Abstract
GGTenu1 mice, deficient in γ-glutamyl transferase and unable to metabolize extracellular glutathione, develop intracellular glutathione deficiency and oxidant stress. We used intratracheal IL-13 to induce airway inflammation and asthma in wild-type (WT) and GGTenu1 mice to determine the effect of altered glutathione metabolism on bronchial asthma. WT and GGTenu1 mice developed similar degrees of lung inflammation. In contrast, IL-13 induced airway epithelial cell mucous cell hyperplasia, mucin and mucin-related gene expression, epidermal growth factor receptor mRNA, and epidermal growth factor receptor activation along with airway hyperreactivity in WT mice but not in GGTenu1 mice. Lung lining fluid (extracellular) glutathione was 10-fold greater in GGTenu1 than in WT lungs, providing increased buffering of inflammation-associated reactive oxygen species. Pharmacologic inhibition of GGT in WT mice produced similar effects, suggesting that the lung lining fluid glutathione protects against epithelial cell induction of asthma. Inhibiting GGT activity in lung lining fluid may represent a novel therapeutic approach for preventing and treating asthma.
Keywords: glutathione, lung lining fluid, asthma
CLINICAL RELEVANCE
We identify gamma-glutamyl transferase (GGT) as a target to treat asthma. GGT regulates glutathione metabolism in lung lining fluid. Inhibition of GGT augments lining fluid glutathione, buffers oxidants derived from inflammation, and attenuates asthma.
Oxidant stress is believed to be important in asthma pathogenesis (1). Nrf2 (nuclear factor, erythroid derived 2, like 2) null mice display increased susceptibility to airway inflammation and airway hyperreactivity in an ovalbumin model of experimental asthma (2). Although this transcription factor regulates an array of antioxidant genes, at least part of this antioxidant imbalance seems to involve glutathione because glutathione content and the ratio of glutathione/oxidized glutathione (redox ratio) increase in the ovalbumin-sensitized lung of normal mice but not in that of Nrf2-null mice. Conversely, wild-type (WT) mice display decreased susceptibility to airway inflammation and airway hyperreactivity in the same experimental model when supplemented with membrane-permeable γ-glutamylcysteinylethyl ester. This glutathione precursor, delivered via the peritoneum, augments lung glutathione content, increases the glutathione redox ratio, and alters Th1/Th2 imbalance to decrease eosinophil migration (3).
Glutathione is normally abundant in lung cells and in the extracellular lining fluid that bathes the gas exchange surface (4). Extracellular glutathione is metabolized by the enzyme gamma-glutamyl transferase (GGT) (EC 2.3.2.2, which is present in the lung lining fluid (5). In the absence of GGT, extracellular pools of glutathione in the blood, urine (6, 7), and lung lining fluid (8) enlarge due to decreased turnover. However, intracellular glutathione is decreased because cysteine availability, derived from glutathione breakdown, becomes limiting for intracellular glutathione synthesis (7). Cellular glutathione deficiency causes intracellular oxidant stress in GGT-deficient GGTenu1 mouse lung in normoxia and hyperoxia (8). We used intratracheal IL-13 to induce experimental asthma (9) in the GGTenu1 mouse to test the hypothesis that the GGTenu1 mutant would be more susceptible to asthma than the WT mouse due to cellular glutathione deficiency. We show, however, that GGTenu1 mice were protected from IL-13–induced asthma, suggesting that increasing lung lining fluid glutathione protects airway epithelial cells from inflammation-induced oxidant stress and thus may provide a new approach to preventing and treating asthma.
MATERIALS AND METHODS
Mouse Model
GGTenu1 mice (C57Bl/6J) were bred and genotyped in the Laboratory and Animal Science Center according to protocols approved by the Institutional Utilization and Animal Care Committee at Boston University School of Medicine (10). Instillation of IL-13 was used as a cytokine-driven model of experimental asthma (9). Mice were transiently anesthetized with Metofane and received 5 μm of IL-13 delivered in 100 μl of saline via the trachea and dosed for three successive days. Controls received saline alone. Airway physiology was assessed on the fourth day under appropriate anesthesia via an intratracheal catheter using the Scireg flexivent apparatus (SCIREQ, Montreal, PQ, Canada). The lungs were exposed to graded doses of methacholine (0, 5, 10, and 15 μg/ml), and airway resistance was measured. In separate experiments, lung lining fluid GGT activity was inhibited in WT mice by intratracheal delivery of acivicin, an irreversible GGT antagonist (11).
Bronchoalveolar Lavage Analysis
The lung was lavaged once with 500 μm of ice-cold saline and aspirated to recover bronchoalveolar lavage fluid (BALF) (99% recovery). Cells were counted on a hemocytometer, and cytospin slides were prepared and stained with Diff-Quik for differential cell counts (8). Cell-free BALF was assessed for IL-13 protein plus eotaxin protein and leukotriene C4 and E4 contents by ELISA using the R&D Systems mouse Quantikine kit and the Cayman Chemical kit (Cayman, Ann Arbor, MI), respectively, using an internal standard curve according to the manufacturer's instructions. Cytokine proteins were assayed using the RayBio32-mouse cytokine array (RayBio, Norcross, GA). Signal intensity was determined by densitometry, and values were normalized to internal standards on each blot and then to the saline-treated WT lung (12). Glutathione, cysteine, and GSNO were assessed by HPLC (8, 13). Protein carbonyl content was assayed by ELISA using a kit from CellBiolabs, Inc. (San Diego, CA) according to manufacturer's instructions using an internal standard curve. Samples were assayed in duplicate or triplicate and normalized to the WT saline-treated lung.
Histology and Immunohistochemistry
Lung tissue was inflation fixed with freshly prepared 4% paraformaldhyde (5, 8). Mucin accumulation was assessed with Alcian blue/periodic acid-Schiff (PAS) stain (14). Native EGF receptor was localized in lung using peptide-specific rabbit antisera from Cell Signaling Technology (Catalogue #2232; Cell Signaling Technology, Beverly, MA) at a dilution of 1:25 and incubation at 4°C overnight. For antigen retrieval, slides were exposed to 50 mM glycine at pH 3.5 and 10 mM EDTA for 10 minutes at 95°C. Activation of EGF receptor was assessed using phospho-specific antisera raised against phosphorylated EGFR from the same company at a dilution of 1:25 and incubation at 4°C overnight (Catalogue #2231; Cell Signaling Technology). For antigen retrieval, slides were exposed to Proteinase K (Zymeda preparation) at a dilution of 1:16 for 8 minutes at 42°C. In each case, signal specificity was confirmed in a competition experiment with the corresponding peptide antigen obtained from Cell Signaling Technology. Sections were stained for peroxidase activity with the Vectabond ABC kit according to the manufacturer's instructions.
Versican was localized with 8 mg/ml of a rabbit anti-mouse versican (GAG b domain) polyclonal antibody (Chemicon International, Temecula, CA). This antibody was raised against a GST fusion protein containing amino acids 1360 through 1439 of mouse versican and recognizes a large molecular weight band by Western blot in aortic and cardiac tissue from mouse and media from mouse smooth muscle cells. All sections were photographed in a Leitz Orthoplan microscope (5, 8).
Real-Time RT-PCR
Lung RNA was isolated with Tri-Reagent, treated with DNase, and quantified by spectrophotometry. Versican mRNA analysis was performed in the laboratory of Dr. Wight using 2 μm of total RNA and reverse transcribed in a 40-μl reaction mix with random primers and the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Relative quantification was performed using the Taqman Gene Expression Assay Mm00490179_m1 to detect all versican splice variants by amplifying across the exon junction of the last C-type lectin domain and the complement control protein module or SUSHI domain in the G3 domain. Gene expression was normalized to eukaryotic 18S rRNA Endogenous Control part no. 4333760 (Applied Biosystems). Messenger RNA analyses for the mucin genes Muc1, Muc2, Muc3, Muc4, Muc5ac, Muc5b, and the mucous granule protein Clca3 (gob-5) were performed in a similar fashion at the Pulmonary Center of Boston University using intron-spanning primer sets with Taqman probes from Applied Biosciences. Target cDNA was quantified using Taqman probes and the standard curve method, and determinations were done with three replicates.
EGF receptor mRNA induction in response to IL-13 was assessed with SYBR green using primers purchased from Superarray Bioscience Corporation (Frederick, MD). Fifty nanograms of cDNA were used in a 50-μl reaction volume with SYBR Green PCR master mix from Applied Biosciences. Forty cycles of amplification, data acquisition, and data analysis were performed on the ABI Prism 7700 Sequence detector (Applied Biosystems). EGFR was normalized to actin mRNA.
Statistics
Nominal data are presented as means with SE and analyzed by ANOVA using STATISTICA release 4.5 (StatSoft, Inc, 1993). Post hoc comparison was done with the Newman-Keuls test, and critical ranges and significant differences among groups are denoted in the figures. P values < 0.05 were considered significant.
RESULTS
IL-13 Induces Airway Inflammation in WT and GGTenu1 Mice
Bronchalveolar lavage cytokines.
IL-13 protein assayed by ELISA was barely detectable in saline-treated mice, but levels rose to ∼1200 pg/ml in IL-13–treated mice of both genotypes. Proinflammatory cytokines assayed by RayBio cytokine protein array were increased by IL-13 to similar levels in WT and GGTenu1 mice (Figure 1A). IL-6 was induced on average 15-fold (P = 0.017) and IL-12 by 5-fold (P = 0.014). This array detected the increase in IL-13 content (P = 0.016). Eotaxin protein, an eosinophil chemotactic factor, was also significantly induced in IL-13–treated mice of both genotypes (see Table E1 in the online supplement).
Figure 1.
Lung inflammatory milieu induced by IL-13. (A) Profile of cytokine proteins induced by IL-13 in wild-type (WT) and GGTenu1 mouse bronchoalveolar lavage fluid (BALF) compared with WT saline control. (B) Cell differentials in BALF from saline-treated and IL-13–treated WT and GGTenu1 mice. (C) Immunohistochemistry for versican protein shows the absence of signal in saline-treated WT (top left) and GGTenu1 lung (bottom left) but abundant signal surrounding the airways after IL-13 treatment in WT (top right) and GGTenu1 lung (bottom right). Original magnifications: ×400.
Inflammatory cells in bronchoalveolar lavage.
IL-13 increased inflammatory cells by 3- to 4-fold in WT and GGTenu1 mice, and differential cell counts were similar in both genotypes (Figure 1B). Macrophages predominated in BALF from saline-treated mice (n = 4) of both genotypes. Eosinophils predominated after IL-13 treatment (P = 0.000006, dagger in Figure 1B, even more so in GGTenu1), macrophages declined (P = 0.000005), and lymphocytes were unchanged.
IL-13 induction of inflammation-associated proteoglycan.
Versican was examined as a proteoglycan that is known to accumulate in the subepithelial layer of human airways in association with inflammation in asthma (15). IL-13 induced versican mRNA by 5- to 6-fold in mice from both genotypes. No immunohistochemical signal for versican was detected in saline-treated mice (Figures 1C and 1E), but an intense signal surrounded the airways (Figures 1D and 1F) and vasculature in IL-13–treated mice of each genotype.
GGT Deficiency Attenuates the Airway Epithelial Cell Response to IL-13
Accumulation of PAS-positive material and Muc5ac mRNA.
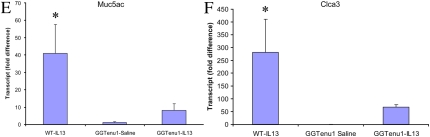
An abundance of PAS-positive material was evident in airway epithelial cells of IL-13–treated WT mice (Figure 2A). In contrast, only sparse amounts of PAS-positive material were present in airway epithelial cells of IL-13–treated GGTenu1 mice (Figure 2B), whereas no signal was evident in saline-treated WT (Figure 2C) or GGTenu1 (Figure 2D) mice. The accumulation of PAS-positive material in epithelial cells after IL-13 treatment was associated with a 35-fold induction of Muc5ac mRNA in WT mice (P < 106; n = 6), but this induction was 4-fold less in GGTenu1 mice (n = 6) (Figure 2E). The increase in Muc5ac mRNA was specific because mRNA for Muc1, Muc2, Muc3, Muc4, or Muc5b were not induced by IL-13 in mice of either genotype (Figure E1).
Figure 2.
IL-13 induces lung mucus accumulation plus mucin and mucin-related gene expression. (A) Periodic acid-Schiff (PAS-positive signal in airway epithelial cells from IL-13–treated WT mice (top left). (B) Absence of PAS-positive material in IL-13–treated GGTenu1 lung. No PAS-positive cells were identified in saline-treated WT (C) or GGTenu1 (D) lung. Original magnifications: ×100. (E) Compared with saline-treated WT control mice, Muc5ac mRNA was significantly induced after IL-13 treatment but only in WT lung (asterisk). (F) Chloride channel 3 mRNA, Clca3 (gob-5), was significantly induced after IL-13 treatment but only in WT lung (asterisk).
Expression of Clca3.
Messenger RNA for the chloride channel Clca3, a gene that is induced at the onset of asthma (16), was induced 281-fold in IL-13–treated WT mice (P = 0.002; n = 6) but was 4-fold less in IL-13–treated GGTenu1 mice (n = 6) (Figure 2F).
EGFR induction and activation.
The effect of IL-13 on EGF receptor was examined because Muc5ac gene induction in asthma is mediated by oxidant-dependent activation of the EGFR (17–19).
EGFR Immunohistochemistry
EGFR protein was identified on the apical surface of ciliated airway epithelial cells in saline-treated and IL-13–treated WT and GGTenu1 lung. Signal from WT lung is shown in Figures 3A and 3B. Specificity was confirmed in a competition experiment coincubating EGFR antisera with the EGFR peptide antigen, which abolished signal (Figure 3C).
Figure 3.
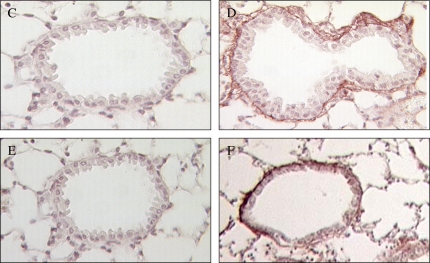
Epidermal growth factor receptor (EGFR) analysis. Native EGF receptor was immunolocalized to ciliated airway epithelial cells in saline-treated WT lung (A, original magnification: ×400; B, original magnification: ×630). This EGFR signal was abolished by coincubation with the peptide antigen in a competition assay (C, original magnification: ×400). Results were the same in saline-treated GGTenu1 lung (data not shown). (D–G) Activated, phosphorylated EGF receptor was detectable in the nuclei of airway epithelial cells from IL-13–treated WT lung (G, other three groups D–F; original magnifications: ×400). Large arrow marks nuclei with intense signal compared with surrounding nuclei. Nuclear EGFR signal was abolished by coincubation with the peptide antigen in a competition assay (H). Real-time PCR for EGFR mRNA ratio in IL-13–treated versus corresponding saline-treated control mice showed a significant induction in WT lung (asterisk) but not in GGTenu1 lung or WT lung treated with acivicin.
Expression of Activated EGFR
EGFR is known to translocate to the nucleus when activated (20). EGFR cellular localization was assessed with peptide-specific antisera raised against a phosphorylated domain of EGFR (21, 20). No nuclear signal was detectable in saline-treated GGTenu1 (Figure 3D) or WT (Figure 3F) mice or in IL-13–treated GGTenu1 mice (Figure 3E), but an intense nuclear signal was present in scattered nuclei of airway epithelial cells of IL-13–treated WT mice. This signal predominated in epithelial cells from larger airways (Figure 3G) and was inhibited by coincubation with peptide (Figure 3H).
Induction of EGF Receptor mRNA
EGFR receptor activation is associated with induction of its mRNA. Real-time PCR (Figure 3I) showed that IL-13 treatment did not induce EGFR mRNA in GGTenu1 lung over that of the saline-treated GGTenu1 control (0.65 ± 0.08; n = 4) but significantly induced EGFR mRNA by 1.7- ± 0.2-fold in WT lung compared with its saline-treated control (P = 0.003; n = 4).
GGT Deficiency Prevents IL-13–Induced Airway Hyperreactivity in GGTenu1 Mice
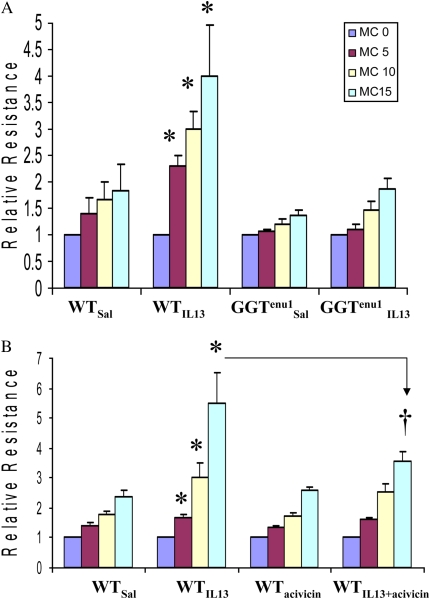
Airway resistance measured during graded exposure to methacholine was significantly higher (asterisks in Figure 4A) in IL-13–treated WT mice (WTIL13, n = 4) at 5 (P = 0.0002), 10 (ANOVA; P = 0.0002), and 15 (P = 0.02) μg/ml of methacholine compared with saline-treated WT (WTsal, n = 5), saline-treated GGTenu1 (GGTenu1SAL, n = 7), and IL-13–treated GGTenu1 (GGTenu1IL13, n = 8) mice (Figure 4A).
Figure 4.
Airway hyperreactivity. (A) Induction of airway hyperreactivity was measured by assaying airway resistance during graded methacholine (MC) exposure. At each methacholine dose, airway resistance was significantly higher (asterisks) in IL-13–treated WT mice compared with saline-treated mice of either genotype and IL-13–treated GGTenu1 mice. (B) Airway resistance measured during graded exposure to methacholine was significantly higher in IL-13–treated WT mice (WTIL13) at all doses compared with saline-treated (WTsal) and acivicin-treated WT mice (WTacivicin) (asterisks). The response at 15 μg/ml was significantly attenuated when IL-13 treatment was combined with acivicin (dagger).
GGT Deficiency Increases Glutathione Content and Decreases Protein Carbonyl Content in Lung Lining Fluid after IL-13
Bronchoalveolar lavage glutathione and protein carbonyls.
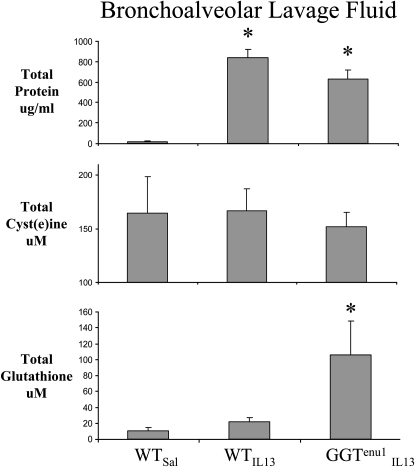
We have previously shown that lung lining fluid glutathione is 2-fold greater in GGTenu1 mice compared with WT control mice (8). Compared with saline-treated WT mice, IL-13 treatment increased protein content in bronchoalveolar lavage (BAL) by 35-fold in mice of each genotype (asterisks in Figure 5) (n = 4; P = 0.0004). Total cysteine content did not differ significantly among the three groups of mice, suggesting that lung cysteine supply was able to meet demand. Compared with saline-treated WT mice, BAL glutathione content did not increase significantly in IL-13–treated WT mice but increased by 10-fold in IL-13–treated GGTenu1 mice (asterisks in Figure 5, n = 4; P = 0.03). Compared with saline-treated WT mice (n = 3), protein carbonyl content increased significantly in IL-13–treated WT mice by 2.7-fold (P = 0.005; n = 7). In contrast, this marker of protein oxidation increased only 1.4-fold in IL-13–treated GGTenu1 mice (n = 4; P = 0.17) (Figure 6) compared with saline-treated GGTenu1 mice (n = 3).
Figure 5.
Total protein, cysteine, and glutathione content in BALF. Compared with saline-treated WT mice, total protein content was elevated 35-fold in IL-13–treated WT and GGTenu1 mice (asterisks). Total nonprotein cysteine content was similar in all three groups. Total glutathione content was significantly elevated 10-fold in IL-13–treated GGTenu1 mice (asterisk).
Figure 6.
Protein carbonyl content in BALF. BAL protein carbonyl content was assayed, and data were normalized to the lowest value of the WT saline-treated control mice (n = 3). This marker of protein oxidation accumulated significantly by 2.7-fold in IL-13–treated WT mice compared with saline-treated WT control mice (asterisk) but not in IL-13–treated GGTenu1 mice compared with GGTenu1 saline-treated control mice.
BAL glutathione-related products.
BALF was examined for accumulation of glutathione-related molecules, including nitrosoglutathione (GSNO), an endogenous bronchodilator, and leukotrienes C4 and E4, bronchoconstrictors induced during inflammation (22). There was no significant accumulation of either in IL-13–treated WT or GGTenu1 mice (GSNO data not shown; leukotriene data shown in Table E2).
Plasma.
IL-13 treatment increased total plasma protein content in both genotypes compared with saline-treated WT control but did not significantly alter the total cysteine content or affect the level of glutathionemia present at baseline in GGTenu1mice (n = 4) (Figure E2).
Inhibition of GGT Activity in Lung Lining Fluid of WT Mice Attenuates EGFR mRNA Induction and Airway Hyperreactivity
Seventy-eighty percent of lung lining fluid GGT activity in WT mice was inactivated for up to 24 hours with intratracheal delivery of the irreversible inhibitor acivicin at 1 to 2.5 −μM (Figure E3). Acivicin treatment significantly attenuated IL-13–induced EGFR mRNA induction (0.99 ± 0.06; n = 3) (see Figure 3I). Airway resistance measured during graded exposure to methacholine was significantly higher in IL-13–treated WT mice (WTIL13; n = 5) at all doses compared with saline-treated (WTsal; n = 6) and acivicin-treated (WTacivicin; n = 10) WT mice (see asterisks in Figure 4), and the response at 15 μg/ml was significantly attenuated when IL-13 treatment was combined with acivicin (ANOVA; P = 0.003; n = 4) (see Figure 4B, dagger).
DISCUSSION
Asthma is characterized by inflammation, and oxidants derived from inflammatory cells contribute to the oxidant-antioxidant imbalance and oxidant stress that are associated with asthma pathogenesis (1). Whether attenuation of oxidant stress alone can affect the phenotype of asthma is unclear (23). Our studies, exposing GGTenu1 mice to IL-13, were designed to explore the selective role of glutathione metabolism and oxidant stress in an experimental model of asthma (9). Glutathione metabolism is regulated by the ectoenzyme GGT. Loss of GGT enzyme activity impairs glutathione metabolism, limits cysteine availability, and causes deficiency of intracellular glutathione pools. This is particularly evident in endothelial cells, alveolar macrophages, and bronchiolar Clara cells in the GGTenu1 lung even in normoxia. Upon exposure to hyperoxia, where metabolism of oxygen increases the level of intracellular reactive oxygen species and induces oxidant stress, these cells are injured more rapidly so that the onset of pulmonary edema, hemorrhage, and death is accelerated in GGTenu1 mice compared with WT mice (8).
We hypothesized that deficiency of cellular glutathione pools would increase oxidant stress and accentuate the asthma phenotype in the GGTenu1 mouse lung after IL-13 treatment. However, our experiments show that the asthma phenotype is attenuated in these mice despite IL-13 delivery, cytokine activation, and inflammatory cell and matrix-associated protein accumulation to levels seen in WT mouse lung. The attenuated asthmatic response occurred specifically in airway epithelial cells as decreased levels of airway mucous cell hyperplasia, mucin protein, and Muc5ac gene induction and EGFR gene induction and receptor activation.
Airway hyperreactivity, one of the hallmarks of asthma, was prevented in the GGTenu1 mice, suggesting that hyperreactivity is linked to oxidant-induced airway epithelial cell signaling rather than airway inflammation and cytokine release. Pharmacologic inhibition of GGT activity in lung lining fluid of WT mice also attenuated IL-13–induced epithelial cell activation and airway hyperreactivity, demonstrating that lung lining fluid GGT activity, rather than other metabolic consequences of the GGTenu1 mutation, prevented IL-13–induced asthma.
GGT regulates extracellular glutathione pools in the plasma (6) and the lung lining fluid (8). In the absence of turnover, the plasma glutathione pool enlarges 5-fold (6, 7) and the lung lining fluid pool 2-fold (8) in the GGT-deficient GGTenu1 mouse compared with WT mice at baseline. This abundance of lung lining fluid glutathione in GGTenu1 mice increased after IL-13 treatment, and the lack of accumulation of protein carbonyls in BALF confirms an augmention of antioxidant buffering capacity against the reactive oxidant species generated by inflammatory cells.
Studies in humans suggest that lung lining fluid glutathione is dynamic in patients with asthma. Total glutathione increases in BALF (24) and in supernatant from induced sputum (25). Extracellular glutathione protects human airway epithelial cells against toxicity from hexamethylene diisocyanate, an in vitro model of isocyanate-induced asthma (26). There is an inverse correlation between BALF glutathione and airway hyperreactivity to methacholine (24). Our results in the GGTenu1 mouse lung support these human data.
We considered that the lack of IL-13–induced airway hyperreactivity in the GGTenu1 mouse lung could involve the accumulation of glutathione-related molecules such as the nitrosothiol GSNO, an endogenous bronchodilator (27). However, we were unable to detect any difference in GSNO content in BALF between IL-13–treated GGTenu1 or WT mice. Enhanced lung lining fluid glutathione content alone could explain our result in the GGTenu1 mouse because its antioxidant activity affects airway tone (28).
Although GGT may be involved in the production of the bronchoconstrictive cysteinyl leukotriene LTD4, recent mouse data show that LTC4 to LTD4 metabolism is mainly regulated by gamma-glutamyl leukotrienase (Ggtla1), a GGT-related enzyme that is encoded by a separate gene (22). Loss of Ggtla1 activity, but not GGT activity, increases airway hyperreactivity as LTC4 accumulates without metabolism. Absence of IL-13–induced airway hyperreactivity in the GGTenu1 mouse lung is unlikely to be related to altered cysteinyl leukotriene metabolism. Our data concur with the hypothesis that the major function of GGT in the mouse is glutathione metabolism.
Our results support a role for enhanced antioxidant activity in the extracellular glutathione pool as a mechanism for attenuation of IL-13–mediated asthma in the GGTenu1 mouse lung. This extracellular pool does not affect the IL-13–induced inflammatory response, which was similar in WT and GGTenu1 mice. Rather, we propose that it protects airway epithelial cells by buffering the oxidizing milieu created by the inflammatory response induced by IL-13. These data directly implicate the epithelial cell in the process that leads to airway hyperreactivity because the absence of EGFR activation is associated with absence of airway hyperreactivity.
Previously glutathione aerosols have be used to alter lung lining fluid glutathione in asthma (29), but these had limited success and induced bronchoconstriction (30). Ingestion of the prodrug N-acetylcysteine failed to elevate lung or lung lining fluid glutathione content (31, 32). Pharmacologic inhibition of lung lining fluid glutathione metabolism seems to be a robust strategy to enhance lung lining fluid glutathione content in the presence of oxidant stress associated with inflammation. A class of γ-phosphono diester glutamate analogues is available as novel GGT inhibitors with greater potency and specificity than acivicin (33). We believe that this mechanism should be studied further as a means of preventing or treating asthma inasmuch as lung lining fluid glutathione has been proposed as an antioxidant shield that protects the airway epithelium (4) and EGFR is a signaling system that is affected by epithelial barrier integrity (34). Although experiments with allergen challenged GGTenu1 mice will be important to extend these results, augmentation of lung lining fluid glutathione by inhibiting metabolism, combined with supplementation of cellular glutathione pools with γ-GCS (3), may be a relevant strategy to protect airway epithelium while modulating immune cell function in asthma and other inflammatory lung diseases, inasmuchas oxidant stress associated with inflammation is emerging as a common factor in the pathogenesis of lung injury (35).
Acknowledgments
The authors thank JunLing Yang, Lillian Cross, and Fengzhi Shao for technical assistance. IL-13 was the generous gift of Wyeth Pharmaceutical, Cambridge, MA, through an MTA with Martin Joyce-Brady.
This work was supported by National Institutes of Health grants DK054787 (R.P.H.) and HL076801 and DE15989 (S.A.) and Program Project PO1 HL47049 (M.J.-B.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2007-0128OC on December 6, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Fujisawa T. Role of oxygen radicals on bronchial asthma. Curr Drug Targets Inflamm Allergy 2005;4:505–509. [DOI] [PubMed] [Google Scholar]
- 2.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 2005;202:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koike Y, Hisada T, Utsugi M, Ishizuka T, Shimizu Y, Ono A, Murata Y, Hamuro J, Mori M, Dobashi K. Glutathione redox regulates airway hyperresponsiveness and airway inflammation in mice. Am J Respir Cell Mol Biol 2007;37:322–329. [DOI] [PubMed] [Google Scholar]
- 4.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high-levels of glutathione. J Appl Physiol 1987;63:152–157. [DOI] [PubMed] [Google Scholar]
- 5.Joyce-Brady M, Takahashi Y, Oakes SM, Rishi AK, Levine RA, Kinlough CL, Hughey RP. Synthesis and release of amphipathic gamma-glutamyl transferase by the pulmonary alveolar type 2 cell: its redistribution throughout the gas exchange portion of the lung indicates a new role for surfactant. J Biol Chem 1994;269:14219–14226. [PubMed] [Google Scholar]
- 6.Harding C, Williams P, Wagner E, Chang DS, Wild K, Colwell RE, Wolff JA. Mice with genetic gamma-glutamyl transpeptidase deficiency exhibit glutathionuria, severe growth failure, reduced life spans, and infertility. J Biol Chem 1997;272:12560–12567. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman M, Wiseman AL, Shi Z, Carter BZ, Barrios R, Ou C, Chevez-Barrios P, Wang Y, Habib GM, Goodman JC, et al. Growth retardation and cysteine deficiency in γ-glutamyl transpeptidase-deficient mice. Proc Natl Acad Sci USA 1996;93:7923–7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jean J, Liu Y, Brown LA, Marc RE, Klings ES, Joyce-Brady M. Gamma-glutamyl transferase deficiency results in lung oxidant stress in normoxia. Am J Physiol Lung Cell Mol Physiol 2002;283:L766–L776. [DOI] [PubMed] [Google Scholar]
- 9.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998;282:2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jean J, Harding CO, Oakes SM, Yu Q, Held PK, Joyce-Brady M. Gamma-glutamyl transferase (GGT) deficiency in the GGTenu1 mouse results from a single point mutation that leads to a stop codon in the first coding exon of GGT mRNA. Mutagenesis 1999;14:31–36. [DOI] [PubMed] [Google Scholar]
- 11.Capraro MA, Hughey RP. Processing of the propeptide form of rat renal gamma-glutamyltranspeptidse. FEBS Lett 1983;157:139–143. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Q, Desta T, Fenton M, Graves DT, Amar S. Cytokine profiling of macrophages exposed to porphyromonas gingivalis, its lipopolysaccharide, or its FimA protein. Infect Immun 2005;73:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortenberry JD, Owens ML, Brown LA. S-nitrosoglutathione enhances neutrophil DNA fragmentation and cell death. Am J Physiol 1999;276:L435–L442. [DOI] [PubMed] [Google Scholar]
- 14.Christensen TG, Lucey EC, Breuer R, Snider GL. Acid-induced secretory cell metaplasia in hamster bronchi. Environ Res 1988;45:78–90. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Olivenstein R, Taha R, Hamid Q, Ludwig M. Enhanced proteoglycan deposition in the airway wall of atopic asthmatics. Am J Respir Crit Care Med 1999;160:725–729. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi A, Morita S, Iwashita H, Sagiya Y, Ashida Y, Shirafuji H, Fujisawa Y, Nishimura O, Fujino M. Role of Gob-5 in mucus overproduction and airway hyperresponsiveness in asthma. Proc Natl Acad Sci USA 2001;98:5175–5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgel PR, Nadel JA. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax 2004;59:992–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casalino-Matsuda SM, Monzon ME, Conner GE, Salathe M, Forteza RM. Role of hyaluronan and reactive oxygen species in tissue kallikrein-mediated epidermal growth factor receptor activation in human airways. J Biol Chem 2004;279:21606–21616. [DOI] [PubMed] [Google Scholar]
- 19.Takeyama K, Dabbaugh K, Shim JJ, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol 2000;164:1546–1552. [DOI] [PubMed] [Google Scholar]
- 20.Lin SY, Makino K, Xia WY, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol 2001;3:802–808. [DOI] [PubMed] [Google Scholar]
- 21.Tyner JW, Kim EY, Ide K, Pelletier MR, Roswit WT, Morton JD, Battaile JT, Patel AC, Patterson GA, Castro M, et al. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest 2006;116:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han B, Luo G, Shi ZZ, Barrios R, Atwood D, Liu W, Habib GM, Sifers RN, Corry DB, Lieberman MW. Gamma-glutamyl leukotrienase, a novel endothelial membrane protein, is specifically responsible for leukotriene D(4) formation in vivo. Am J Pathol 2002;161:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mak JC, Chan-Yeung MM. Reactive oxidant species in asthma. Curr Opin Pulm Med 2006;12:7–11. [DOI] [PubMed] [Google Scholar]
- 24.Smith LJ, Houston M, Anderson J. Increased levels of glutathione in bronchoalveolar lavage fluid from patients with asthma. Am Rev Respir Dis 1993;147:1461–1464. [DOI] [PubMed] [Google Scholar]
- 25.Beier J, Beeh KM, Semmler D, Beike N, Buhl R. Increased concentrations of glutathione in induced sputum of patients with mild or moderate allergic asthma. Ann Allergy Asthma Immunol 2004;92:459–463. [DOI] [PubMed] [Google Scholar]
- 26.Wisnewski AV, Liu Q, Liu J, Redlich CA. Glutathione protects human airway proteins and epithelial cells from isocyanates. Clin Exp Allergy 2005;35:352–357. [DOI] [PubMed] [Google Scholar]
- 27.Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS. Protection from experimental asthma by an endogenous bronchodilator. Science 2005;308:1618–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kloek J, van Ark I, Bloksma N, De Clerck F, Nijkamp FP, Folkerts G. Glutathione and other low-molecular-weight thiols relax guinea pig trachea ex vivo: interactions with nitric oxide? Am J Physiol Lung Cell Mol Physiol 2002;283:403–408. [DOI] [PubMed] [Google Scholar]
- 29.Buhl R, Vogelmeier C, Critenden M, Hubbard RC, Hoyt RF, Wilson EM, Cantin AM, Crystal RG. Augmentation of glutathione in the fluid lining the epithelium of the lower respiratory tract by directly administering glutathione aerosol. Proc Natl Acad Sci USA 1990;87:4063–4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marrades RM, Roca J, Barbera JA, de Jover L, MacNee W, Rodriguez-Roisin R. Nebulized glutathione induces bronchoconstriction in patients with mild asthma. Am J Respir Crit Care Med 1997;156:425–430. [DOI] [PubMed] [Google Scholar]
- 31.Bridgeman MM, Marsden M, Selby C, Morrison D, MacNee W. Effect of N-acetyl cysteine on the concentrations of thiols in plasma, bronchoalveolar lavage fluid, and lung tissue. Thorax 1994;49:670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cotgreave IA, Eklund A, Larsson K, Moldeus PW. No penetration of orally administered N-acetylcysteine into bronchoalveolar lavage fluid. Eur J Respir Dis 1987;70:73–77. [PubMed] [Google Scholar]
- 33.Han L, Hiratake J, Kamiyama A, Sakata K. Design, synthesis, and evaluation of gamma-phosphono diester analogues of glutamate as highly potent inhibitors and active site probes of gamma-glutamyl transpeptidase. Biochemistry 2007;46:1432–1447. [DOI] [PubMed] [Google Scholar]
- 34.Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, Welsh MJ. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 2003;422:322–326. [DOI] [PubMed] [Google Scholar]
- 35.Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal 2006;8:76–87. [DOI] [PubMed] [Google Scholar]