Abstract
Arsenic trioxide (As2O3) is a potent inducer of apoptosis of malignant cells in vitro and in vivo, but the precise mechanisms by which it mediates such effects are not well defined. We provide evidence that As2O3 induces phosphorylation/activation of the MAPK signal-integrating kinases (Mnks) 1 and 2 in leukemia cell lines. Such activation is defective in cells with targeted disruption of the p38α MAPK gene, indicating that it requires upstream engagement of the p38 MAPK pathway. Studies using Mnk1–/– or Mnk2–/–, or double Mnk1–/–Mnk2–/– knock-out cells, establish that activation of Mnk1 and Mnk2 by arsenic trioxide regulates downstream phosphorylation of the eukaryotic initiation factor 4E at Ser-209. Importantly, arsenic-induced apoptosis is enhanced in cells with targeted disruption of the Mnk1 and/or Mnk2 genes, suggesting that these kinases are activated in a negative-feedback regulatory manner, to control generation of arsenic trioxide responses. Consistent with this, pharmacological inhibition of Mnk activity enhances the suppressive effects of arsenic trioxide on primary leukemic progenitors from patients with acute leukemias. Taken together, these findings indicate an important role for Mnk kinases, acting as negative regulators for signals that control generation of arsenic trioxide-dependent apoptosis and antileukemic responses.
The use of arsenic-containing compounds in the treatment of leukemias and other malignancies dates several decades back in time (1). Despite the known existence of arsenic compounds for hundreds of years, only recently has a derivative of this heavy metal, arsenic trioxide (As2O3), found an established role in the treatment of a human disease. Extensive work has now established that As2O3 exhibits potent pro-apoptotic effects against malignant cells and has important antineoplastic activities in vitro and in vivo (2–6). Importantly, As2O3 has been approved for the treatment of acute promyelocytic leukemia (APL)2 in humans, and its introduction in the therapy of this form of acute leukemia has had a major impact in medical oncology (1, 7–10). Notably, APL is a form of leukemia with unusual sensitivity to the effects of arsenic trioxide. It is well established that induction of differentiation of APL cells occurs at low concentrations (0.5 μm), whereas higher (≥2 μm) concentrations are required for the generation of its pro-apoptotic effects in other cell types (2–6). As2O3 is also currently under investigation for the treatment of other hematological malignancies and clonal disorders, including chronic myelogenous leukemia, multiple myeloma, and myelodysplastic syndromes (2, 4, 11–13). A remaining challenge in introducing arsenic trioxide in the treatment of other malignancies is the development of means to enhance arsenic-dependent apoptosis at lower final concentrations. Thus, identification of cellular pathways that could be targeted to enhance the antineoplastic properties of As2O3 are of high translational potential and interest.
Previous work has suggested that the pro-apoptotic effects of As2O3 on APL cells correlate with targeting and degradation of the abnormal PML-RARα fusion protein (5, 14, 15), although independent mechanisms also exist (16). Among the genes regulated by the PML-RARα fusion protein is the mitogen-activated protein kinase (MAPK)-interacting kinase 1 (Mnk1) (17, 18), a kinase that was recently shown to be post-translationally stabilized by PML-RARα fusion protein and participate in the control of differentiation of myeloid cells (19). Mnk1 and the related Mnk2 are known to be activated downstream of MAPKs, via phosphorylation at Thr-197 and Thr-202 located in their activation loop (20–23), and after their activation, they in turn phosphorylate the cap binding eukaryotic initiation factor 4E (eIF4E) at Ser-209 in response to mitogens and stress signals (22, 23).
In previous work, we had demonstrated that the p38 MAPK is activated in response to treatment of leukemic cells with As2O3 (24) and shown that such activation occurs in a negative feedback regulatory manner, to control and limit arsenic-dependent apoptosis. This was established by studies demonstrating that pharmacological inhibitors of p38 promote generation of As2O3-dependent apoptosis (24), whereas pro-apoptotic responses are enhanced in p38α knock-out cells (25). We have been interested in identifying downstream effectors of the p38 MAPK that may be engaged during As2O3 treatment of leukemic cells to counteract its antileukemic effects, because such proteins could be conceivably targeted for the treatment of hematological malignancies and other tumors. In the present study we sought to identify downstream effectors of p38 that may account for the negative regulatory properties of the p38 pathway in the induction of arsenic trioxide responses. Our data demonstrate that the kinases Mnk1 and Mnk2 are activated in an As2O3-inducible manner and regulate downstream phosphorylation of eIF4E. Such engagement of Mnk1 by As2O3 requires upstream activation of the p38 MAPK pathway, as evidenced in studies using p38α knock-out cells. The induction of arsenic trioxide-induced apoptosis is enhanced in cells with targeted disruption of the Mnk1 and/or Mnk2 genes, indicating that activation of Mnk kinases negatively regulates As2O3-dependent apoptosis. Consistent with this, inhibition of Mnk kinase activity or siRNA-mediated knockdown of Mnk1/Mnk2 expression enhances the antileukemic properties of As2O3 on primitive hematopoietic progenitors from patients with acute leukemia in vitro, raising the possibility that targeting Mnk kinases may be an effective approach to enhance the antileukemic properties of As2O3 in vivo.
MATERIALS AND METHODS
Cells and Reagents—The CML-derived K562 cell line, the NB-4 human APL cell line and the U937 human acute myelomonocytic leukemia cell lines were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum and antibiotics. Immortalized mouse embryonic fibroblasts from p38α knock-out mice (26) were kindly provided from Dr. Angel Nebreda (CNIO (Spanish National Cancer Center), Madrid, Spain). Immortalized fibroblasts from Mnk1/Mnk2 double knock-out and Mnk1 and Mnk2 single knock-out mice (27) were grown in Dulbecco's modified Eagle's medium with 10% fetal bovine serum and antibiotics. Arsenic trioxide (As2O3) was purchased from Sigma. Antibodies against the phosphorylated forms of Mnk1 (Thr-197/202), p42/p44 MAPK (Thr-202/Tyr204), p38 MAPK (Thr-180/Tyr-182), and eIF4E (Ser-209) were obtained from Cell Signaling Technology, Inc. (Danvers, MA). Antibodies against eIF4E were also obtained from Cell Signaling Technology, Inc. Antibodies against p38α MAPK and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and from Chemicon International (Temecula, CA), respectively. The Mnk1 inhibitor, CGP57380, the MEK1/2 inhibitor, U0126, and anisomycin were purchased from Calbiochem.
Cell Lysis and Immunoblotting—Cells were treated with the indicated doses of As2O3 for the indicated times and subsequently lysed in the phosphorylation lysis buffer as previously described (28–30). In the experiments in which pharmacological inhibitors were used, the cells were pretreated for 60 min at the indicated final concentrations of inhibitors and subsequently treated for the indicated times with As2O3, in the continuous presence of the inhibitors, prior to cell lysis in phosphorylation lysis buffer. Immunoblotting using an enhanced chemiluminescence (ECL) method was done as previously described (28–30).
Cell Proliferation Assays—Cells were treated with the indicated doses of As2O3, in the presence or absence of CGP57380 (2 μm), for 7 days. Cell proliferation assays using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide method were performed as in our previous studies (31, 32).
Evaluation of Apoptosis—Cells were exposed to the indicated doses of As2O3 for the indicated time period. Flow cytometric assays to evaluate apoptosis by Annexin and propidium iodide staining were done essentially as previously described (33).
Human Hematopoietic Progenitor Cell Assays—Bone marrow or peripheral blood from patients with acute leukemia were collected after obtaining consent approved by the Institutional Review Board of Northwestern University. The effects of arsenic trioxide on the growth of leukemic progenitors were assessed by clonogenic assays in methylcellulose, as in previous studies (24, 31, 33, 34). The cells were cultured in the presence or absence of As2O3 (0.5 μm) in the presence or absence of the indicated concentration of CGP57380 (10 μm). Leukemic CFU-blast (CFU-L) colonies were scored on day 14 of culture.
siRNA-mediated Knockdown of Mnk1/2 in Human Leukemic Cells—For knockdown of Mnk1 and Mnk2 simultaneously, U937 cells were transfected with a mixed pool of pre-designed Mnk1- and Mnk2-specific siRNAs from Dharmacon (Lafayette, CO), using Amaxa Biosystems Nucleofector Kit V (Gaithersburg, MD), whereas the control set of U937 cells was transfected with a non-targeted siRNA pool. Cells were subsequently cultured for 48 h prior following quantification real-time reverse transcription-PCR method using Mnk1/2-specific primers. These studies were performed as previously described (61). The suppressive effects of arsenic trioxide on leukemic progenitor (CFU-L) colony formation from U937 leukemic cells transfected with Mnk1/2-specific siRNAs were assessed by clonogenic assays in methylcellulose (24, 31, 33, 34). The cells were cultured in the presence or absence of As2O3 (0.5 μm) and leukemic CFU-blast (CFU-L) colonies were scored on day 5 of culture.
RESULTS
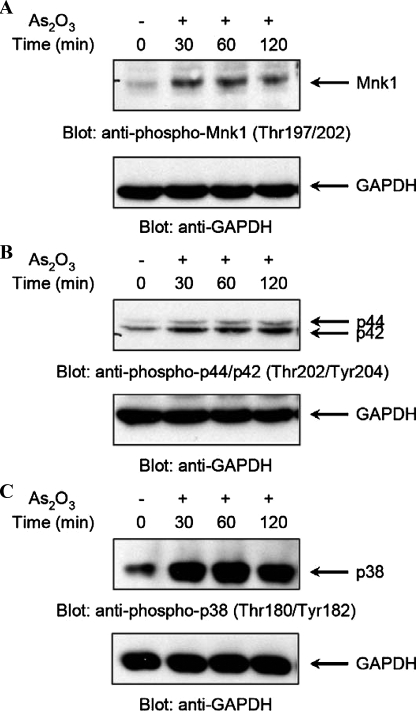
We initially determined whether As2O3 treatment of leukemic cells induces phosphorylation/activation of Mnk1. NB4 cells were treated with arsenic trioxide for different times, ranging from 30 to 120 min, and after cell lysis, total lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form of Mnk1 on Thr-197 and Thr-202. As2O3 treatment triggered phosphorylation of Mnk1 that occurred rapidly, within 30 min, and was still detectable after 120 min of treatment of the cells (Fig. 1A). Such a time pattern of Mnk1 phosphorylation was similar to the patterns seen for arsenic-inducible phosphorylation of MAPKs, including Erk1/Erk2 (Fig. 1B) and p38 (Fig. 1C). Thus, Mnk1 is phosphorylated/activated during treatment of leukemia cells with As2O3, suggesting that it may play a role in the generation of arsenic trioxide responses.
FIGURE 1.
As2O3-dependent phosphorylation of the Mnk1 in NB4 acute promyelocytic leukemia cells. A, NB-4 cells were incubated in the absence or presence of As2O3(2 μm) for the indicated times. Equal amounts of total cell lysates were resolved by SDS-PAGE and immunoblotted with an anti-phospho-Mnk1 (Thr-197/202) antibody (upper panel). The same blot was re-probed with an anti-GAPDH antibody to control for protein loading (lower panel). B, NB-4 cells were incubated in the absence or presence of As2O3(2 μm) for the indicated times. Equal amounts of total cell lysates were resolved by SDS-PAGE and immunoblotted with an anti-phospho-p44/p42 (Thr-202/Tyr-204) antibody (upper panel). The same blot was re-probed with an anti-GAPDH antibody to control for loading (lower panel). C, NB-4 cells were incubated in the absence or presence of As2O3(2 μm) for the indicated times. Equal amounts of total cell lysates were analyzed by SDS-PAGE and immunoblotted with an anti-phospho-p38 (Thr-180/Tyr-182) antibody (upper panel). The same blot was re-probed with an anti-GAPDH antibody to control for loading (lower panel).
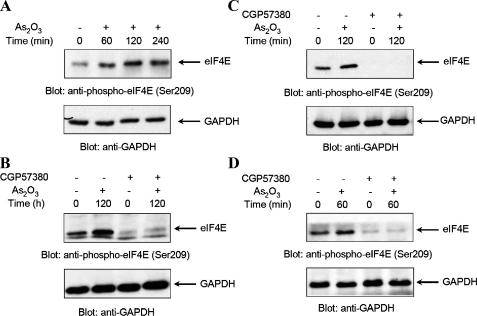
In response to mitogen and diverse cellular stresses, phosphorylated/activated Mnk1, and the related kinase, Mnk2, regulate the phosphorylation of the cap-binding eIF4E at the physiologically relevant site, Ser-209 (22, 23, 35). Consistent with the observed phosphorylation/activation of Mnk1, As2O3 treatment of NB4 cells was found to induce strong phosphorylation of eIF4E (Fig. 2A). In experiments in which cells were pretreated with the specific Mnk kinase inhibitor (36, 37), CGP57380, we found that the As2O3-dependent phosphorylation of eIF4E was blocked (Fig. 2B), indicating that Mnk activity is essential for such phosphorylation. As2O3-dependent inducible phosphorylation of eIF4E was also detected in other hematopoietic cell lines of diverse origin, including the CML-derived K562 cell line (Fig. 2C), and the acute myelomonocytic leukemia-derived U937 cell line (Fig. 2D). As in the case of NB4 cells, cotreatment of either cell line with the Mnk kinase inhibitor, CGP57380, resulted in abrogation of eIF4E phosphorylation (Fig. 2, C and D), establishing a requirement for Mnk activity in As2O3-dependent engagement of eIF4E.
FIGURE 2.
As2O3induces phosphorylation of eIF4E in an Mnk1-dependent manner in leukemia cell lines. A, NB-4 cells were incubated in the absence or presence of As2O3(2 μm) for the indicated times. Equal amounts of total cell lysates were resolved by SDS-PAGE and immunoblotted with an anti-phospho-eIF4E (Ser-209) antibody (upper panel). The same blot was re-probed with an anti-GAPDH antibody to control for protein loading (lower panel). B, NB-4 cells were pre-treated for 60 min with CGP57380 (20 μm), before treatment with As2O3(2 μm) for 120 min, as indicated. Equal amounts of total cell lysates were resolved by SDS-PAGE and immunoblotted with an anti-phospho-eIF4E (Ser-209) antibody (upper panel). The same blot was reprobed with an anti-GAPDH antibody to control for loading (lower panel). C, K562 cells were pre-treated for 60 min with CGP57380 (20 μm), before treatment with As2O3(2 μm) for 120 min, as indicated. Equal amounts of total cell lysates were resolved by SDS-PAGE and immunoblotted with an anti-phospho-eIF4E (Ser-209) antibody (upper panel). The same blot was reprobed with an anti-GAPDH antibody to control for loading (lower panel). D, U937 cells were pre-treated for 60 min with CGP57380 (20 μm), before treatment with As2O3(5 μm) for 60 min, as indicated. Equal amounts of total cell lysates were analyzed by SDS-PAGE and immunoblotted with an anti-phospho-eIF4E (Ser-209) antibody (upper panel). The same blot was re-probed with an anti-GAPDH antibody to control for protein loading (lower panel).
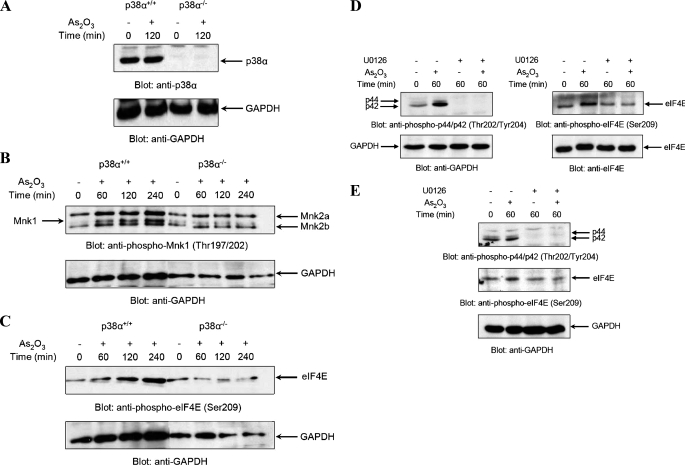
It is well established that phosphorylation of Mnk1 in response to stress signals requires upstream activation of MAPKs (21). As in previous work we had demonstrated that As2O3 induces activation of p38 MAPK (24, 25), we sought to determine if p38 activity is required for activation of Mnk1 in response to arsenic trioxide. To address this, we used immortalized mouse embryonic fibroblasts (MEFs), obtained from mice with targeted deletion of the p38α gene (26). As expected, there was no detection of the predominant p38 isoform (38), p38α, in such knock-out MEFs (Fig. 3A). Subsequently, experiments were performed in which p38α+/+ and p38α–/– MEFs were incubated either in the presence or absence of As2O3 for different times, and after cell lysis, lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form the protein on Thr-197/202. As shown in Fig. 3B, Mnk1 was rapidly phosphorylated in response to arsenic treatment of the parental, p38α+/+, cells (Fig. 3B). In addition, As2O3 treatment induced phosphorylation of the two forms of Mnk2, Mnk2a and Mnk2b, which were expressed in such MEFs (Fig. 3B). On the other hand, As2O3-inducible phosphorylation of both Mnk1 and Mnk2 was defective in the p38α–/– cells, indicating a requirement for p38α MAPK in As2O3-dependent Mnk phosphorylation. Consistent with the suppression of Mnk1/2 phosphorylation/activation in p38 knock-out cells, phosphorylation of eIF4E by As2O3 was completely abrogated in the p38α knock-out MEFs (Fig. 3C). Taken altogether, these studies established that As2O3 phosphorylates/activates both Mnk1 and Mnk2, and that such activation requires upstream engagement of the p38 MAPK pathway.
FIGURE 3.
As2O3-dependent phosphorylation/activation of Mnk1 and Mnk2 and phosphorylation of the eIF4E is MAPK-dependent. A, total cell lysates from As2O3(2 μm)-treated p38α+/+ or p38α–/– MEFs were resolved by SDS-PAGE and immunoblotted with an anti-p38α antibody (upper panel). The same blot was re-probed with an anti-GAPDH antibody to control for loading (lower panel). B, p38α+/+ and p38α–/– MEFs were treated with As2O3(2 μm) for the indicated times. Equal amounts of total cell lysates were analyzed by SDS-PAGE and immunoblotted with an anti-phospho-Mnk1 (Thr197/202) antibody (upper panel). The same blot was reprobed with an anti-GAPDH antibody to control for loading (lower panel). C, p38α+/+ and p38α–/– MEFs were treated with As2O3(2 μm) for the indicated times or with anisomycin (1 μg/ml) as indicated. Equal amounts of total cell lysates were analyzed by SDS-PAGE and immunoblotted with an anti-phospho-eIF4E (Ser-209) antibody (upper panel). The same blot was reprobed with an anti-GAPDH antibody to control for loading (lower panel). D, NB4 cells were pre-treated for 60 min with MEK1/2 inhibitor, U0126 (10 μm), before treatment with As2O3(1 μm) for indicated times. Equal amounts of total cell lysates were resolved by SDS-PAGE and immunoblotted either with an anti-phospho-Erk (Thr-202/Tyr-204) antibody (left side; upper panel) or with an anti-phospho-eIF4E (Ser-209) antibody (right side; upper panel). Blots were then stripped and reprobed with either an anti-GAPDH antibody (left side; lower panel) or with an anti-eIF4E antibody (right side; lower panel) to control for loading. E, U937 cells were pre-treated for 60 min with MEK1/2 inhibitor, U0126 (10 μm), prior to treatment with As2O3(1 μm) for the indicated times, in the continuous presence or absence of the MEK inhibitor. Equal amounts of total cell lysates were resolved by SDS-PAGE, and the upper part of the blot was immunoblotted with an anti-phospho-Erk (Thr-202/Tyr-204) antibody (upper panel), while the lower part of blot was immunoblotted with an anti-phospho-eIF4E (Ser-209) antibody (central panel). The upper part of blot was then stripped and reprobed with an anti-GAPDH antibody to control for loading (lower panel).
Mnk1 and Mnk2 have been previously identified as Erk kinase substrates in other systems (20, 21) and are known to be phosphorylated/activated in vitro by both Erk and p38 in response various stimuli such as growth factors, cellular stresses and inflammatory cytokines (20, 21, 22, 35). Because Erk1/2 are phosphorylated/activated by As2O3, we determined whether Erk kinase activity is required for As2O3-dependent Mnk1/2 activation. To address this, experiments were performed using the specific MEK inhibitor U0126. Pretreatment of cells with U0126 inhibited phosphorylation of both Erk1/2 and eIF4E in both NB4 (Fig. 3D) and U937 cells (Fig. 3E), strongly suggesting that, beyond p38, Erk activity is required for As2O3-dependent engagement of the Mnk/eIF4E pathway.
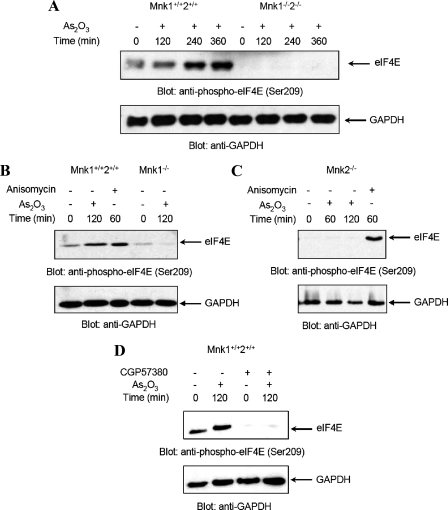
In subsequent studies, we sought to determine the functional relevance of Mnk1 and Mnk2 in the generation of arsenic trioxide responses. For that purpose, we used cells with targeted deletion of either the Mnk1 or the Mnk2 genes alone, or double knock-out cells for both Mnk1 and Mnk2 (27). In initial experiments, wild-type Mnk1+/+Mnk2+/+ MEFs and double knockout Mnk1–/–Mnk2–/– MEFs were treated for different times with As2O3, and the phosphorylation of eIF4E was assessed. Arsenic trioxide treatment induced strong phosphorylation o eIF4E (Fig. 4A) in parental MEFs, but not in Mnk1–/– Mnk2–/– MEFs. Interestingly, such As2O3-inducible phosphorylation of eIF4E was also blocked in either single Mnk1 (Fig. 4B) or Mnk2 (Fig. 4C) knock-out cells, indicating that the functions of both Mnk1 and Mnk2 are essential for eIF4E phosphorylation in response to arsenic trioxide. It should be also noted that pretreatment of Mnk1+/+ Mnk2+/+ MEFs with CGP57380, also resulted in suppression of eIF4E phosphorylation (Fig. 4D).
FIGURE 4.
As2O3-dependent phosphorylation of the eIF4E in Mnk1+/+2+/+, Mnk1–/–2–/–, Mnk1–/–, and Mnk2–/– MEFs. A, Mnk1+/+2+/+ and Mnk1–/–2–/– MEFs were incubated in the absence or presence of As2O3(2 μm) for the indicated times. Equal amounts of total cell lysates were resolved by SDS-PAGE and immunoblotted with an anti-phospho-eIF4E (Ser-209) antibody (upper panel). The blot was then stripped and re-probed with an anti-GAPDH antibody to control for loading (lower panel). B, Mnk1+/+2+/+ and Mnk1–/– MEFs were incubated in the absence or presence of As2O3(2 μm) for indicated times or in the presence of anisomycin (1 μg/ml) for 60 min. Equal amounts of total cell lysates were analyzed by SDS-PAGE and immunoblotted with an anti-phospho-eIF4E (Ser-209) antibody (upper panel). The same blot was reprobed with an anti-GAPDH antibody to control for loading (lower panel). C, Mnk2–/– MEFs were incubated in the absence or presence of As2O3(2 μm) for indicated times or in the presence of anisomycin (1 μg/ml) for 60 min. Equal amounts of total cell lysates were analyzed by SDS-PAGE and immunoblotted with an anti-phospho-eIF4E (Ser-209) antibody (upper panel). The same blot was then stripped and reprobed with an anti-GAPDH antibody to control for loading (lower panel). D, Mnk1+/+2+/+ MEFs were pre-treated for 60 min with CGP57380 (20 μm), before treatment with As2O3(2 μm) for 120 min as indicated. Equal amounts of total cell lysates were analyzed by SDS-PAGE and immunoblotted with an anti-phospho-eIF4E (Ser-209) antibody (upper panel). The same blot was reprobed with an anti-GAPDH antibody to control for loading (lower panel).
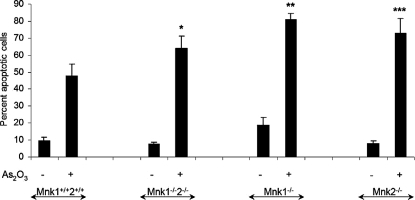
In subsequent studies, we sought to determine the functional role of Mnk1 and Mnk2 in the generation of As2O3-mediated apoptosis. When the induction of apoptosis by arsenic trioxide was determined in immortalized MEFs with targeted disruption of the Mnk1 and/or Mnk2 genes, we found enhanced As2O3-induced apoptosis in cells lacking either Mnk1, or Mnk2 and in double knock-out MEFs for both Mnk1 and Mnk2, as compared with the parental cells (Fig. 5). Interestingly, there was slightly more apoptosis in the single Mnk1 knock-out MEFs as compared with the single Mnk2 knockouts, or the double Mnk1/Mnk2 knockouts, but the generation of arsenic-dependent apoptosis was enhanced in all of them when compared with wild-type MEFs. Paired t test analysis of Mnk1–/–2–/–, Mnk1–/–, and Mnk2–/– cells compared with Mnk1+/+2+/+ cells displayed p values of 0.0192, 0.0078, and 0.0037, respectively (Fig. 5), firmly establishing that both Mnk1 and Mnk2 play essential roles in the generation of anti-apoptotic signals in response to As2O3.
FIGURE 5.
Targeted disruption of the Mnk1 and Mnk2 genes potentiates As2O3-induced apoptosis. Mnk1+/+2+/+, Mnk1–/–2–/–, Mnk1–/–, and Mnk2–/– MEFs were incubated in the absence or presence of As2O3(10 μm) for 48 h. The percentage of apoptotic cells was determined by flow cytometric analysis for annexin V/propidium iodide staining. Data are expressed as means ± S.E. of six independent experiments. Paired t test analysis of Mnk1–/–2–/–, Mnk1–/–, and Mnk2–/– MEFs compared with Mnk1+/+2+/+ MEFs (columns marked with asterisks), showed p = 0.0192, p = 0.0078, and p = 0.0037, respectively.
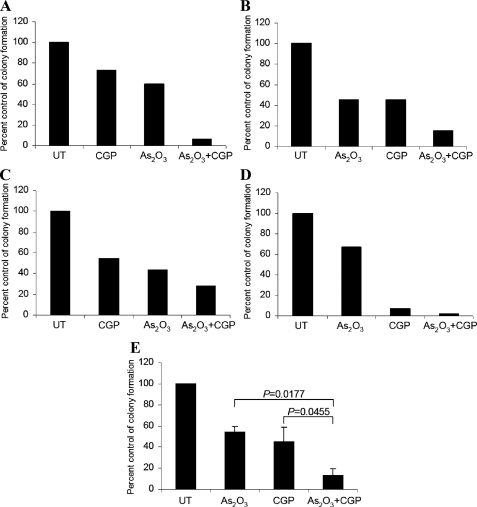
To further explore the role of Mnk kinases in the generation of the effects of As2O3 in a more physiologically relevant system, we examined the effects of pharmacological inhibition of Mnk1 and Mnk2 in the induction of the suppressive effects of As2O3 on primary leukemic progenitors from patients with AML. Bone marrow or peripheral blood from different AML patients were exposed to As2O3 in the absence or presence of the pharmacological Mnk inhibitor, CGP57380, and leukemic progenitor colony formation (CFU-L) was assessed by clonogenic assays in methylcellulose. Addition of As2O3 to the cultures suppressed leukemic progenitor colony formation in all different cases (Fig. 6, A–D). Interestingly, the Mnk inhibitor CGP58370 also suppressed leukemic colony formation to a similar degree (Fig. 6). However, concomitant addition of CGP58370 and As2O3 resulted in strong synergistic effects, as shown by paired t test analysis (p = 0.0177 for the combination of As2O3 plus CGP57380 versus As2O3 alone; and p = 0.0455 versus CGP57380 alone) (Fig. 6E).
FIGURE 6.
Pharmacological inhibition of Mnk1 promotes As2O3-induced growth suppression of CFU-L colony formation from AML patients and promotes sensitivity of leukemic blasts at very low concentrations of As2O3. A–D, bone marrow or peripheral blood mononuclear cells from four AML patients were plated in a methylcellulose assay system with As2O3(0.5 μm), in the presence or absence of CGP57380 (10 μm). The data are expressed as percent control of CFU-L colony numbers for untreated cells. E, means ± S.E. of the values from the experiments using different patient samples (A–D) are shown. Paired t test analysis showed p = 0.0177 for the combination of CGP57380 and As2O3versus As2O3alone and p = 0.0455 for the combination of CGP57380 and As2O3versus CGP57380 alone.
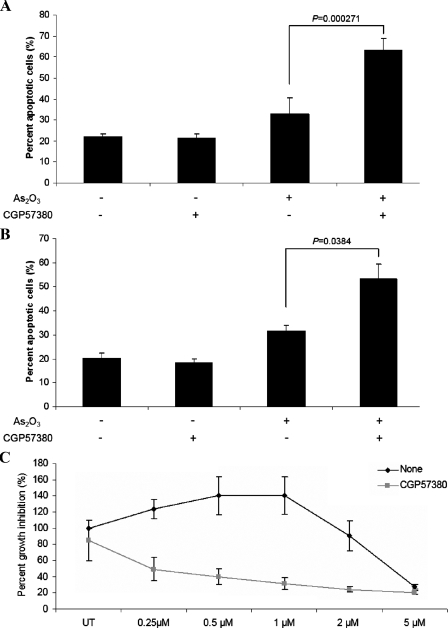
To better understand the mechanisms of enhanced generation of the antileukemic effects by the combination of As2O3 with CGP58370, the effects of such combination on the generation of As2O3-dependent apoptosis in different leukemic cell lines were assessed. As expected, treatment of U937 (Fig. 7A) or K562 (Fig. 7B) cells with arsenic trioxide resulted in induction of apoptosis, but concomitant addition of the Mnk inhibitor, CGP58370, led to enhanced apoptosis that was significant (paired p value = 0.000271 for U937 and paired p value = 0.0384 for K562 cells) (Fig. 7, A and B). We also performed dose-response experiments, to determine whether pharmacological inhibition of the kinases Mnk1 and Mnk2 promotes generation of antileukemic effects in response to As2O3 on AML cells, at low concentrations that normally do not result in such effects. Due to limitations in availability of primary leukemic progenitors, such studies were also performed using U937 acute myelomonocytic leukemia cells. As shown in Fig. 7C arsenic trioxide treatment did not result in any significant growth inhibitory effects on such cells when used at concentrations <2 μm. However, concomitant addition of CGP57380 dramatically enhanced arsenic-dependent growth suppression, resulting in substantial growth inhibition at the very low concentration of 0.25 μm (Fig. 7C). Thus, pharmacological inhibition of Mnk kinases strongly enhances the antileukemic properties of As2O3 and results in the generation of antileukemic responses at very low concentrations of As2O3.
FIGURE 7.
Pharmacological inhibition of Mnk1 promotes As2O3-induced apoptosis and growth suppression of human leukemic cell lines. A, U937 cells were pre-treated for 60 min with CGP57380 (20 μm) and then incubated in the absence or presence of As2O3(2 μm) for 96 h. The percentage of apoptotic cells was determined by flow cytometric analysis for annexin V/propidium iodide staining. Data are expressed as means ± S.E. of five independent experiments. Paired t test analysis showed p = 0.000271 for the combination of CGP57380 and As2O3versus As2O3alone. B, K562 cells were pre-treated for 60 min with CGP57380 (20 μm) and then incubated in the absence or presence of As2O3(2.5 μm) for 96 h. The percentage of apoptotic cells was determined by flow cytometric analysis for annexin V/propidium iodide staining. Data are expressed as means ± S.E. of three independent experiments. Paired t test analysis showed p = 0.0384 for the combination of CGP57380 and As2O3versus As2O3alone. C, U937 cells were incubated for 7 days in the presence or absence of the indicated doses of As2O3, in the presence or absence of CGP57380 (2 μm). Cell proliferation was assessed by using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Data are expressed as means ± S.E. of five independent experiments.
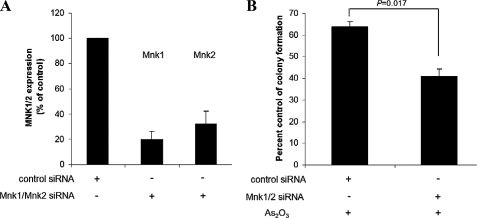
To directly assess the functional relevance of Mnk1 and Mnk2 in the generation of antileukemic effects of As2O3, we determined whether siRNA-mediated knock-down of Mnk1 and Mnk2 enhances the suppressive effects of As2O3 on leukemic progenitor (CFU-L) colony formation. U937 cells were transfected with either nonspecific siRNA or siRNAs specific targeting Mnk1 and Mnk2 (Fig. 8A). The cells were exposed to As2O3, and CFU-L colony formation was assessed in clonogenic assays in methylcellulose. As2O3-dependent suppression of CFU-L colony formation was strongly enhanced in cells transfected with Mnk1/Mnk2 siRNAs over controls (paired p = 0.017) (Fig. 8B), firmly establishing that the Mnk pathway plays a critical regulatory role in the generation of the effects of As2O3 on leukemic progenitors.
FIGURE 8.
Knockdown of Mnk kinases enhances the suppressive effects of As2O3 on leukemic progenitor (CFU-L) growth. A, U937 cells were transfected with control siRNA or a mixture of Mnk1- and Mnk2-specific siRNAs. 48 h after transfection, expression of mRNAs for Mnk1 and Mnk2 genes was evaluated by quantitative real-time reverse transcription-PCR using GAPDH gene for normalization. Data are expressed as -fold increase over control samples and represent means ± S.E. of two independent experiments. B, U937 cells were transfected with control siRNA or a mixture of Mnk1- and Mnk2-specific siRNAs and were subsequently incubated in methylcellulose in the presence or absence of As2O3(0.5 μm), and leukemic CFU-L colony formation was assessed. Data are expressed as percent control colony formation of untreated samples and represent means ± S.E. of three independent experiments are shown. Paired t test analysis comparing the effects of As2O3 in the presence or absence of Mnk1/Mnk2 siRNAs showed a paired p value = 0.017.
DISCUSSION
It is well established that arsenic trioxide induces apoptosis and suppresses the growth of different types of neoplastic cells in vitro and in vivo (1–6, 39). Despite that, the precise up-stream signals that control induction of programmed cell death by As2O3 remain to be elucidated. Previous work has demonstrated that decreasing the mitochondrial membrane potential may be a key mechanism by which arsenic trioxide induces its pro-apoptotic effects (11–13). Such an As2O3-dependent decrease of mitochondrial membrane potential results in the release of cytochrome c and activation of caspases, which ultimately lead to apoptotic cell death (1–6, 11–13, 40–42). Other work has established that activation of the JNK MAPK pathway is essential for As2O3-dependent apoptosis (43), whereas inhibition of transcriptional NF-κB activity is also involved in the generation of arsenic trioxide responses (44–46). It should be also noted that the generation of reactive oxygen species depends on cellular glutathione stores (47) and that ascorbic acid (48) or buthionine sulfoximine (49) enhance the pro-apoptotic effects of As2O3.
Despite the potent pro-apoptotic and antitumor effects of arsenic trioxide, it is well documented that malignant cells develop resistance to its effects in vitro and in vivo (1–6), but the precise cellular mechanisms responsible for such resistance remain unknown. In previous work, we demonstrated that the p38 MAPK (24) and its upstream effectors Mkk3 and Mkk6 (25) are activated during treatment of different cell types with As2O3, whereas pharmacological or molecular targeting of theses kinase was found to enhance As2O3-mediated apoptosis and antiproliferative responses (24, 25). These findings have strongly suggested that this MAPK cascade may be negatively regulating generation of As2O3 responses, likely as a component of a negative feedback regulatory loop. The identification of the downstream effector signaling elements that mediate induction of anti-apoptotic responses is therefore of considerable interest, as it may lead to more potent and specific approaches to target anti-apoptotic mechanisms and promote generation of As2O3-dependent antitumor activities.
In the present study, we provide the first evidence that Mnk1 and Mnk2 are activated during treatment of cells with arsenic trioxide. Our data establish that such activation is rapid, occurring within 30 min of treatment of cells, and results in downstream phosphorylation of eIF4E on serine 209. We also demonstrate that activation of Mnk kinases/downstream eIF4E phosphorylation in response to arsenic trioxide is defective in cells with targeted disruption of the p38α MAPK gene and is blocked by pharmacological inhibition of the MEK/Erk pathway, establishing that it requires upstream engagement of the p38 and Erk MAPK pathways. In studies using knock-out cells for Mnk1, Mnk2, or both, we found that the absence of these kinases results in increased generation of As2O3-dependent pro-apoptotic signals and programmed cell death. Consistent with this, our data demonstrate that inhibition of Mnk1/Mnk2 activity in primitive progenitors from patients with AML results in enhanced suppression of leukemic growth and arsenic-dependent apoptosis. Similarly, siRNA targeting of Mnk kinases was also found to enhance the suppressive effects of arsenic trioxide on U937-derived leukemic progenitor colony formation. These findings indicate that Mnk1/Mnk2 are critical regulators of the biological effects of arsenic trioxide and, along the kinase Msk1 (33), may be the key signaling elements downstream of MAPKs that block induction of antileukemic responses.
Mnk1 and Mnk2 are serine threonine kinases that regulate phosphorylation of eIF4E on serine 209 (27), a site important for the activation of the protein (50). Previous work has established that engagement of these kinases in growth factor signaling is MAPK-dependent and regulated by the p38 and/or the Mek/Erk kinase cascades (20, 21). The ability of Mnks to phosphorylate eIF4E may be of importance in the regulation of mRNA translation, as it has been previously demonstrated that eIF4E phosphorylation is relevant to cap-dependent translation and protein synthesis in different cellular systems (51–55). Consistent with this, recent studies have established that Mnk1-mediated phosphorylation of eIF4E occurs in response to oxidants (56). In addition, there is evidence that such phosphorylation is associated with enhanced herpes simplex virus-1 mRNA translation and replication (56) and tumor necrosis factor-α biosynthesis (57). There is also evidence that Mnks phosphorylate AU-rich elementbinding proteins such as hnRNP A1, resulting in decreased binding to tumor necrosis factor-α AU-rich element in vitro and tumor necrosis factor-α mRNA in vivo (57). On the other hand, there have been studies demonstrating that, under certain circumstances, Mnks may negatively regulate protein translation (37) or that Mnk-mediated phosphorylation in response to oxidant stress negatively regulates global protein synthesis (58). Notably, the functions of Mnk1 and Mnk2 may be regulated by a novel Mnk-specific regulatory mechanism, involving autoinhibition by a reprogrammed activation segment (59).
Despite the significant advances on the mechanisms of activation and function of Mnks, the precise roles of members of this family of kinases in various physiological and pathophysiological processes remains to be established. Our studies suggest a novel function for Mnk1 and Mnk2, acting as negative regulators of arsenic trioxide-dependent apoptosis. Such a function of Mnks appears to be also important for the generation of the antileukemic effects of arsenic trioxide, as shown by our studies with primitive leukemic precursors enriched from the bone marrows or peripheral blood of patients with acute leukemia. Interestingly, a recent study demonstrated that loss of Mnk function sensitizes fibroblasts to serum withdrawal-induced apoptosis (60). The results of this study, taken together with our findings, suggest that Mnk1 and Mnk2 may be important elements in the induction of anti-apoptotic responses, possibly via regulation of translation of anti-apoptotic genes. Although the identities of anti-apoptotic proteins whose expression may be regulated by Mnk kinases remain to be defined in future studies, our data raise the possibility that targeting of Mnks may be a way to enhance antileukemic responses and reverse the resistance that malignant cells develop to the effects of arsenic trioxide, and possibly other antitumor agents.
This work was supported by National Institutes of Health Grants CA121192, CA77816, and CA100579 (to L. C. P.) and a merit review grant by the Dept. of Veterans Affairs (to L. C. P.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: APL, acute promyelocytic leukemia; MAPK, mitogen-activated protein kinase; Mnk1, MAPK-interacting kinase 1; eIF4E, eukaryotic initiation factor 4E; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; CFU-L, leukemic CFU-blast; siRNA, small interference RNA; MEF, mouse embryonic fibroblast; Mek, MAPK/Erk kinase; Erk, extracellular signal-regulated kinase.
References
- 1.Kandel, E. V., and Leroy, G. V. (1937) Arch. Intern. Med. 60 846–866 [Google Scholar]
- 2.Douer, D., and Tallman, M. S. (2005) J. Clin. Oncol. 23 2396–2410 [DOI] [PubMed] [Google Scholar]
- 3.Miller, W. H., Schipper, H. M., Lee, J. S., Singer, J., and Waxman, S. (2002) Cancer Res. 62 3893–3903 [PubMed] [Google Scholar]
- 4.Chen, Z., Chen, G. Q., Shen, Z. X., Sun, G. L., Tong, J. H., Wang, Z. Y., and Chen, S. J. (2002) Semin. Hematol. 39 22–26 [DOI] [PubMed] [Google Scholar]
- 5.Tallman, M. S., Nabhan, C., Feusner, J. H., and Rowe, J. M. (2002) Blood 99 759–767 [DOI] [PubMed] [Google Scholar]
- 6.Tallman, M. S. (2001) Blood Rev. 15 133–142 [DOI] [PubMed] [Google Scholar]
- 7.Sun, H. D., Ma, L., Hu, X.-C., and Zhang, T.-D. (1992) Chin. J. Integr. Chin. West Med. 12 170–172 [Google Scholar]
- 8.Shen, Z. X., Chen, G. Q., Ni, J. H., Li, X. S., Xiong, S. M., Qiu, Q. Y., Zhu, J., Tang, W., Sun, G. L., Yang, K. Q., Chen, Y., Zhou, L., Fang, Z. W., Wang, Y. T., Ma, J., Zhang, P., Zhang, T. D., Chen, S. J., Chen, Z., and Wang, Z. Y. (1997) Blood 89 3354–3360 [PubMed] [Google Scholar]
- 9.Niu, C., Yan, H., Yu, T., Sun, H. P., Liu, J. X., Li, X. S., Wu, W., Zhang, F. Q., Chen, Y., Zhou, L., Li, J. M., Zeng, X. Y., Yang, R. R., Yuan, M. M., Ren, M. Y., Gu, F. Y., Cao, Q., Gu, B. W., Su, X. Y., Chen, G. Q., Xiong, S. M., Zhang, T., Waxman, S., Wang, Z. Y., and Chen, S. J. (1999) Blood 94 3315–3324 [PubMed] [Google Scholar]
- 10.Soignet, S. L., Frankel, S. R., Douer, D., Tallman, M. S., Kantarjian, H., Calleja, E., Stone, R. M., Kalaycio, M., Scheinberg, D. A., Steinherz, P., Sievers, E. L., Coutre, S., Dahlberg, S., Ellison, R., and Warrell, R. P., Jr. (2001) J. Clin. Oncol. 19 3852–3860 [DOI] [PubMed] [Google Scholar]
- 11.Novick, S. C., and Warrell, R. P., Jr. (2000) Semin. Oncol. 27 495–501 [PubMed] [Google Scholar]
- 12.List, A., Beran, M., DiPersio, J., Slack, J., Vey, N., Rosenfeld, C. S., and Greenberg, P. (2003) Leukemia 17 1499–1507 [DOI] [PubMed] [Google Scholar]
- 13.Evens, A. M., Tallman, M. S., and Gartenhaus, R. B. (2004) Leuk. Res. 28 891–900 [DOI] [PubMed] [Google Scholar]
- 14.Chen, G. Q., Zhu, J., Shi, X. G., Ni, J. H., Zhong, H. J., Si, G. Y., Jin, X. L., Tang, W., Li, X. S., Xong, S. M., Shen, Z. X., Sun, G. L., Ma, J., Zhang, P., Zhang, T. D., Gazin, C., Naoe, T., Chen, S. J., Wang, Z. Y., and Chen, Z. (1996) Blood 88 1052–1061 [PubMed] [Google Scholar]
- 15.Shen, Y., Shen, Z. X., Yan, H., Chen, J., Zeng, X. Y., and Li, J. M. (2001) Leukemia 15 735–741 [DOI] [PubMed] [Google Scholar]
- 16.Nervi, C., Ferrara, F. F., Fanelli, M., Rippo, M. R., Tomassini, B., Ferrucci, P. F., Ruthardt, M., Gelmetti, V., Gambacorti-Passerini, C., Diverio, D., Grignani, F., Pelicci, P. G., and Testi, R. (1998) Blood 92 2244–2251 [PubMed] [Google Scholar]
- 17.Muller-Tidow, C., Schwable, J., Steffen, B., Tidow, N., Brandt, B., Becker, K., Schulze-Bahr, E., Halfter, H., Vogt, U., Metzger, R., Schneider, P. M., Buchner, T., Brandts, C., Berdel, W. E., and Serve, H. (2004) Clin. Cancer Res. 10 1241–1249 [DOI] [PubMed] [Google Scholar]
- 18.Muller-Tidow, C., Steffen, B., Cauvet, T., Tickenbrock, L., Ji, P., Diederichs, S., Sargin, B., Kohler, G., Stelljes, M., Puccetti, E., Ruthardt, M., deVos, S., Hiebert, S. W., Koeffler, H. P., Berdel, W. E., and Serve, H. (2004) Mol. Cell. Biol. 24 2890–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Worch, J., Tickenbrock, L., Schwäble, J., Steffen, B., Cauvet, T., Mlody, B., Buerger, H., Koeffler, H. P., Berdel, W. E., Serve, H., and Müller-Tidow, C. (2004) Oncogene 23 9162–9172 [DOI] [PubMed] [Google Scholar]
- 20.Fukunaga, R., and Hunter, T. (1997) EMBO J. 16 1921–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waskiewicz, A. J., Flynn, A., Proud, C. G., and Cooper, J. A. (1997) EMBO J. 16 1909–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheper, G. C., Morrice, N. A., Kleijn, M., and Proud, C. G. (2001) Mol. Cell. Biol. 21 743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waskiewicz, A. J., Johnson, J. C., Penn, B., Mahalingam, M., Kimball, S. R., and Cooper, J. A. (1999) Mol. Cell. Biol. 19 1871–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma, A., Mohindru, M., Deb, D. K., Sassano, A., Kambhampati, S., Ravandi, F., Minucci, S., Kalvakolanu, D. V., and Platanias, L. C. (2002) J. Biol. Chem. 277 44988–44995 [DOI] [PubMed] [Google Scholar]
- 25.Giafis, N., Katsoulidis, E., Sassano, A., Tallman, M. S., Higgins, L. S., Nebreda, A. R., Davis, R. J., and Platanias, L. C. (2006) Cancer Res. 66 6763–6771 [DOI] [PubMed] [Google Scholar]
- 26.Adams, R. H., Porras, A., Alonso, G., Jones, M., Vintersten, K., Panelli, S., Valladares, A., Perez, L., Klein, R., and Nebreda, A. R. (2000) Mol. Cell 6 109–116 [PubMed] [Google Scholar]
- 27.Ueda, T., Watanabe-Fukunaga, R., Fukuyama, H., Nagata, S., and Fukunaga, R. (2004) Mol. Cell. Biol. 24 6539–6549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uddin, S., Yenush, L., Sun, X. J., Sweet, M. E., White, M. F., and Platanias, L. C. (1995) J. Biol. Chem. 270 15938–15941 [DOI] [PubMed] [Google Scholar]
- 29.Ahmad, S., Alsayed, Y., Druke, B. J., and Platanias, L. C. (1997) J. Biol. Chem. 272 29991–29994 [DOI] [PubMed] [Google Scholar]
- 30.Verma, A., Deb, D. K., Sassano, A., Uddin, S., Varga, J., Wickrema, A., and Platanias, L. C. (2002) J. Biol. Chem. 277 7726–7735 [DOI] [PubMed] [Google Scholar]
- 31.Mayer, I. A., Verma, A., Grumbach, I. M., Uddin, S., Lekmine, F., Ravandi, F., Majchrzak, B., Fujita, S., Fish, E. N., and Platanias, L. C. (2001) J. Biol. Chem. 276 28570–28577 [DOI] [PubMed] [Google Scholar]
- 32.Uddin, S., Fish, E. N., Sher, D., Gardziola, C., Colamonici, O. R., Kellum, M., Pitha, P. M., White, M. F., and Platanias, L. C. (1997) Blood 90 2574–2582 [PubMed] [Google Scholar]
- 33.Kannan-Thulasiraman, P., Katsoulidis, E., Tallman, M. S., Arthur, J. S., and Platanias, L. C. (2006) J. Biol. Chem. 281 22446–22452 [DOI] [PubMed] [Google Scholar]
- 34.Yoon, P., Giafis, N., Smith, J., Mears, H., Katsoulidis, E., Sassano, A., Altman, J., Redig, A. J., Tallman, M. S., and Platanias, L. C. (2006) Mol. Cancer Ther. 5 2815–2823 [DOI] [PubMed] [Google Scholar]
- 35.Wang, X., Flynn, A., Waskiewicz, A. J., Webb, B. L., Vries, R. G., Baines, I. A., Cooper, J. A., and Proud, C. G. (1998) J. Biol. Chem. 273 9373–9377 [DOI] [PubMed] [Google Scholar]
- 36.Tschopp, C., Knauf, U., Brauchle, M., Zurini, M., Ramage, P., Glueck, D., New, L., Han, J., and Gram, H. (2003) Mol. Cell. Biol. Res. Commun. 3 205–211 [DOI] [PubMed] [Google Scholar]
- 37.Knauf, U., Tschopp, C., and Gram, H. (2001) Mol. Cell. Biol. 21 5500–5511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Platanias, L. C. (2003) Pharmacol. Ther. 98 129–142 [DOI] [PubMed] [Google Scholar]
- 39.Jing, Y., and Waxman, S. (2007) Curr. Top. Microbiol. Immunol. 313 245–269 [DOI] [PubMed] [Google Scholar]
- 40.Davison, K., Mann, K. K., and Miller, W. H., Jr. (2002) Semin. Hematol. 39 3–7 [DOI] [PubMed] [Google Scholar]
- 41.Akao, Y., Mizoguchi, H., Kojima, S., Naoe, T., Ohishi, N., and Yagi, K. (1998) Br. J. Haematol. 102 1055–1060 [DOI] [PubMed] [Google Scholar]
- 42.Jing, Y., Dai, J., Chalmers-Redman, R. M., Tatton, W. G., and Waxman, S. (1999) Blood 94 2102–2111 [PubMed] [Google Scholar]
- 43.Davison, K., Mann, K. K., Waxman, S., and Miller, W. H., Jr. (2004) Blood 103 3496–3502 [DOI] [PubMed] [Google Scholar]
- 44.Mathas, S., Lietz, A., Janz, M., Hinz, M., Jundt, F., Scheidereit, C., Bommert, K., and Dorken, B. (2003) Blood 102 1028–1034 [DOI] [PubMed] [Google Scholar]
- 45.Kerbauy, D. M., Lesnikov, V., Abbasi, N., Seal, S., Scott, B., and Deeg, H. J. (2005) Blood 106 3917–3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei, L. H., Lai, K. P., Chen, C. A., Cheng, C. H., Huang, Y. J., Chou, C. H., Kuo, M. L., and Hsieh, C. Y. (2005) Oncogene 24 390–398 [DOI] [PubMed] [Google Scholar]
- 47.Grad, J. M., Bahlis, N. J., Reis, I., Oshiro, M. M., Dalton, W. S., and Boise, L. H. (2001) Blood 98 805–813 [DOI] [PubMed] [Google Scholar]
- 48.Gartenhaus, R. B., Prachand, S. N., Paniaqua, M., Li, Y., and Gordon, L. I. (2002) Clin. Cancer Res. 8 566–572 [PubMed] [Google Scholar]
- 49.Ramos, A. M., Fernandez, C., Amran, D., Sancho, P., de Blas, E., and Aller, P. (2005) Blood 105 4013–4020 [DOI] [PubMed] [Google Scholar]
- 50.Flynn, A., and Proud, C. G. (1995) J. Biol. Chem. 270 21684–21688 [DOI] [PubMed] [Google Scholar]
- 51.Reiling, J. H., Doepfner, K. T., Hafen, E., and Stocker, H. (2005) Curr. Biol. 15 24–30 [DOI] [PubMed] [Google Scholar]
- 52.Bonneau, A. M., and Sonenberg, N. (1987) J. Biol. Chem. 262 11134–11139 [PubMed] [Google Scholar]
- 53.Beretta, L., Singer, N. G., Hinderer, R., Gingras, A. C., Richardson, B., Hanash, S. M., and Sonenberg, N. (1998) J. Immunol. 160 3269–3273 [PubMed] [Google Scholar]
- 54.Pyronnet, S., Dostie, J., and Sonenberg, N. (2001) Genes Dev. 15 2083–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takei, N., Kawamura, M., Hara, K., Yonezawa, K., and Nawa, H. (2001) J. Biol. Chem. 276 42818–42825 [DOI] [PubMed] [Google Scholar]
- 56.Walsh, D., and Mohr, I. (2004) Genes Dev. 18 660–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buxadé, M., Parra, J. L., Rousseau, S., Shpiro, N., Marquez, R., Morrice, N., Bain, J., Espel, E., and Proud, C. G. (2005) Immunity 23 177–189 [DOI] [PubMed] [Google Scholar]
- 58.Shenberger, J. S., Zhang, L., Hughlock, M. K., Ueda, T., Watanabe-Fukunaga, R., and Fukunaga, R. (2007) Int. J. Biochem. Cell Biol. 39 1828–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jauch, R., Cho, M. K., Jäkel, S., Netter, C., Schreiter, K., Aicher, B., Zweckstetter, M., Jäckle, H., and Wahl, M. C. (2006) EMBO J. 25 4020–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chrestensen, C. A., Eschenroeder, A., Ross, W. G., Ueda, T., Watanabe-Fukunaga, R., Fukunaga, R., and Sturgill, T. W. (2007) Genes Cells 12 1133–1140 [DOI] [PubMed] [Google Scholar]
- 61.Kaur, S., Lal, L., Sassano, A., Majchrzak-Kita, B., Srikanth, M., Baker, D. P., Petroulakis, E., Hay, N., Sonenberg, N., Fish, E. N., and Platanias, L. C. (2007) J. Biol. Chem. 282 1757–1768 [DOI] [PubMed] [Google Scholar]