Abstract
Perioperative factors including hypoxia, hypocapnia, and certain anesthetics have been suggested to contribute to Alzheimer disease (AD) neuropathogenesis. Desflurane is one of the most commonly used inhalation anesthetics. However, the effects of desflurane on AD neuropathogenesis have not been previously determined. Here, we set out to assess the effects of desflurane and hypoxia on caspase activation, amyloid precursor protein (APP) processing, and amyloid β-protein (Aβ) generation in H4 human neuroglioma cells (H4 naïve cells) as well as those overexpressing APP (H4-APP cells). Neither 12% desflurane nor hypoxia (18% O2) alone affected caspase-3 activation, APP processing, and Aβ generation. However, treatment with a combination of 12% desflurane and hypoxia (18% O2) (desflurane/hypoxia) for 6 h induced caspase-3 activation, altered APP processing, and increased Aβ generation in H4-APP cells. Desflurane/hypoxia also increased levels of β-site APP-cleaving enzyme in H4-APP cells. In addition, desflurane/hypoxia-induced Aβ generation could be reduced by the broad caspase inhibitor benzyloxycarbonyl-VAD. Finally, the Aβ aggregation inhibitor clioquinol and γ-secretase inhibitor L-685,458 attenuated caspase-3 activation induced by desflurane/hypoxia. In summary, desflurane can induce Aβ production and caspase activation, but only in the presence of hypoxia. Pending in vivo confirmation, these data may have profound implications for anesthesia care in elderly patients, and especially those with AD.
An estimated 200 million patients worldwide undergo surgery each year. Several reports have suggested that anesthesia and surgery may facilitate development of Alzheimer disease (AD)4 (1–3). A recent study also reported that patients having coronary artery bypass graft surgery under general anesthesia are at increased risk for AD as compared with those having percutaneous transluminal coronary angioplasty under local anesthesia (4).
Genetic evidence, confirmed by neuropathological and biochemical findings, indicates that excessive production and/or accumulation of amyloid β-protein (Aβ) play a fundamental role in the pathology of AD (reviewed in Refs. 5 and 6). Aβ is produced via serial proteolysis of amyloid precursor protein (APP) by aspartyl protease β-site APP-cleaving enzyme (BACE), or β-secretase, andγ-secretase. BACE cleaves APP to generate a 99-residue membrane-associated C terminus fragment (APP-C99). APP-C99 is further cleaved by γ-secretase to release 4-kDa Aβ and β-amyloid precursor protein intracellular domain (7–9). Presenilin and γ-secretase co-fractionate as a detergent-sensitive, high molecular weight complex (10) that includes at least three other proteins, nicastrin/APH-2, APH-1, and PEN-2, all of which are necessary and sufficient for γ-secretase activity (11–13). Increasing evidence indicates that apoptosis is associated with a variety of neurodegenerative disorders, including AD (Refs. 14–17; reviewed in Ref. 18). Aβ has been shown to cause caspase activation and apoptosis, which can in turn potentiate Aβ generation (16, 19–28). Finally, fibrillar aggregates of Aβ and oligomeric species of Aβ are more neurotoxic (29–37).
Perioperative factors, including hypoxia (38–42), hypocapnia (43), and anesthetics (44–47), have been reported to potentially contribute to AD neuropathogenesis. These perioperative factors may also cause post-operative cognitive dysfunction, a dementia associated with surgery and anesthesia, by triggering AD neuropathogenesis.
Isoflurane, sevoflurane, and desflurane are the most commonly used inhalation anesthetics. It has been reported that isoflurane enhances the oligomerization and cytotoxicity of Aβ (44) and induces apoptosis (48–51). Our recent studies have shown that a clinically relevant concentration of isoflurane can lead to caspase-3 activation, decrease cell viability, alter APP processing, and increase Aβ generation in human H4 neuroglioma cells overexpressing human APP (45–47). Loop et al. (49) reported that isoflurane and sevoflurane, but not desflurane, can induce caspase activation and apoptosis in human T lymphocytes. However, effects of desflurane and desflurane plus other perioperative risk factors, e.g. hypoxia, on APP processing and Aβ generation have not been assessed.
In the present study, we set out to determine effects of desflurane, hypoxia, and the combination of the two (desflurane/hypoxia) on caspase-3 activation, APP processing, and Aβ generation in H4 human neuroglioma cells (H4 naïve cells) and H4 naïve cells stably transfected to express full-length (FL) APP (H4-APP cells). We also investigated whether the caspase inhibitor, Z-VAD, the γ-secretase inhibitor L-685,458, and the Aβ aggregation inhibitor clioquinol could attenuate desflurane/hypoxia-induced caspase-3 activation and Aβ generation.
EXPERIMENTAL PROCEDURES
Cell Lines—We employed H4 human neuroglioma cells (H4 naïve cells) and H4 naïve cells stably transfected to express full-length (FL) APP (H4-APP cells) in the experiments. All of the cell lines were cultured in Dulbecco's modified Eagle's medium (high glucose) containing 9% heat-inactivated fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mm l-glutamine. Stably transfected H4 cells were additionally supplemented with 200 μg/ml G418.
Cell Treatment—The cells were treated for 6 h with following three conditions: desflurane alone (21% O2, 5% CO2, and 12% desflurane), hypoxia alone (18% O2 and 5% CO2), and desflurane/hypoxia (18% O2, 5% CO2, and 12% desflurane). Control conditions included 5% CO2 plus 21% O2, which did not affect caspase-3 activation, cell viability, APP processing, and Aβ generation (45–47). We used 18% O2 to establish mildly hypoxic conditions. In the interaction studies, the cells were treated with Z-VAD (50 μm,), L-685,458 (0.5 μm), clioquinol (CQ) (1 μm), and Aβ40 (7.5 μm) plus Aβ42 (7.5 μm) 1 h before desflurane/hypoxia treatment. The controls for Z-VAD, L685,458, and CQ were Me2SO, whereas the control condition for Aβ was phosphate-buffered saline. Control conditions for desflurane, hypoxia, and desflurane/hypoxia were 5% CO2 plus 21% O2, which did not affect caspase-3 activation, cell viability, APP processing, and Aβ generation (data not shown).
Cell Lysis and Protein Amount Quantification—Cell pellets were detergent-extracted on ice using immunoprecipitation buffer (10 mm Tris-HCl, pH 7.4, 150 mm NaCl, 2 mm EDTA, 0.5% Nonidet P-40) plus protease inhibitors (1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin A). The lysates were collected, centrifuged at 12,000 rpm for 10 min, and quantified for total proteins by BCA protein assay kit (Pierce).
Western Blots Analysis—The cells were harvested at the end of the experiments and were subjected to Western blots analyses as described by Xie et al. (52). Antibodies A8717 (1:1,000; Sigma) and C66 (1:1,000, generous gift of Dr. Dora Kovacs at Massachusetts General Hospital and Harvard Medical School) were used to visualize FL-APP (110 kDa) and APP-CTFs (10–12 kDa). Antibody anti-β-actin (1:2,000, Sigma) was used to detect β-actin (42 kDa). A caspase-3 antibody (1:1,000 dilution; Cell Signaling Technology, Inc., Beverly, MA) was used to recognize FL-caspase-3 (35–40 kDa) and caspase-3 fragment (17–20 kDa) resulting from cleavage at asparate position 175. Rabbit polyclonal anti-BACE-1 antibody (1:1,000; Abcam, Cambridge, MA) was used to detect protein levels of BACE (65 kDa). The quantification of Western blots was performed as described by Xie et al. (52). Briefly, intensity of signals was analyzed by using the National Institutes of Health image program (NIH Image 1.62). We quantified Western blots using two steps. First, we used levels of β-actin to normalize (e.g. determining ratio of FL-APP amount to β-actin amount) levels of FL-APP, APP-CTFs, FL-caspase-3, caspase-3 fragment, and BACE to control for any loading differences in total protein amounts. Second, we presented changes in levels of FL-APP, APP-CTFs, FL-caspase-3, caspase-3 fragment, and BACE in treated cells as a percentage of those in cells treated with controls.
Quantification of Aβ Using Sandwich Enzyme-linked Immunosorbent Assay—Secreted Aβ was measured with a Sandwich enzyme-linked immunosorbent assay by using an Aβ measurement kit (Invitrogen), and by the Aβ enzyme-linked immunosorbent assay core facility at the Center for Neurological Diseases, Harvard Institute of Medicine, Harvard Medical School, Boston, Massachusetts, as described by Xie et al. (53). Specifically, 96-well plates were coated with mouse monoclonal antibodies specific to Aβ40 (2G3) or Aβ42 (21F12). Following blocking with Block Ace, wells were incubated overnight at 4 °C with test samples of conditioned cell culture media, and then an anti-Aβ (α-Aβ-HR1) conjugated to horseradish peroxidase was added. The plates were then developed with TMB reagent, and well absorbance was measured at 450 nm. Aβ levels in test samples were determined by comparison with signal from unconditioned media spiked with known quantities of Aβ40 and Aβ42.
Statistics—Given the presence of background caspase-3 activation and cell death in cells cultured in serum-free medium, we did not use absolute values to describe changes in caspase-3 activation. Instead, 100% caspase-3 activation, FL-APP, APP-CTFs, and BACE in the manuscript refer to control levels for the purpose of comparison to experimental conditions. The data were expressed as the means ± S.D. The number of samples varied from 3 to 10, and the samples were normally distributed. We used a two-tailed t test to compare differences between experimental groups and control groups. p values less than 0.05 (* or #) and 0.01 (** or ##) were considered statistically significant.
RESULTS
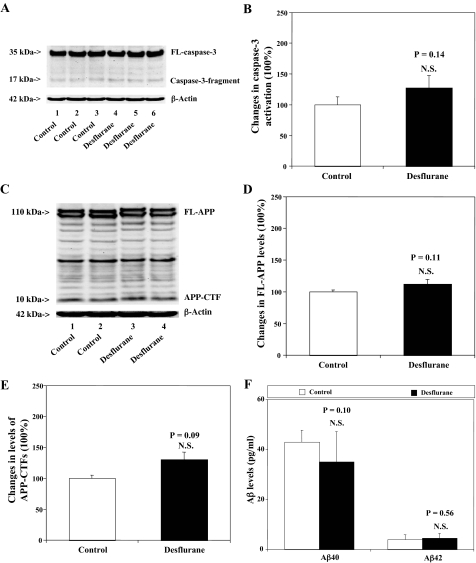
Desflurane Does Not Cause Caspase-3 Activation, APP Processing, or Secreted Aβ Levels in H4-APP Cells—We previously reported that isoflurane can induce apoptosis and increase secreted Aβ levels in H4-APP cells (45–47). The effects of other commonly used inhalation anesthetics, including sevoflurane and desflurane, on cellular apoptosis, APP processing, and Aβ generation have not been reported. We therefore set out to determine these effects in H4-APP cells. Because caspase-3 activation is one of the final steps of cellular apoptosis (54), we assessed effects of desflurane on caspase-3 activation by quantitative Western blot analyses. In the present experiment, treatment with 12% desflurane for 6 h only induced modest caspase-3 cleavage (activation) (Fig. 1A), as assessed by determining the ratio of cleaved (activated) caspase-3 fragment (17–19 kDa) to FL-caspase-3 (35–40 kDa). Quantification of the Western blots, based on the ratio of caspase-3 fragment to FL-caspase-3, revealed that the desflurane treatment did not significantly induce caspase-3 activation as compared with control conditions: 100% versus 127% (p = 0.14) (Fig. 1B). APP immunoblotting revealed no significant differences in levels of FL-APP and APP-CTFs between desflurane-treated and control H4-APP cells (Fig. 1, C–E). Finally, the desflurane treatment did not increase secreted levels of Aβ40 or Aβ42 as compared with control conditions (Fig. 1F). These results indicate that desflurane alone neither induces apoptosis nor increases Aβ generation.
FIGURE 1.
Desflurane does not affect caspase-3 activation, APP processing, nor Aβ levels in H4-APP cells. A, treatment with 12% desflurane for 6 h (lanes 4–6) does not induce caspase-3 cleavage (activation) as compared with control conditions (lanes 1–3) in H4-APP cells. There is no significant difference in the amounts of β-actin in control conditions or desflurane-treated H4-APP cells. B, caspase-3 activation assessed by quantifying ratio of caspase-3 fragment to FL-caspase-3 in the Western blots. Quantification of the Western blot shows that desflurane treatment (black bar) does not increase caspase-3 activation compared with control conditions (white bar)(p = 0.14, NS), normalized to β-actin levels. C, desflurane (lanes 3 and 4) does not affect APP processing as compared with control conditions (lanes 1 and 2) in H4-APP cells. There is no significant difference in amounts of β-actin in control conditions or desflurane-treated H4-APP cells. D, APP processing assessed by quantifying levels of FL-APP in the Western blots. Quantification of the Western blot shows that desflurane treatment (black bar) does not alter levels of FL-APP as compared with control conditions (white bar) (p = 0.11, NS), normalized to β-actin levels. E, APP processing assessed by quantifying levels of APP-CTFs in the Western blots. Quantification of the Western blot shows that desflurane treatment (black bar) does not alter levels of APP-CTFs as compared with control conditions (white bar)(p = 0.09, NS), normalized to β-actin levels. F, desflurane (black bar) does not increase generation of Aβ40 (p = 0.10, NS) and Aβ42 (p = 0.56, NS) as compared with control conditions (white bar).
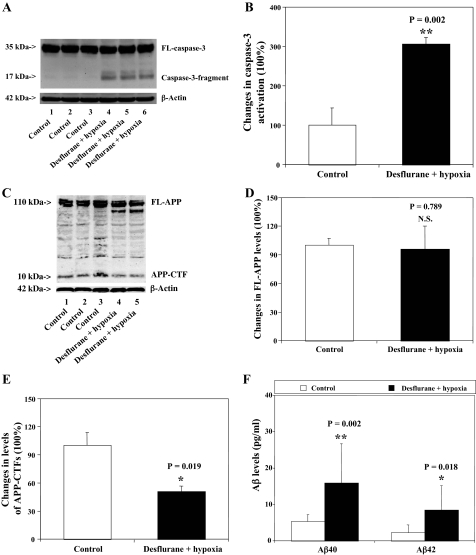
Desflurane/Hypoxia Induces Caspase-3 Activation, Affects APP Processing, and Increases Secreted Aβ Levels in H4-APP Cells—Because hypoxia has been reported to potentiate AD neuropathogenesis (43, 55), we assessed the effects of desflurane/hypoxia on caspase-3 activation, APP processing, and secreted Aβ levels in H4-APP cells. We chose 18% O2 to assess the effects of desflurane on apoptosis and Aβ generation under mildly hypoxic conditions. Caspase-3 immunoblotting showed visible increases in protein levels of caspase-3 fragment following treatment with 12% desflurane/hypoxia (18%) for 6 h as compared with control conditions (Fig. 2A). Quantification of the results by determining ratio of cleaved (activated) caspase-3 fragment (17–19 kDa) to FL-caspase-3 (35–40 kDa) revealed that desflurane/hypoxia treatment led to a 306% increase in caspase-3 cleavage (activation) as compared with control conditions (p = 0.002). APP immunoblotting showed visible decreases in protein levels of APP-CTFs (10–12 kDa), but not FL-APP (110 kDa), following the desflurane/hypoxia treatment as compared with control conditions (Fig. 2C). Quantification of the results showed that the desflurane/hypoxia treatment led to a 49% decrease in levels of APP-CTFs as compared with control conditions (Fig. 2E, p = 0.019). Finally, the desflurane/hypoxia treatment increased secreted Aβ40: 5.3 pg/ml versus 15.9 pg/ml, p = 0.002 and Aβ42: 1.9 pg/ml versus 6.7 pg/ml, p = 0.018 (Fig. 2F). These findings suggest that the combination of desflurane and hypoxia induces apoptosis, alters APP processing, and increases Aβ generation.
FIGURE 2.
Desflurane/hypoxia induces caspase-3 activation, affects APP processing, and increases Aβ levels in H4-APP cells. A, treatment with 12% desflurane/hypoxia (18%) for 6 h (lanes 4–6) induces caspase-3 cleavage (activation) as compared with control conditions (lanes 1–3) in H4-APP cells. There is no significant difference in the amounts of β-actin in control conditions or desflurane/hypoxia-treated H4-APP cells. B, quantification of the Western blot shows that desflurane/hypoxia treatment (black bar) (**, p = 0.002) increases caspase-3 activation compared with control conditions (white bar), normalized to β-actin levels. C, desflurane/hypoxia (lanes 4 and 5) reduces levels of APP-CTFs, but not FL-APP, as compared with control conditions (lanes 1–3) in H4-APP cells. There is no significant difference in amounts of β-actin in control conditions or desflurane/hypoxia-treated H4-APP cells. D, quantification of the Western blot shows that desflurane/hypoxia (black bar) does not alter levels of FL-APP as compared with control conditions (white bar)(p = 0.789, NS), normalized to β-actin levels. E, quantification of the Western blot shows that desflurane/hypoxia (black bar) decreases levels of APP-CTFs as compared with control conditions (white bar) (*, p = 0.019), normalized to β-actin levels. F, desflurane/hypoxia (black bar) increases generation of both Aβ40 (**, p = 0.002) and Aβ42 (*, p = 0.018) as compared with control conditions (white bar).
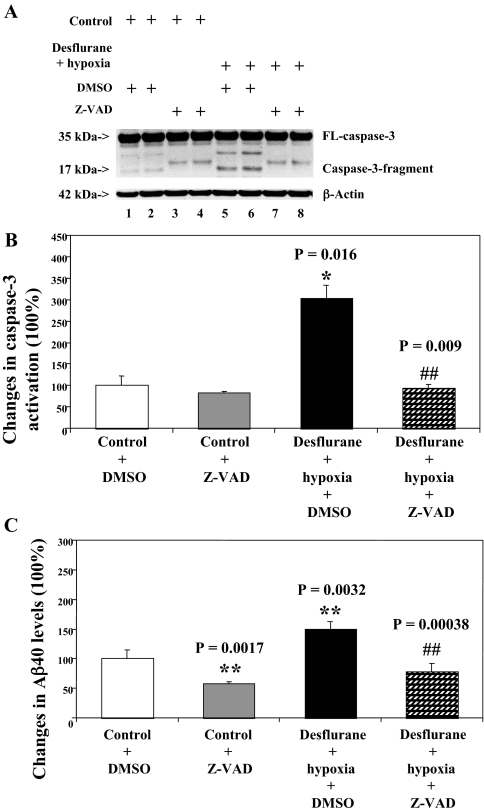
Caspase Inhibitor Z-VAD Attenuates the Desflurane/Hypoxia-induced Caspase-3 Activation—Given the findings that desflurane/hypoxia can induce apoptosis and increase Aβ generation, we next asked whether the desflurane/hypoxia-induced alteration in APP processing and Aβ generation is dependent on caspase-3 activation. For this purpose, we set out to assess the effects of the caspase inhibitor Z-VAD on desflurane/hypoxia-induced caspase-3 activation and Aβ generation. Z-VAD treatment attenuated caspase-3 activation induced by desflurane/hypoxia treatment, whereas Z-VAD alone did not induce caspase-3 activation as compared with control conditions (Fig. 3A). Quantification of the Western blots revealed that Z-VAD attenuated desflurane/hypoxia-induced caspase-3 activation: 304% versus 94%, p = 0.009 (Fig. 3B). Z-VAD also reduced desflurane/hypoxia-induced increases in secreted Aβ levels in H4-APP cells, p = 0.00038 (Fig. 3C).
FIGURE 3.
Caspase inhibitor Z-VAD attenuates caspase-3 activation induced by desflurane/hypoxia in H4-APP cells. A, treatment with desflurane (12%) plus hypoxia (18%) for 6 h (lanes 5 and 6) induces caspase-3 cleavage (activation) as compared with control conditions (lanes 1 and 2) or Z-VAD (50 μm) treatment (lanes 3 and 4). Z-VAD treatment (lane 7 and 8) attenuates caspase-3 cleavage induced by desflurane/hypoxia. There is no significant difference in amounts of β-actin in H4-APP cells with above treatments. B, quantification of the Western blot shows that desflurane/hypoxia (black bar) increases caspase-3 activation as compared with control conditions (white bar) (*, p = 0.016), normalized to β-actin levels. The desflurane/hypoxia-induced caspase-3 activation is reduced by Z-VAD (50 μm) treatment (net bar; ##, p = 0.009). C, desflurane/hypoxia (black bar) increases secreted Aβ levels in H4-APP cells as compared with control conditions (white bar) (**, p = 0.0032). Z-VAD (net bar) reduces the desflurane/hypoxia-induced increases in secreted Aβ levels in H4-APP cells (##, p = 0.00038). DMSO, dimethyl sulfoxide.
Next, we asked whether desflurane/hypoxia can induce caspase-3 activation in the absence of APP overexpression in naïve H4 cells. Caspase-3 immunoblotting showed that desflurane/hypoxia induced caspase-3 activation as compared with control conditions in both H4 naïve and H4-APP cells (Fig. 4A). Quantification of the Western blot revealed that desflurane/hypoxia treatment led to a 306% and a 195% increase in caspase-3 activation as compared with control conditions in H4 naïve cells (Fig. 4B, p = 0.025) and H4-APP cells (Fig. 4C, p = 0.007), respectively. APP immunoblotting did not show detectable changes in levels of APP-CTFs and FL-APP between desflurane/hypoxia treatment and control conditions in H4 naïve cells (Fig. 4D). As a positive control, APP immunoblotting showed that desflurane/hypoxia (lane 4) reduced levels of APP-CTFs without significant changes in FL-APP levels as compared with control conditions (lane 3) in H4-APP cells (Fig. 4D). Quantification of the Western blots revealed that desflurane/hypoxia treatment did not change the ratio of APP-CTFs to FL-APP in H4 naïve cells (Fig. 4E) but led to a 24% decrease in the ratio of APP-CTFs to FL-APP in H4-APP cells (Fig. 4F, p = 0.03). Aβ levels were too low to be detected in H4 naïve cells in these experiments (data not shown). Taken together, these findings suggest that whereas desflurane/hypoxia can induce caspase-3 activation in the absence of alterations in APP processing and Aβ generation in H4 naïve cells, desflurane/hypoxia-induced increases in Aβ generation are dependent on the desflurane/hypoxia-induced caspase-3 activation in H4-APP cells.
FIGURE 4.
Desflurane/hypoxia treatment induces caspase-3 activation independent of APP processing in H4 naïve cells. A, treatment with desflurane (12%) plus hypoxia (18%) for 6 h (lanes 3 and 4) induces caspase-3 cleavage (activation) as compared with control conditions (lanes 1 and 2) in H4 naïve cells. The same treatment (lanes 7 and 8) also induces caspase-3 activation in H4-APP cells as compared with control conditions (lanes 5 and 6). B, quantification of the Western blot shows that desflurane/hypoxia (black bar) increases caspase-3 activation as compared with control conditions (white bar) (*, p = 0.025), normalized to β-actin levels, in H4 naïve cells. C, quantification of the Western blot shows that desflurane/hypoxia (black bar) (**, p = 0.007) increases caspase-3 activation as compared with control conditions (white bar), normalized to β-actin levels, in H4-APP cells. D, desflurane/hypoxia (lane 2) does not alter levels of APP-CTFs as compared with control conditions (lane 1) in H4 naïve cells. Desflurane/hypoxia (lane 4) reduces levels of APP-CTFs as compared with control conditions (lane 3) in H4-APP cells. E, quantification of the Western blot shows that desflurane/hypoxia (black bar) does not alter ratio of APP-CTFs to FL-APP as compared with control conditions (white bar) in H4 naïve cells (p = 0.35, NS). F, quantification of the Western blot shows that desflurane/hypoxia (black bar) decreases ratio of APP-CTFs to FL-APP in H4-APP cells (*, p = 0.03).
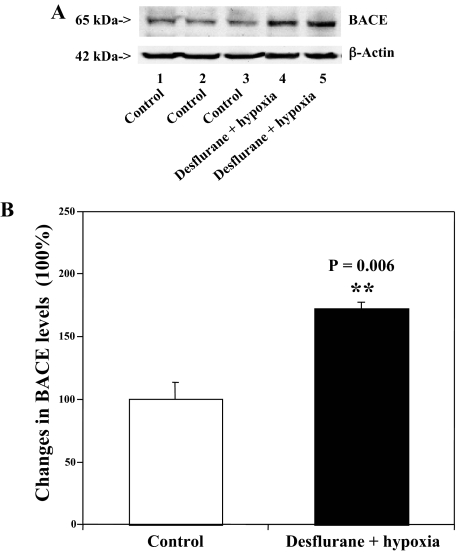
Desflurane/Hypoxia Increases Levels of BACE in H4-APP Cells—We next asked whether desflurane/hypoxia can increase levels of BACE. Treatment with 12% desflurane/hypoxia (18% O2) for 6 h caused visible increases in BACE levels as compared with control conditions (Fig. 5A). Quantification of the Western blot showed that desflurane/hypoxia treatment led to a 172% increase in BACE levels as compared with control conditions (p = 0.006, Fig. 5B). These results suggest that desflurane/hypoxia can activate caspase-3 and increase Aβ generation by enhancing BACE levels.
FIGURE 5.
Desflurane/hypoxia increases levels of BACE in H4-APP cells. A, desflurane/hypoxia (lanes 4 and 5) increases levels of BACE (65 kDa) as compared with control conditions (lanes 1–3). There is no significant difference in amounts of β-actin in control conditions or desflurane/hypoxia-treated H4-APP cells. B, quantification of the Western blot shows that desflurane/hypoxia (black bar) increases BACE levels as compared with control conditions (white bar) (**, p = 0.006), normalized to β-actin levels.
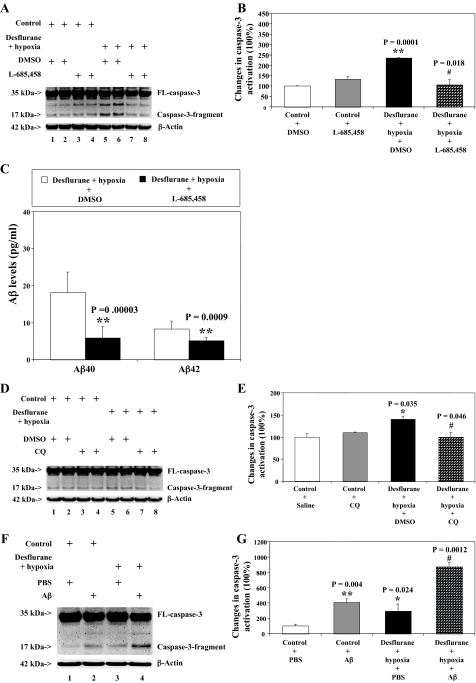
Desflurane/Hypoxia-induced Caspase-3 Activation Is Attenuated by the γ-Secretase Inhibitor L-685,458 or the Aβ Aggregation Blocker Clioquinol but Is Potentiated by Aβ—We next asked whether pharmacologically based reductions in Aβ generation or Aβ aggregation can attenuate desflurane/hypoxia-induced caspase-3 activation. For this purpose, we assessed the effects of the γ-secretase inhibitor L-685,458 and Aβ aggregation blocker CQ on desflurane/hypoxia-induced caspase-3 activation in H4-APP cells. L-685,458 reduced caspase-3 activation induced by desflurane/hypoxia: 106% versus 235%, p = 0.018 (Fig. 6, A and B). L-685,458 alone did not decrease caspase-3 fragment levels as compared with control conditions (Fig. 6, A and B). As expected, L-685,458 attenuated hypoxia/desflurane-induced increases in secreted Aβ levels: 18.11 pg/ml versus 5.87 pg/ml (Aβ40, p = 0.00003); 8.26 pg/ml versus 5.16 pg/ml (Aβ42, p = 0.00009) (Fig. 6C). Next, CQ treatment led to reductions of desflurane/hypoxia-induced caspase-3 activation: 140% versus 101%, p = 0.046) (Fig. 6, D and E). CQ alone did not cause caspase-3 activation as compared with control conditions (Fig. 6, D and E). Finally, we asked whether a pro-apoptotic stimulus, 7.5 μm Aβ40 plus 7.5 μm Aβ42, could potentiate desflurane/hypoxia-induced caspase-3 activation. We were able to show that the Aβ treatment potentiated desflurane/hypoxia-induced caspase-3 activation in H4 naïve cells: 295% versus 806%, p = 0.0027 (Fig. 6, F and G). These results suggest that desflurane/hypoxia treatment may induce apoptosis via enhancement of Aβ generation and aggregation.
FIGURE 6.
γ-Secretase inhibitor L-685,458 and Aβ aggregation inhibitor CQ reduce the desflurane/hypoxia-induced caspase-3 activation, but Aβ potentiates the desflurane/hypoxia-induced caspase-3 activation in H4-APP cells. A, treatment with desflurane (12%) plus hypoxia (18%) for 6 h (lanes 5 and 6) induces caspase-3 cleavage (activation) as compared with control conditions (lanes 1 and 2) or L-685,458 (0.5 μm) treatment (lanes 3 and 4). L-685,458 (0.5 μm) treatment (lane 7 and 8) attenuates caspase-3 cleavage induced by desflurane/hypoxia. There is no significant difference in amounts of β-actin in H4-APP cells with above treatments. B, quantification of the Western blot shows that desflurane/hypoxia (black bar) increases caspase-3 activation as compared with control conditions (white bar) (**, p = 0.0001), normalized to β-actin levels. The desflurane/hypoxia-induced caspase-3 activation is reduced by L-685,458 (0.5 μm) (net bar) (#, p = 0.018). C, L-685,458 (0.5 μm) reduces the desflurane/hypoxia-induced increases in secreted Aβ40 (**, p = 0.00003) and Aβ42 (**, p = 0.0009) levels in H4-APP cells. D, desflurane/hypoxia (lanes 5 and 6) induces caspase-3 cleavage (activation) as compared with control conditions (lanes 1 and 2) or CQ (1 μm) treatment (lanes 3 and 4). CQ (1 μm)(lanes 7 and 8) treatment attenuates caspase-3 cleavage induced by desflurane/hypoxia. There is no significant difference in amounts of β-actin in H4-APP cells with above treatments. E, quantification of the Western blot shows that desflurane/hypoxia (black bar) increases caspase-3 activation as compared with control conditions (white bar) (*, p = 0.035), normalized to β-actin levels. The desflurane/hypoxia-induced caspase-3 activation is attenuated by treatment of CQ (1 μm) (net bar) (#, p = 0.046). F, desflurane/hypoxia (lane 3) induces caspase-3 cleavage (activation) as compared with control conditions (lane 1). Aβ40 (7.5 μm) plus Aβ42 (7.5 μm) treatment potentiates caspase-3 activation induced by desflurane/hypoxia (lane 4). There is no significant difference in amounts of β-actin in H4-APP cells with the above treatments. G, quantification of the Western blot shows that desflurane/hypoxia (black bar; *, p = 0.024) increases caspase-3 activation as compared with control conditions (white bar), normalized to β-actin levels. The desflurane/hypoxia-induced caspase-3 activation is potentiated by treatment of Aβ (net bar) (##, p = 0.0012). DMSO, dimethyl sulfoxide.
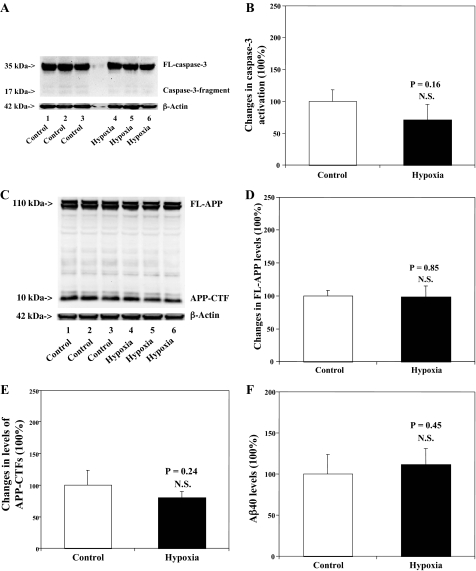
Hypoxia (18% O2) Alone Does Not Affect Caspase-3 Activation, APP Processing, or Aβ Levels in H4-APP Cells—Finally, we assessed whether hypoxia (18%) alone can also induce caspase-3 activation, alter APP processing, and increase Aβ generation in H4-APP cells. Treatment with hypoxia (18% O2) for 6 h did not induce caspase-3 activation (Fig. 7, A and B), did not alter APP processing (Fig. 7, C–E), and did not increase Aβ generation (Fig. 7F). Taken together, these findings suggest that the apoptosis, APP processing and Aβ generation induced by desflurane/hypoxia are most likely due to synergistic effects of desflurane/hypoxia.
FIGURE 7.
Hypoxia (18%) does not affect caspase-3 activation, APP processing, nor Aβ generation in H4-APP cells. A, hypoxia treatment alone (lanes 4–6) does not induce caspase-3 cleavage (activation) as compared with control conditions (lanes 1–3) in H4-APP cells. There is no significant difference in amounts of β-actin in control conditions or hypoxia-treated H4-APP cells. B, quantification of the Western blot shows that hypoxia treatment (black bar) does not increase caspase-3 activation compared with control conditions (white bar)(p = 0.16, NS), normalized to β-actin levels. C, hypoxia (lanes 4–6) does not affect APP processing as compared with control conditions (lanes 1–3) in H4-APP cells. There is no significant difference in amounts of β-actin in control conditions or hypoxia-treated H4-APP cells. D, quantification of the Western blot shows that hypoxia treatment (black bar) does not alter levels of FL-APP as compared with control conditions (white bar) (p = 0.85, NS), normalized to β-actin levels. E, quantification of the Western blot shows that hypoxia treatment (black bar) does not alter levels of APP-CTFs as compared with control conditions (white bar)(p = 0.24, NS), normalized to β-actin levels. F, hypoxia (black bar) does not increase generation of Aβ40 as compared with control conditions (white bar)(p = 0.45, NS).
DISCUSSION
The commonly used inhalation anesthetic isoflurane has previously been shown to promote Aβ aggregation and to enhance toxicity of Aβ (44). We have shown that isoflurane can induce cellular apoptosis and increase Aβ generation in H4-APP cells (45–47). Because desflurane is another commonly used inhalation anesthetic, we set out to assess the effects of desflurane on apoptosis, APP processing, and Aβ generation in H4-APP cells. We were able to show that a 6-h treatment with a clinically relevant concentration of desflurane (12%) did not induce caspase-3 activation and affected neither APP processing nor secreted Aβ levels in H4-APP cells. These results are consistent with findings of other studies, which illustrated that isoflurane and sevoflurane, but not desflurane, can induce apoptosis (49). However, it is still possible that different treatments of desflurane in other cell lines may lead to apoptosis and enhancement of Aβ generation.
Next, we found that treatment with 12% desflurane/hypoxia (18% O2) for 6 h can induce caspase-3 activation, alter APP processing, and increase Aβ generation in H4-APP cells, whereas treatment with either 12% desflurane or hypoxia alone did not lead to similar effects in H4-APP cells. Collectively, these findings suggest that desflurane may promote AD neuropathogenesis only under hypoxic conditions. It is possible that treatment with 12% desflurane alone for 6 h had undetectable effects on apoptosis and APP/Aβ, which were potentiated by hypoxic conditions. Hypoxia and cerebral blood supply insufficiency remain prevalent concerns in perioperative care for patients, because they can result from hypotension, shunting (e.g. one lung ventilation), and other pathological conditions. Therefore, these data, once confirmed in vivo, suggest that it would be prudent to vigilantly avoid these pathological conditions in patients when desflurane is used as an inhalation anesthetic.
We employed 18% O2 to assess the effects of desflurane on apoptosis and Aβ generation under mildly hypoxic conditions. Future studies will be necessary to assess whether severe hypoxic conditions, e.g. 1% O2, alone would have similar effects on apoptosis and Aβ generation.
Z-VAD, a broad caspase activation inhibitor, was able to attenuate both caspase-3 activation and Aβ generation induced by desflurane/hypoxia. These results suggest that the desflurane/hypoxia-induced alterations in APP processing and Aβ generation are largely dependent on the ability of desflurane/hypoxia to induce apoptosis. To further explore the mechanism by which desflurane/hypoxia induces apoptosis, affects APP processing, and increases Aβ generation, we assessed the effects of desflurane/hypoxia on BACE. Desflurane/hypoxia treatment for 6 h increased protein levels of BACE in H4-APP cells. Previously, we showed that inhalation anesthetic isoflurane can induce apoptosis which in turn enhances BACE levels to facilitate APP processing and to increase Aβ generation (47). Recent studies have shown that subsequent to caspase activation during ischemia, reductions in protein levels of Golgi-localized γ-ear-containing ARF-binding protein (GGA)-3, a protein that can alter trafficking and metabolism of BACE, is associated with increased levels of BACE and activity of β-secretase (56). It is interesting to speculate whether isoflurane and desflurane/hypoxia treatments might likewise reduce levels of GGA-3 to enhance BACE levels and β-secretase activity subsequent to caspase activation.
We have previously shown that an amyloid fibril-binding dye, Congo Red (32), a β-sheet breaker peptide iAβ5 (57), and a metal protein attenuation compound CQ (58) can attenuate, whereas Aβ can potentiate, apoptosis induced by isoflurane (46, 47). We therefore assessed whether inhibition of Aβ aggregation and generation can attenuate desflurane/hypoxia-induced caspase-3 activation in H4-APP cells by determining the effects of CQ (an Aβ aggregation inhibitor) and L-685,458 (a γ-secretase inhibitor) on caspase-3 activation induced by desflurane/hypoxia in H4-APP cells. L-685,458 inhibited both caspase-3 activation and Aβ generation induced by desflurane/hypoxia. CQ also inhibited caspase-activation induced by desflurane/hypoxia. Collectively, these results suggest that reductions in Aβ generation and aggregation can attenuate desflurane/hypoxia-induced apoptosis. Conversely, treatment with Aβ potentiated desflurane/hypoxia-induced caspase-3 activation, suggesting a vicious cycle of apoptosis and Aβ generation that can be triggered by desflurane/hypoxia.
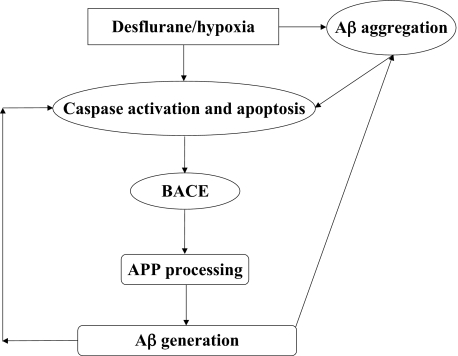
In conclusion, we have found that the combination of desflurane and hypoxia, but neither alone, induces apoptosis, alters APP processing, and increases Aβ generation. Fig. 8 shows a scheme in which desflurane/hypoxia activates caspase, which then leads to increases in BACE and Aβ levels. Increased Aβ levels would then lead to further caspase activation, resulting in a vicious cycle. Further investigation, especially via in vivo studies, will be necessary to assess the potential role of desflurane and/or hypoxia in triggering or driving AD neuropathogenesis. These efforts should promote further attempts to determine the effects of anesthetics on AD neuropathogenesis, ultimately leading to provision of safer anesthesia care to patients, especially elderly patients, who are particularly susceptible to the incidence of post-operative cognitive dysfunction and risk for AD.
FIGURE 8.
Hypothetical pathway by which desflurane/hypoxia induces a vicious cycle of apoptosis and Aβ generation/aggregation. Desflurane/hypoxia induces caspase-3 activation/apoptosis. Caspase activation, in turn, increases BACE levels, which serves to increase Aβ generation. Desflurane/hypoxia also enhances Aβ aggregation, which further induces caspase-3 activation and apoptosis. Elevated Aβ generation and Aβ aggregation then further induces apoptosis leading to a vicious cycle of desflurane/hypoxia-induced apoptosis and Aβ generation/aggregation.
Acknowledgments
Antibody C66 was a generous gift from Dr. Dora Kovacs at Massachusetts General Hospital and Harvard Medical School.
This work was supported by Grant R37MH 60009 from the National Institutes of Health and the Cure Alzheimer's Fund (to R. E. T.) and by Grant K08 NS048140 from the National Institutes of Health, and Jahnigen Career Development Award from the American Geriatrics Society, an Investigator Initiated research grant from the Alzheimer's Association, and the William Milton Fund (Harvard University) (to Z. X.). The cost of the anesthetic desflurane and partial financial support of Yuanlin Dong and Bin Zhang were generously provided by the Department of Anesthesia and Critical Care in Massachusetts General Hospital and Harvard Medical School. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: AD, Alzheimer disease; FL, full-length; APP, amyloid β precursor protein; BACE, β-site APP-cleaving enzyme; Aβ, amyloid β-protein; CQ, clioquinol; Z, benzyloxycarbonyl; NS, not significant.
References
- 1.Bohnen, N. I., Warner, M. A., Kokmen, E., Beard, C. M., and Kurland, L. T. (1994) J. Am. Geriatr. Soc. 42 198–201 [DOI] [PubMed] [Google Scholar]
- 2.Bohnen, N., Warner, M. A., Kokmen, E., and Kurland, L. T. (1994) Int. J. Neurosci. 77 181–185 [DOI] [PubMed] [Google Scholar]
- 3.Muravchick, S., and Smith, D. S. (1995) Anesthesiology 82 305–307 [DOI] [PubMed] [Google Scholar]
- 4.Lee, T. A., Wolozin, B., Weiss, K. B., and Bednar, M. M. (2005) J. Alzheimers Dis. 7 319–324 [DOI] [PubMed] [Google Scholar]
- 5.Tanzi, R. E., and Bertram, L. (2005) Cell 120 545–555 [DOI] [PubMed] [Google Scholar]
- 6.Selkoe, D. J. (2001) Physiol. Rev. 81 741–766 [DOI] [PubMed] [Google Scholar]
- 7.Gu, Y., Misonou, H., Sato, T., Dohmae, N., Takio, K., and Ihara, Y. (2001) J. Biol. Chem. 276 35235–35238 [DOI] [PubMed] [Google Scholar]
- 8.Sastre, M., Steiner, H., Fuchs, K., Capell, A., Multhaup, G., Condron, M. M., Teplow, D. B., and Haass, C. (2001) EMBO Rep. 2 835–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu, C., Kim, S. H., Ikeuchi, T., Xu, H., Gasparini, L., Wang, R., and Sisodia, S. S. (2001) J. Biol. Chem. 276 43756–43760 [DOI] [PubMed] [Google Scholar]
- 10.Li, Y. M., Lai, M. T., Xu, M., Huang, Q., DiMuzio-Mower, J., Sardana, M. K., Shi, X. P., Yin, K. C., Shafer, J. A., and Gardell, S. J. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 6138–6143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis, R., McGrath, G., Zhang, J., Ruddy, D. A., Sym, M., Apfeld, J., Nicoll, M., Maxwell, M., Hai, B., Ellis, M. C., Parks, A. L., Xu, W., Li, J., Gurney, M., Myers, R. L., Himes, C. S., Hiebsch, R., Ruble, C., Nye, J. S., and Curtis, D. (2002) Dev. Cell 3 85–97 [DOI] [PubMed] [Google Scholar]
- 12.Steiner, H., Winkler, E., Edbauer, D., Prokop, S., Basset, G., Yamasaki, A., Kostka, M., and Haass, C. (2002) J. Biol. Chem. 277 39062–39065 [DOI] [PubMed] [Google Scholar]
- 13.Yu, G., Nishimura, M., Arawaka, S., Levitan, D., Zhang, L., Tandon, A., Song, Y. Q., Rogaeva, E., Chen, F., Kawarai, T., Supala, A., Levesque, L., Yu, H., Yang, D. S., Holmes, E., Milman, P., Liang, Y., Zhang, D. M., Xu, D. H., Sato, C., Rogaev, E., Smith, M., Janus, C., Zhang, Y., Aebersold, R., Farrer, L. S., Sorbi, S., Bruni, A., Fraser, P., and St George-Hyslop, P. (2000) Nature 407 48–54 [DOI] [PubMed] [Google Scholar]
- 14.Masliah, E., Mallory, M., Alford, M., Tanaka, S., and Hansen, L. A. (1998) J. Neuropathol. Exp. Neurol. 57 1041–1052 [DOI] [PubMed] [Google Scholar]
- 15.Yang, F., Sun, X., Beech, W., Teter, B., Wu, S., Sigel, J., Vinters, H. V., Frautschy, S. A., and Cole, G. M. (1998) Am. J. Pathol. 152 379–389 [PMC free article] [PubMed] [Google Scholar]
- 16.Gervais, F. G., Xu, D., Robertson, G. S., Vaillancourt, J. P., Zhu, Y., Huang, J., LeBlanc, A., Smith, D., Rigby, M., Shearman, M. S., Clarke, E. E., Zheng, H., Van Der Ploeg, L. H., Ruffolo, S. C., Thornberry, N. A., Xanthoudakis, S., Zamboni, R. J., Roy, S., and Nicholson, D. W. (1999) Cell 97 395–406 [DOI] [PubMed] [Google Scholar]
- 17.Shimohama, S., Tanino, H., and Fujimoto, S. (1999) Biochem. Biophys. Res. Commun. 256 381–384 [DOI] [PubMed] [Google Scholar]
- 18.Raina, A. K., Hochman, A., Ickes, H., Zhu, X., Ogawa, O., Cash, A. D., Shimohama, S., Perry, G., and Smith, M. A. (2003) Prog. Neuropsychopharmacol. Biol. Psychiatry 27 251–254 [DOI] [PubMed] [Google Scholar]
- 19.LeBlanc, A. (1995) J. Neurosci. 15 7837–7846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeBlanc, A., Liu, H., Goodyer, C., Bergeron, C., and Hammond, J. (1999) J. Biol. Chem. 274 23426–23436 [DOI] [PubMed] [Google Scholar]
- 21.Galli, C., Piccini, A., Ciotti, M. T., Castellani, L., Calissano, P., Zaccheo, D., and Tabaton, M. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 1247–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sodhi, C. P., Rampalli, S., Perez, R. G., Koo, E. H., Quinn, B., and Gottardi-Littell, N. R. (2004) Brain Res. Mol. Brain Res. 128 201–211 [DOI] [PubMed] [Google Scholar]
- 23.Tesco, G., Koh, Y. H., and Tanzi, R. E. (2003) J. Biol. Chem. 278 46074–46080 [DOI] [PubMed] [Google Scholar]
- 24.Guo, Q., Sopher, B. L., Furukawa, K., Pham, D. G., Robinson, N., Martin, G. M., and Mattson, M. P. (1997) J. Neurosci. 17 4212–4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Florent, S., Malaplate-Armand, C., Youssef, I., Kriem, B., Koziel, V., Escanye, M. C., Fifre, A., Sponne, I., Leininger-Muller, B., Olivier, J. L., Pillot, T., and Oster, T. (2006) J. Neurochem. 96 385–395 [DOI] [PubMed] [Google Scholar]
- 26.Kriem, B., Sponne, I., Fifre, A., Malaplate-Armand, C., Lozac'h-Pillot, K., Koziel, V., Yen-Potin, F. T., Bihain, B., Oster, T., Olivier, J. L., and Pillot, T. (2005) FASEB J. 19 85–87 [DOI] [PubMed] [Google Scholar]
- 27.Pillot, T., Drouet, B., Queille, S., Labeur, C., Vandekerchkhove, J., Rosseneu, M., Pincon-Raymond, M., and Chambaz, J. (1999) J. Neurochem. 73 1626–1634 [DOI] [PubMed] [Google Scholar]
- 28.Sponne, I., Fifre, A., Drouet, B., Klein, C., Koziel, V., Pincon-Raymond, M., Olivier, J. L., Chambaz, J., and Pillot, T. (2003) J. Biol. Chem. 278 3437–3445 [DOI] [PubMed] [Google Scholar]
- 29.De Felice, F. G., Vieira, M. N., Saraiva, L. M., Figueroa-Villar, J. D., Garcia-Abreu, J., Liu, R., Chang, L., Klein, W. L., and Ferreira, S. T. (2004) FASEB J. 18 1366–1372 [DOI] [PubMed] [Google Scholar]
- 30.Pike, C. J., Burdick, D., Walencewicz, A. J., Glabe, C. G., and Cotman, C. W. (1993) J. Neurosci. 13 1676–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grace, E. A., Rabiner, C. A., and Busciglio, J. (2002) Neuroscience 114 265–273 [DOI] [PubMed] [Google Scholar]
- 32.Lorenzo, A., and Yankner, B. A. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 12243–12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambert, M. P., Barlow, A. K., Chromy, B. A., Edwards, C., Freed, R., Liosatos, M., Morgan, T. E., Rozovsky, I., Trommer, B., Viola, K. L., Wals, P., Zhang, C., Finch, C. E., Krafft, G. A., and Klein, W. L. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, H. W., Pasternak, J. F., Kuo, H., Ristic, H., Lambert, M. P., Chromy, B., Viola, K. L., Klein, W. L., Stine, W. B., Krafft, G. A., and Trommer, B. L. (2002) Brain Res. 924 133–140 [DOI] [PubMed] [Google Scholar]
- 35.Walsh, D. M., Klyubin, I., Fadeeva, J. V., Rowan, M. J., and Selkoe, D. J. (2002) Biochem. Soc. Trans. 30 552–557 [DOI] [PubMed] [Google Scholar]
- 36.Kim, H. J., Chae, S. C., Lee, D. K., Chromy, B., Lee, S. C., Park, Y. C., Klein, W. L., Krafft, G. A., and Hong, S. T. (2003) FASEB J. 17 118–120 [DOI] [PubMed] [Google Scholar]
- 37.Kayed, R., Head, E., Thompson, J. L., McIntire, T. M., Milton, S. C., Cotman, C. W., and Glabe, C. G. (2003) Science 300 486–489 [DOI] [PubMed] [Google Scholar]
- 38.Kokmen, E., Whisnant, J. P., O'Fallon, W. M., Chu, C. P., and Beard, C. M. (1996) Neurology 46 154–159 [DOI] [PubMed] [Google Scholar]
- 39.Nagy, Z., Esiri, M. M., Jobst, K. A., Morris, J. H., King, E. M., McDonald, B., Joachim, C., Litchfield, S., Barnetson, L., and Smith, A. D. (1997) J. Neuropathol. Exp. Neurol. 56 165–170 [DOI] [PubMed] [Google Scholar]
- 40.Snowdon, D. A., Greiner, L. H., Mortimer, J. A., Riley, K. P., Greiner, P. A., and Markesbery, W. R. (1997) J. Am. Med. Assoc. 277 813–817 [PubMed] [Google Scholar]
- 41.Jendroska, K., Hoffmann, O. M., and Patt, S. (1997) Ann. N. Y. Acad. Sci. 826 401–405 [DOI] [PubMed] [Google Scholar]
- 42.Kalaria, R. N. (2000) Neurobiol. Aging 21 321–330 [DOI] [PubMed] [Google Scholar]
- 43.Xie, Z., Moir, R. D., Romano, D. M., Tesco, G., Kovacs, D. M., and Tanzi, R. E. (2004) Neurodegener. Dis. 1 29–37 [DOI] [PubMed] [Google Scholar]
- 44.Eckenhoff, R. G., Johansson, J. S., Wei, H., Carnini, A., Kang, B., Wei, W., Pidikiti, R., Keller, J. M., and Eckenhoff, M. F. (2004) Anesthesiology 101 703–709 [DOI] [PubMed] [Google Scholar]
- 45.Xie, Z., Dong, Y., Maeda, U., Alfille, P., Culley, D. J., Crosby, G., and Tanzi, R. E. (2006) Anesthesiology 104 988–994 [DOI] [PubMed] [Google Scholar]
- 46.Xie, Z., Dong, Y., Maeda, U., Moir, R., Inouye, S. K., Culley, D. J., Crosby, G., and Tanzi, R. E. (2006) J. Gerontol. A Biol. Sci. Med. Sci. 61 1300–1306 [DOI] [PubMed] [Google Scholar]
- 47.Xie, Z., Dong, Y., Maeda, U., Moir, R. D., Xia, W., Culley, D. J., Crosby, G., and Tanzi, R. E. (2007) J. Neurosci. 27 1247–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kvolik, S., Glavas-Obrovac, L., Bares, V., and Karner, I. (2005) Life Sci. 77 2369–2383 [DOI] [PubMed] [Google Scholar]
- 49.Loop, T., Dovi-Akue, D., Frick, M., Roesslein, M., Egger, L., Humar, M., Hoetzel, A., Schmidt, R., Borner, C., Pahl, H. L., Geiger, K. K., and Pannen, B. H. (2005) Anesthesiology 102 1147–1157 [DOI] [PubMed] [Google Scholar]
- 50.Wei, H., Kang, B., Wei, W., Liang, G., Meng, Q. C., Li, Y., and Eckenhoff, R. G. (2005) Brain Res. 1037 139–147 [DOI] [PubMed] [Google Scholar]
- 51.Matsuoka, H., Kurosawa, S., Horinouchi, T., Kato, M., and Hashimoto, Y. (2001) Anesthesiology 95 1467–1472 [DOI] [PubMed] [Google Scholar]
- 52.Xie, Z., Romano, D. M., and Tanzi, R. E. (2005) J. Mol. Neurosci. 25 67–77 [DOI] [PubMed] [Google Scholar]
- 53.Xie, Z., Romano, D. M., and Tanzi, R. E. (2005) J. Biol. Chem. 280 15413–15421 [DOI] [PubMed] [Google Scholar]
- 54.Thornberry, N. A. (1998) Chem. Biol. 5 R97–R103 [DOI] [PubMed] [Google Scholar]
- 55.Sun, X., He, G., Qing, H., Zhou, W., Dobie, F., Cai, F., Staufenbiel, M., Huang, L. E., and Song, W. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 18727–18732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tesco, G., Koh, Y. H., Kang, E. L., Cameron, A. N., Das, S., Sena-Esteves, M., Hiltunen, M., Yang, S. H., Zhong, Z., Shen, Y., Simpkins, J. W., and Tanzi, R. E. (2007) Neuron 54 721–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soto, C., Sigurdsson, E. M., Morelli, L., Kumar, R. A., Castano, E. M., and Frangione, B. (1998) Nat. Med. 4 822–826 [DOI] [PubMed] [Google Scholar]
- 58.Cherny, R. A., Atwood, C. S., Xilinas, M. E., Gray, D. N., Jones, W. D., McLean, C. A., Barnham, K. J., Volitakis, I., Fraser, F. W., Kim, Y., Huang, X., Goldstein, L. E., Moir, R. D., Lim, J. T., Beyreuther, K., Zheng, H., Tanzi, R. E., Masters, C. L., and Bush, A. I. (2001) Neuron 30 665–676 [DOI] [PubMed] [Google Scholar]