levels and stimulates growth cone motility. Neuron. 2001;31:261–275. doi: 10.1016/s0896-6273(01)00357-9. [DOI] [PubMed] [Google Scholar]