Abstract
Cysteine dioxygenase (CDO) catalyzes the conversion of cysteine to cysteinesulfinic acid and is important in the regulation of intracellular cysteine levels in mammals and in the provision of oxidized cysteine metabolites such as sulfate and taurine. Several crystal structure studies of mammalian CDO have shown that there is a cross-linked cofactor present in the active site of the enzyme. The cofactor consists of a thioether bond between the γ-sulfur of residue cysteine 93 and the aromatic side chain of residue tyrosine 157. The exact requirements for cofactor synthesis and the contribution of the cofactor to the catalytic activity of the enzyme have yet to be fully described. In this study, therefore, we explored the factors necessary for cofactor biogenesis in vitro and in vivo and examined what effect cofactor formation had on activity in vitro. Like other cross-linked cofactor-containing enzymes, formation of the Cys-Tyr cofactor in CDO required a transition metal cofactor (Fe2+) and O2. Unlike other enzymes, however, biogenesis was also strictly dependent upon the presence of substrate. Cofactor formation was also appreciably slower than the rates reported for other enzymes and, indeed, took hundreds of catalytic turnover cycles to occur. In the absence of the Cys-Tyr cofactor, CDO possessed appreciable catalytic activity, suggesting that the cofactor was not essential for catalysis. Nevertheless, at physiologically relevant cysteine concentrations, cofactor formation increased CDO catalytic efficiency by ∼10-fold. Overall, the regulation of Cys-Tyr cofactor formation in CDO by ambient cysteine levels represents an unusual form of substrate-mediated feed-forward activation of enzyme activity with important physiological consequences.
Recent biochemical and crystallographic studies have revealed that some enzymes contain unusual modifications to the amino acid residues residing within their active sites. Also known as amino acid-derived cofactors, these altered residues represent a new and exciting area for research in the field of protein post-translational modifications. Unlike other post-translational modifications, which are made by attaching extrinsic molecules onto target proteins through reactions that are specifically catalyzed by third party enzymes, amino acid cofactors are generated directly from the amino acids within the proteins that contain them and are required for the production of fully competent enzymes (1, 2). From a biological perspective, amino acid-derived cofactors are significant because they can create novel structural motifs within the enzyme active site as well as alter the chemical properties of the unmodified parent amino acid residues and thus expand the otherwise limited range of catalytic reactions in which the common amino acids can participate.
One recent addition to the list of enzymes known to contain an amino acid cofactor is cysteine dioxygenase (CDO).3 CDO is an iron (Fe2+)-dependent thiol dioxygenase that uses molecular oxygen to oxidize the sulfhydryl group of cysteine to generate cysteinesulfinic acid (see Fig. 1A for reaction). In mammals, the enzyme plays an important role in the regulation of intracellular free cysteine levels and catalyzes the first step in the biosynthesis of essential metabolites of cysteine, such as sulfate, hypotaurine, and taurine (3–5). It has also been shown that CDO protein levels are highly regulated in response to cysteine in both cultured cells and in living animals (6, 7). This regulation occurs almost exclusively by changes in the rate at which CDO is ubiquitinated and degraded by the 26 S proteasome system.
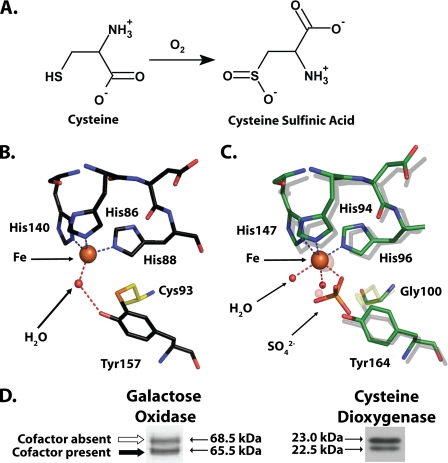
FIGURE 1.
A, reaction catalyzed by CDO. B, cutaway view of the rat CDO active site (PDB entry 2B5H (16)), showing the cross-linked amino acid cofactor composed of residue Cys93 covalently bound to Tyr157. Also visible is the iron cofactor of CDO and a single molecule of water hydrogen bonded to the iron and Tyr157. C, superimposition of select active site residues from a bacterial CDO (C. necator, indicated by a green carbon backbone; PDB entry 2GM6) and rat CDO (indicated by a gray carbon backbone). The indicated residue numbers are for C. necator. Note that C. necator CDO is unable to form a cross-linked amino acid cofactor because it contains a glycine in the position where there is a cysteine in rat CDO. D, both galactose oxidase (modified from Fig. 2 of Whittaker and Whittaker (13)) and rat CDO (obtained from rat liver) run as a double band when analyzed by SDS-PAGE. In the case of galactose oxidase, the apparent migration difference is because of the presence of a thioether-containing cross-linked cofactor in the bottom band and its absence in the top band (13).
Crystallographic studies have revealed that the amino acid cofactor in the active site of CDO consists of two cross-linked residues, cysteine 93 and tyrosine 157 (Fig. 1B). The γ-sulfur of cysteine 93 (Cys93-Sγ) is covalently bonded to the ortho-position of tyrosine 157 (Tyr157-Cε2). The cis configuration of the coplanar bond between Cys93-Sγ and Tyr157-Cε2 suggests that it may have partial double bond character. A similar thioether-containing cofactor has been previously reported in galactose oxidase (8), glyoxal oxidase (9, 10), and in NirA, a sulfite reductase (11).
There are two major aspects of the cross-linked cofactor in CDO that have yet to be adequately characterized: how the cofactor is synthesized and the role that it plays in CDO catalysis. Inadequate characterization of cofactor biosynthesis is not a problem that is unique to CDO, however, as the mechanisms by which most protein-derived cofactors are formed remain poorly understood (12). Galactose oxidase, however, forms a cross-linked cofactor that is identical to CDO, and the process by which it is synthesized has been examined. The in vitro formation of the cross-linked cofactor in galactose oxidase is a self-assembly process that is distinct from the biologically relevant catalytic activity of the enzyme and requires only the enzyme itself, molecular oxygen, and copper(I) (13, 14). The processing event is very fast (t½ = 3.9 s at pH 7), and formation of the cofactor is required before galactose oxidase is able to achieve catalytic competency (13). Another interesting finding from studies of galactose oxidase is that gel electrophoresis can be a useful analytical tool for the evaluation of cross-linked cofactor formation. Upon SDS-PAGE, recombinant galactose oxidase runs as a double band (Fig. 1D). Both mutational and mass spectrometry studies have verified that the apparent mass difference is an aberration caused by the presence of the cross-linked cofactor exclusively in the lower band.
Multiple reports have indicated that the CDO protein expressed by a variety of mammalian tissues runs as a double band on SDS-PAGE (6, 7, 15), a pattern that is very similar to that of galactose oxidase (Fig. 1D). This banding pattern had been attributed to an artifact of SDS-PAGE analysis after attempts to determine whether the difference in migration between the two bands represented an actual post-translational modification failed to identify any canonical small molecular weight modifications such as phosphorylation or glycosylation (15). In light of the recent discovery that CDO possesses a cross-linked cofactor, it seemed very likely that the underlying cause for the anomalous SDS-PAGE migration pattern of CDO is similar to that of galactose oxidase and is actually because of two separate populations of protein being expressed in vivo, one with the cofactor and one without. Whether the mechanism of cofactor synthesis is also similar to that of galactose oxidase is not known, although proposals have been made that it is and requires only oxygen and ferrous iron (16, 17).
The second unresolved issue regarding the cross-linked cofactor of CDO is its exact purpose. It has been postulated that the Cys93–Tyr157 cross-link plays an important structural role in the positioning of Tyr157 for participation in catalysis and in the maintenance of a tetrahedral coordination geometry around the iron metallocenter of the enzyme (16–18). Ye et al. (18) recently claimed that support for this hypothesis could be found in crystallographic observations of a CDO that is expressed by the bacterium Cupriavidus necator (PDB entry 2GM6). This bacterial CDO is unable to form a cross-linked cofactor, because its Cys93 equivalent is a glycine residue, and the enzyme displays a distorted octahedral geometry around its metallocenter as opposed to the tetrahedral geometry found in mammalian CDOs. Nevertheless, a characterization of the catalytic competencies of four separate bacterial CDOs, all of which have glycine substituted for Cys93 and hence are unable to form a thioether cross-link, has shown that these enzymes are highly active and show substrate specificities that are identical to mammalian CDO (19). Moreover, a close examination of the superimposed active sites of Rattus norvegicus CDO (PDB entry 2B5H) and C. necator CDO shows that the absence of the thioether bond results in only a slight displacement of the Tyr157 equivalent in C. necator CDO (Fig. 1C). Therefore, on the basis of structural observations alone, it is difficult to definitively deduce the precise role of the cross-linked cofactor. Another proposal is that the Cys-Tyr bond effectively stabilizes a radical intermediate and prevents extraneous reactions from damaging the enzyme (18), although there has been no direct evidence to support this hypothesis.
In this work, we report a series of investigations that explored the formation of the cross-linked cofactor of CDO in vitro using purified recombinant protein and rat liver lysate and in vivo using transfected hepatoma cells and intact rats. Using a panel of recombinant mutant proteins, we also explored the contribution of the cofactor to catalytic efficiency as well as its effect on catalytic half-life. These studies have revealed new insights into the mechanism of cofactor formation and its role in CDO activity. They also suggest that the synthesis of the cross-linked cofactor represents another level of post-translational control of the enzyme in response to substrate.
EXPERIMENTAL PROCEDURES
Cell Culture—HepG2/C3A human hepatocellular carcinoma cells (ATCC CRL-10741) were cultured in a humidified incubator at 37 °C and 5% CO2 in sulfur amino acid-free Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 1 mm sodium pyruvate, 0.1 mm l-methionine, 0.3 mm cysteine, 1× minimum Eagle's medium nonessential amino acid solution, and 0.05 mm bathocuproine disulfonate.
Animal Feeding Studies—For the animal study, Sprague-Dawley rats (170–210 g) were purchased from Harlan Sprague-Dawley (Indianapolis, IN). These rats were housed individually in polycarbonate cages containing wood chips and paper bedding in a room maintained at 20 °C and 60–70% humidity with light from 2100 to 0900 h. The rats had free access to water at all times but had access to food only during the dark cycle. For 2 weeks, the rats were fed a semipurified diet that contained 100 g of casein/kg (low protein). At the end of the light cycle on day 15, the diet was switched to one that contained 400 g of casein/kg (high protein). Rats were killed at 12, 24, 48, 72, or 144 h after the start of the new diet. Liver was removed, rinsed with ice-cold saline, weighed, immediately frozen in liquid nitrogen, and stored at –80 °C until analysis. Diets were prepared by Dyets, Inc. (Bethlehem, PA). The detailed composition of these diets has been reported previously by Lee et al. (20).
Plasmids/CDO Expression Constructs—For the expression of recombinant CDO protein in Escherichia coli, the open reading frame of wild-type (WT) rat CDO was subcloned into a modified version of the pET-32a expression plasmid as described previously (21). Single point mutants of this construct were engineered using the QuikChange® II site-directed mutagenesis kit (Stratagene) and the appropriate primer sets (supplemental Fig. 1). To transiently express CDO protein in HepG2/C3A cells, the open reading frame of wild-type rat CDO was subcloned into NotI/BamHI restriction sites of the p3XFLAG-CMV-7.1 vector (Sigma). This expression vector added a 3.5-kDa 3× FLAG peptide sequence to the N terminus of CDO, yielding a fusion protein with a final molecular mass of ∼26 kDa. R60A, C93S, and C164A mutants were made of the p3X-FLAG WT CDO mammalian expression vector using the QuikChange® II site-directed mutagenesis kit (Stratagene) and the appropriate primer sets (supplemental Fig. 1).
Recombinant Protein Expression and Purification—Recombinant protein was prepared as described previously (21) but with the minor modification that the ion-exchange step was omitted. Protein was cleaved from its thioredoxin-His6 tag by treatment with factor Xa (Calbiochem) and purified over a nickel immobilized metal affinity chromatography column as described previously (21).
Transfection Conditions for HepG2/C3A Cultures—Cells were seeded into 12-well tissue culture plates at a density of 2 × 105 cells/ml and allowed to attach for 24 h in the maintenance medium. When cells were at a confluency of ∼95% they were then transfected with the CDO expression constructs using Lipofectamine 2000 (Invitrogen). Cultures were allowed to take up the plasmid DNA complexes for 6 h, after which time the cell monolayers were washed twice with phosphate-buffered saline, and the experimental medium was added. The experimental medium was analogous to the maintenance medium with the exception that the concentration of cysteine was varied (0, 0.2, or 0.5 mm). Cells were incubated in the experimental medium for 24 h prior to harvesting.
Measurement of Total Free Thiol Content of Recombinant CDO Protein—Total free thiol content of recombinant CDO protein was assayed using dithio-bis(2-nitrobenzoic acid) (DTNB) as outlined by Whittaker and Whittaker (13). For this assay, the final concentration of protein was 5 μm in a solution of 4 m guanidinium chloride buffered with 50 mm MES, pH 5.3. Thiol concentrations were quantified by the use of a cysteine standard curve. Cysteine standards were processed in the same fashion as recombinant protein samples to obviate the effects of guanidinium chloride on the λmax of DTNB.
Molecular Weight Determination of Whole CDO Protein—Purified recombinant R60A CDO protein was initially dissolved in 20 mm ammonium acetate at a concentration of 22.2 μm (0.5 mg/ml). The protein was then diluted with 50% (v/v) acetonitrile (ACN), 20 mm ammonium acetate, 0.1% (v/v) formic acid to yield a final concentration of 10 pmol/μl. The diluted sample was infused at 2 μl/min into a three-dimensional ion trap mass spectrometer, Esquire-LC (Bruker, Billerica, MA). The instrument was operated under positive ion mode. The temperature was set to 280 °C, with spray voltage as 4 kV and nebulizer gas as 6 p.s.i./min. The instrument was optimized using 5 pmol/μl of standard lysozyme in the same solvent. Mass spectrometry (MS) spectra were acquired across the mass range of m/z 600–1800, and the molecular weight of identified proteins was determined by deconvolution using Bruker Daltonics DataAnalysis version 3.0.
Two-dimensional Gel Electrophoresis and In-gel Digestion for Excised Two-dimensional Gel Spots—The two isoforms of R60A CDO protein were resolved into two separate spots by two-dimensional gel electrophoresis. Following visualization of the two-dimensional gel, the protein spots were excised and placed into microtubes for subsequent in-gel digestion and manual extraction. The in-gel digestion and tryptic/chymotryptic peptide extractions were performed following a protocol from Shevchenko et al. (22) that was slightly modified. The gel pieces were washed and destained using the following sequence of solutions: 50 μl of water; 50 μl of 50% (v/v) ACN in 25 mm ammonium bicarbonate, pH 7.8; and 50 μl of 100% ACN. The samples were reduced with dithiothreitol and alkylated by treatment with iodoacetamide. Samples were then dried completely, and 0.2 μg of modified trypsin (Promega) or chymotrypsin (Sigma) in 20 μl of 50 mm ammonium bicarbonate, pH 7.8, in 10% (v/v) ACN was added to each tube. Samples were left on ice for 15 min and then incubated overnight at 37 °C.
Enzymatic Peptide Mapping by MALDI TOF-TOF and NanoLC/MS/MS Analyses—Digested sample was reconstituted in 5 μl of 50% ACN with 0.1% trifluoroacetic acid prior to MS analysis, and 1 μl was used to spot a MALDI target plate. Before the spotted sample could dry on the plate, 0.5 μl of saturated matrix (10 mg/ml α-cyano-4-hydroxycinnamic acid in 50% ACN with 0.1% trifluoroacetic acid and 1 mm ammonium phosphate) was spotted on top of each sample and then allowed to dry completely. The samples were then subjected to MALDI MS/MS analysis using a 4700 Proteomics Analyzer equipped with TOF-TOF ion optics (Applied Biosystem, Framingham, MA) and 4700 Explorer version 3.0. The instrument was operated in 1-kV reflector positive ion mode and calibrated with a calibration kit (Applied Biosystems, Framingham, MA) containing a mixture of six standard peptides, which was used as the default for chymotryptic samples. An additional internal calibration of two trypsin autolysis ions (m/z 842.508 and m/z 1045.564) was used for spectra acquisition of tryptic samples. The laser power was set to 2000 for MS and 3000 for MS/MS with collision-induced dissociation off. MS spectra were acquired across the mass range of 800–4000 Da. MS/MS spectra were acquired for the precursor ions of interest with a total accumulation of 2000 laser shots.
The remainder of each sample was dried and reconstituted in 10 μl of 2% ACN with 0.1% trifluoroacetic acid for LC-ESI-MS/MS analysis. NanoLC was carried out by a LC Packings Ultimate integrated capillary high performance liquid chromatography system equipped with a Switchos valve switching unit (Dionex, Sunnyvale, CA). The reconstituted peptides (6.4 μl) were injected using a Famous auto sampler onto a C18 PepMap trap column (5 μm, 300 μm × 5 mm, Dionex) for on-line desalting and then separated on a PepMap C-18 RP nano column. Peptides were eluted from the column over the course of 30 min using a linear gradient of 5–45% ACN in 0.1% formic acid at a flow rate of 250 nl/min. The nanoLC was connected in-line to a hybrid triple quadrupole linear ion trap mass spectrometer, 4000 Q Trap (ABI/MDS Sciex, Framingham, MA) equipped with a Micro Ion Spray Head ion source.
MS data acquisition was performed using Analyst 1.4.1 software (Applied Biosystems) in the positive ion mode for information-dependent acquisition (IDA) analysis. The nanospray voltage was set to 2.0 kV and used in positive ion mode for all experiments. Nitrogen was used as the curtain (value of 10) and collision gas (set to high) with heated interface on. The declustering potential was set at 50 eV and Gas1 was 15 p.s.i. In IDA analysis, after each survey scan for m/z 400 to m/z 1550 and an enhanced resolution scan, the three highest intensity ions with multiple charge states were selected for tandem MS (MS/MS) with rolling collision energy applied for detected ions based on different charge states and m/z values.
MS Data Analysis—The MS spectra from MALDI-TOF/TOF on each sample were manually inspected and compared with the predicted in silico tryptic/chymotryptic digest of R60A CDO. The MS/MS spectra of individual peptides were also inspected for differences between the two CDO isoforms. The MS/MS data generated from nanoLC/ESI-based IDA analysis were submitted to Mascot 2.1 for data base searching using in-house licensed Mascot local server, and the search was performed to query to the NCBInr (taxonomy, Mammals) data base with one missed cleavage site by trypsin and two missed cleavage sites by chymotrypsin allowed. The peptide tolerance was set to 1.2 Da and MS/MS tolerance was set to 0.6 Da. Carboxyamidomethyl modifications of cysteine and a methionine oxidation were set as variable modifications. Only significant scores for the peptides defined by Mascot probability analysis greater than “identity” were considered for the peptide identifications. For the mutant samples, the error tolerant search was performed to confirm identification.
For identification of any other potential biological modifications, the MS/MS data generated from nanoLC/ESI-based IDA analysis were also searched against the Mammals FASTA data base using ProteinPilot™ software 2.0 (Applied Biosystems, Foster City, CA) and the paragon algorithm. The carboxyamidomethyl modifications of cysteine and a methionine oxidation were used for thorough Protein ID searching with additional biological modifications. Peptide identifications were confirmed with scores representing >95% statistical significance.
CDO Activity Assays—Purified recombinant protein was assayed for its ability to oxidize cysteine to cysteinesulfinic acid in vitro. The basic assay conditions consisted of 100 μl of protein, 0.1–10 nmol of CDO (as quantified by a bicinchoninic acid assay) diluted to a final volume of 400 μl in a buffer system that contained the following reagents (values indicate final concentrations): 50 mm MES, 0.3 mm ferrous sulfate, 0–50 mm cysteine, and 62.5 μm bathocuproine disulfonate. The final concentration of protein used in the reaction varied for each construct and was adjusted to ensure that no more than 10% of cysteine was consumed during the reaction. Reactions were initiated by the addition of cysteine and incubated at 37 °C on an Eppendorf thermomixer (Brinkmann Instruments) with shaking at 900 rpm. Reactions were terminated by the addition of 200 μl of 5% (w/v) sulfosalicylic acid, and tubes were centrifuged at 16,000 × g to pellet precipitated protein. Then 10 μl of supernatant was removed and diluted with 10 μl of 200 μm asparagine (an internal standard) and 380 μl of 200 mm borate buffer, pH 10.4. Cysteinesulfinic acid and asparagine levels in this mixture were measured by high performance liquid chromatography with detection of the fluorescent derivatives of these amino acids. Chromatography of derivatized samples was carried out on a 4.6 × 150-mm column packed with Nova-Pack C18 packing materials (4 μm spherical particles; Waters) equipped with a C18 cartridge (5 μm spherical particles; All-tech). Using an automatic sample injector (WISP model 717 plus; Waters), 70 μl of o-phthalaldehyde/β-mercaptoethanol derivatization reagent and 30-μl volume of sample solution were injected into the pre-column tubing. The o-phthalaldehyde/β-mercaptoethanol/sample solution was allowed to react for 1 min prior to injection into the eluent stream. The derivatization reagent was prepared fresh daily by first mixing 3.5 mg of o-phthalaldehyde with 150 μl of 100% ethanol and then adding 5 ml of 200 mm borate buffer, pH 10.4, and 10 μl of β-mercaptoethanol.
Separation of amino acids was accomplished by gradient elution at a flow rate of 1.0 ml/min and a column temperature of 30 °C. Buffer A of the gradient consisted of 50 mm potassium phosphate buffer with 3% (v/v) tetrahydrofuran, final pH 7.0, and buffer B consisted of 50 mm potassium phosphate buffer with 3% tetrahydrofuran and 60% ACN, final pH 7.0. Buffers were filtered through a 0.45-μm filter prior to use. The mobile phase was started at 100% buffer A and then linearly increased to 30% buffer B over the first 10 min. 30% buffer B was then maintained for a total of 3 min after which it was linearly increased to 100% buffer B over 3 min and then maintained at 100% buffer B for 2 min. The gradient was then switched back to 100% buffer A over the course of an additional 3 min, and the column was allowed to equilibrate for 6 min prior to the next injection. Detection of derivatized amino acids was done using a multiple wavelength fluorescence detector (model 2475, Waters) at an excitation wavelength of 360 nm and an emission wavelength of 455 nm. The fluorometer was connected to a personal computer equipped with Empower 2 software (Waters) for integration of chromatographic peaks and interpolation of analyte concentrations. Cysteinesulfinic acid values obtained by this method were first normalized by the internal asparagine standard and then quantified against an external cysteinesulfinic acid standard curve before finally being used to calculate CDO activity.
SDS-PAGE and Immunoblotting—To obtain adequate resolution of the CDO isoforms, either a 12 or 15% (w/v) polyacrylamide gel was used. WT and mutant recombinant CDOs were detected using Coomassie Blue staining of gels following separation. Western blots were used to evaluate CDO expression in either mammalian cell culture or in rat liver using SDS-PAGE and immunoblotting with an affinity-purified polyclonal CDO antibody as described previously (6).
RESULTS
Resolution of Non-cofactor- and Cofactor-containing Species of CDO—In previous studies, we have consistently observed that CDO protein endogenously expressed in mammalian tissues migrates as a double band on SDS-PAGE (Fig. 2) (6, 15, 23). With the recent discovery of a cross-linked cofactor in the active site of CDO, we hypothesized that the difference in migration could be due to two separate populations of CDO protein as follows: one population containing the internal cross-link and the other lacking the cross-link.
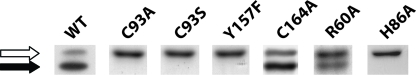
FIGURE 2.
SDS-PAGE analysis of purified recombinant WT and mutant CDO proteins. In this panel of proteins, the position of the upper band is indicated by a white arrow and the position of the lower band is indicated by a black arrow. Proteins were separately resolved by SDS-PAGE on a 12% (w/v) polyacrylamide gel and visualized by Coomassie Blue staining. Assessment of upper versus lower band positions for each construct was determined by comparison to a WT CDO standard that was run on each gel. A total of 2 μgof protein was loaded.
To further explore whether the SDS-PAGE migration difference was because of the presence of a cross-link between Cys93 and Tyr157, we used recombinant CDO protein that could be heterologously expressed, cleaved from its thioredoxin-His6 tag, and purified to homogeneity (>95%). As shown in Fig. 2, recombinant WT CDO ran as a double band by SDS-PAGE, although most of the purified protein was found in the faster migrating lower band. Mutation of Cys93 to either an alanine (C93A) or a serine (C93S) resulted in only a single protein band that corresponded to the upper band of WT CDO. Similarly, mutation of Tyr157 to a phenylalanine (Y157F) produced a single band that migrated to the same apparent molecular weight as the upper band of WT. These results were consistent with the hypothesis that the cross-link between Cys93 and Tyr157 is responsible for the production of the CDO lower band.
To eliminate the possibility that any active site mutation would result in the disruption of the double band migration pattern of CDO on SDS-PAGE, we mutated other active site residues that are not directly part of the thioether cofactor (Fig. 2). Mutation of residue Cys164, which lies near the opening of the CDO active site, to an alanine produced a mutant (C164A) that showed a double band migration pattern that was indistinguishable from that of WT CDO. Substitution of alanine for residue Arg60 (R60A), which has been implicated as an important residue in coordinating substrate in the active site (16–18), resulted in the formation of two bands with a nearly equal distribution of protein between the two bands. On the other hand, substitution of His86, one of the three histidine residues involved in the coordination of the iron cofactor in CDO, with an alanine produced an inactive CDO that migrated as a single protein species that corresponded to the upper band of WT. These results clearly demonstrated that the double band phenomenon was not indiscriminately disrupted by active site point mutations. They also indirectly suggested that the formation of the lower band could be tied to the catalytic activity of the protein (see below).
An assessment of the total free thiol content of several of the recombinant CDO proteins was done to probe for the presence of a thioether cross-link (Table 1). Because of the presence of four cysteine residues in the protein, one molecule of WT CDO protein should theoretically contain four accessible free thiols in the absence of a cross-link between Cys93 and Tyr157. Our data indicated, however, that there were only 2.8 DTNB-reactive free thiol groups per molecule of WT CDO. This suggests that the free thiol group of a single cysteine residue was being blocked, perhaps by incorporation into the Cys93–Tyr157 cofactor. This hypothesis was bolstered by the fact that the C93S and Y157F constructs were the only mutants found to contain the expected number of free cysteines. Of the remaining mutants, R60A had a value that was intermediate between WT and Y157F, possibly because of the fact that only about half of the purified protein was expected to contain the cofactor as inferred from its relative upper and lower band distribution on SDS-PAGE. C164A, like WT, was one short of the expected number of three free cysteine residues, also consistent with the presence of most of this protein in the cofactor-containing form.
TABLE 1.
Total accessible free thiol values for recombinant CDO proteins
Values for observed number of free thiols are expressed as means ± S.D. and were derived from measurements on three independent preparations of recombinant protein.
| Construct | Expected No. of free thiols | Observed No. of free thiols |
|---|---|---|
| mol —SH/mol protein | mol —SH/mol protein | |
| Wild type | 4 | 2.8 ± 0.3 |
| R60A | 4 | 3.3 ± 0.2 |
| C93S | 3 | 2.6 ± 0.5 |
| Y157F | 4 | 3.8 ± 0.1 |
| C164A | 3 | 1.9 ± 0.3 |
To obtain additional direct evidence for the existence of the amino acid cofactor, a series of mass spectrometry experiments was conducted. The R60A mutant was used for these experiments because preparations of this protein consistently provided nearly equivalent amounts of upper and lower band. First, we sought to determine the molecular weight of each of the two protein species. According to the SDS-PAGE migration patterns, the apparent molecular mass difference between the two species of CDO was estimated to be on the order of several hundred daltons. But if the differential migration pattern was because of the presence or absence of a thioether linkage, then the actual mass difference would be predicted to be only 2 Da. Electrospray ionization-mass spectrometry (ESI-MS) of R60A CDO showed one major protein component with a molecular mass of 22.9 kDa (ESI-MS spectra and deconvolution data shown in supplemental Fig. 2). This observation is in agreement with the hypothesis that the two protein species seen by SDS-PAGE analysis differed by a very small mass that could not be resolved by the MS instrumentation, which had a mass accuracy of ±4 Da after deconvolution for the 23-kDa protein.
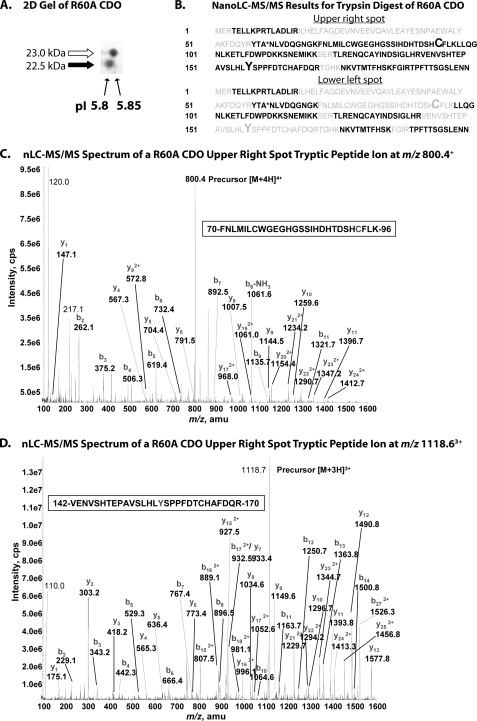
Our second set of mass spectrometry experiments focused on directly detecting the presence of cross-linked peptide fragments in R60A protein that was digested by either trypsin or chymotrypsin endopeptidases and subjected to nanoLC-MS/MS and /TOF analysis. The R60A sample was first resolved by two-dimensional electrophoresis, with isoelectric focusing as the first dimension and SDS-PAGE as the second dimension, to avoid potential cross-contamination of the two isoforms. The results for this separation, which revealed that the lower band has a slightly lower pI than the upper band, are shown in Fig. 3A. In the tryptic digest of the upper band, all possibly detectable peptides were observed, and their sequences were verified (Fig. 3B); note that the peptide covering residues 18–52 was too large to detect with the bottom-up analysis approach. Because the MS/MS spectra of identified peptides included those containing residues Cys93 (Fig. 3C) and Tyr157 (Fig. 3D), we could definitively discount the possibility of a thioether cross-link in the upper band. For the tryptic map of the bottom band, however, the peptides covering Cys93 and Tyr157 could not be detected (Fig. 3B), consistent with the two peptides being linked. Indeed, it is not surprising that the cross-linked peptide (56 amino acid residues) was not detected because the peptide would have been at the limits of detection with the bottom-up approach. We therefore explored an alternative digestion with chymotrypsin, which theoretically would produce a smaller cross-linked peptide fragment (22–24 residues) within the limits of our detection.
FIGURE 3.
A, two-dimensional gel (isoelectric focusing, SDS-PAGE) analysis of recombinant R60A CDO illustrating differences in pI between the upper and lower band species of CDO protein. B, composite summaries of amino acid sequences identified in peptide fragments of R60A CDO generated by trypsin digestion of the upper right and lower left spots resolved in A followed by nanoLC-MS/MS analysis. Amino acid residues positively identified in peptide fragments are indicated in black, and those that were not detected are indicated in gray. Residues Cys93 and Tyr157 are indicated in an enlarged font. The mutated residue (R60A) is indicated by an asterisk. C, MS/MS spectrum of the doubly charged peptide (FNLMILCWGEGHGSSIHDHTDSHCFLK) ion, acquired from nanoLC-MS/MS analysis of tryptic digest of R60A CDO upper band. MS/MS spectrum showed that both Cys76 and Cys93 residues are carboxyamidomethyl cysteine. D, MS/MS spectrum of the doubly charged peptide (VENVSHTEPAVSLHLYSPPFDTCHAFDQR) ion, acquired from nanoLC-MS/MS analysis of tryptic digest of R60A CDO upper band. The Cys164 residue is carboxyamidomethyl cysteine. Abbreviations used are as follows: cps, counts/s; amu, atomic mass unit.
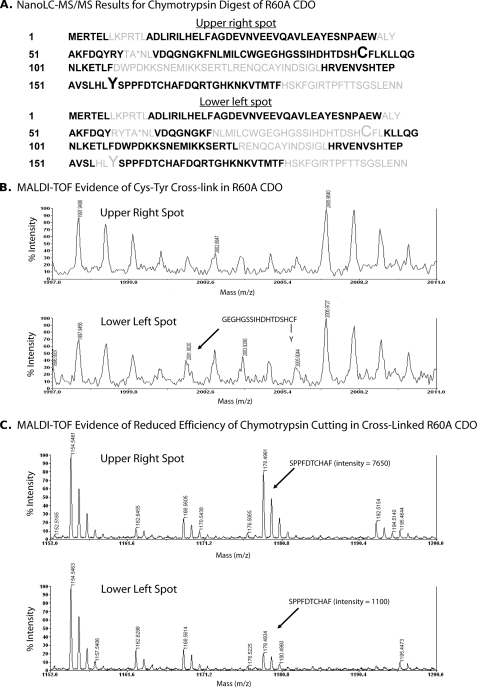
Analogous to the tryptic mapping pattern, chymotryptic digest of the upper band again revealed the presence of separate Cys93- and Tyr157-containing peptides, suggesting that there was no cross-link in the upper band (Fig. 4A). Both individual peptide ions were confirmed by MS/MS spectra (data not shown). Also, the separate Cys93 and Tyr157 peptide fragments were not detected in the lower band, again suggesting that the two could be covalently linked (Fig. 4A). Unfortunately, we could not obtain explicable MS/MS data for a cross-linked chymotryptic peptide. But there was some weak MALDI-TOF evidence at m/z 2001.9 ion indicating the presence of a cross-linked chymotryptic peptide in the lower band but not in the upper band (Fig. 4B). Further evidence was a greatly reduced efficiency for chymotrypsin cleavage of the peptide bond between Tyr157 and Ser158 in the lower band versus the upper band (Fig. 4C), which could have been caused by a cross-link that was sterically impairing chymotrypsin activity. This inefficiency of cutting is also consistent with the weak signal observed for the expected cross-linked peptide ion.
FIGURE 4.
A, composite summary of amino acid sequences identified in peptide fragments of R60A CDO generated by chymotrypsin digestion of the upper right and lower left spots from two-dimensional gel electrophoresis followed by nanoLC-MS/MS analysis. Amino acid residues positively identified in peptide fragments are indicated in black, and those that were not detected are indicated in gray. Residues Cys93 and Tyr157 are indicated in an enlarged font. The mutated residue (R60A) is indicated by an asterisk. B, MALDI evidence for the presence of a Cys93–Tyr157 cross-linked peptide in the lower left spot but not in the upper right spot of R60A CDO. C, MALDI data indicating a reduced cutting efficiency of chymotrypsin in the lower left spot of R60A CDO at the covalent bond between Tyr157 and Ser158, resulting in a poor yield of the SPPFDTCHAF peptide compared with the yield for the upper right spot of R60A CDO.
To obtain stronger MS-based evidence for a cross-linked fragment in the lower band, a double mutant (D48K/T148K) was engineered. The two mutations introduced additional trypsin cut sites that would hypothetically make a smaller cross-linked peptide fragment (30 amino acids long) following tryptic digest. The double mutant ran as a double band on SDS-PAGE, and tryptic mapping of the individual bands revealed separate Cys93- and Tyr157-containing peptides in the upper band of this mutant that were clearly absent in the lower band (data not shown). Again, however, we were unsuccessful in obtaining definitive MS/MS data for a cross-linked peptide in the lower band of the D48K/T148K mutant.
It should be noted that similar difficulties in obtaining definitive MS evidence for a cross-linked cofactor have been recently reported for NirA (11). This protein contains a Cys-Tyr cofactor that is identical in structure to CDO and galactose oxidase. WT NirA also shows a double band migration pattern by SDS-PAGE that collapses to a single upper band following mutation of either of the cross-linked residues.
Collectively, our data argue that the apparent mass difference between the two bands of CDO, based on their separation by SDS-PAGE, is not because of a large molecular weight post-translational modification of the protein, including a cleavage event at the N or C terminus. Rather the migration pattern appears to be due to the differential presence of an internal cross-link between Cys93 and Tyr157, which is present in the lower band and absent in the upper band.
Effect of Cofactor Formation on CDO Activity—With the ability to distinguish the cofactor- and non-cofactor-containing species of CDO, we decided to reevaluate the contribution of the cross-linked cofactor to catalysis by performing a series of kinetic studies on recombinant WT and mutant CDO proteins using a wide range of cysteine concentrations.
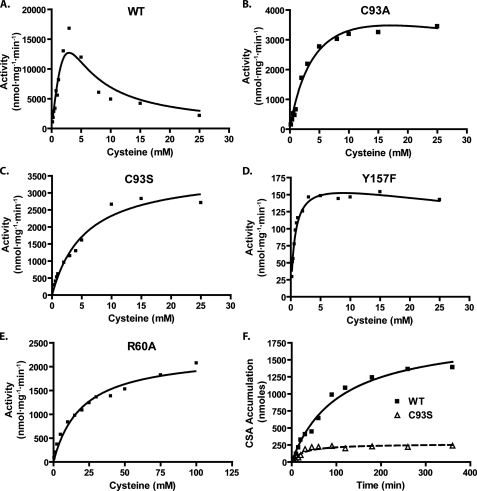
Within the range of physiologically relevant cysteine concentrations (0–0.5 mm), WT CDO exhibited high amounts of activity (Fig. 5A). At supraphysiological levels of cysteine, however, the enzyme showed signs of strong substrate inhibition. Because of this, estimates of catalytic efficiency (kcat/ Km) were done using low substrate levels where first-order reaction conditions still applied (Table 2). Prevention of cofactor formation by mutation of Cys93 to either an alanine or serine failed to abolish the activity of the enzyme. This mutation did, however, result in a dramatic reduction of CDO activity to ∼10% of that observed for WT CDO (Table 2). At supraphysiological substrate levels, however, the Cys93 mutants demonstrated significantly less substrate inhibition than WT CDO (Fig. 5, B and C). Interestingly, as a consequence of the reduced substrate inhibition, the activities of the C93S mutants were ∼50–75% of WT at the highest levels of cysteine tested in our assays.
FIGURE 5.
Effect of various point mutations on CDO activity and catalytic half-life. Cysteinesulfinic acid (CSA) production was measured at various cysteine concentrations for the following purified recombinant proteins: WT (A), C93A (B), C93S (C), Y157F (D), and R60A (E). The final concentration of protein used for the activity assay experiments was as follows: WT, 1 μm; C93A/C93S, 5 μm; Y157F, 100 μm; R60A, 10 μm. Curve fitting was done using Prism4 software (GraphPad) and an integrated algorithm of the Michaelis-Menten equation for substrate inhibition. F, evaluation of the linearity of cysteinesulfinic acid production over time for the WT and C93S CDO proteins. For this last series of activity assays, cysteine was used at a final concentration of 7.5 mm, and the final concentration of CDO protein used was 1 μm WT and 4 μm C93S. The curve fit to the figure in F was done with hyperbolic regression analysis using Prism4. All assays were conducted in MES buffer, pH 6.1, with 0.3 mm ferrous iron.
TABLE 2.
Catalytic efficiency of recombinant CDO mutants
Values for kcat/Km were calculated using activity data shown in Fig. 5, A–E. Because of the high amount of substrate inhibition shown by WT CDO, only activity data that were obtained from low amounts of substrate ([S] ≪ Km) and that showed first-order kinetics were used. Tangents were fitted to the data with an intersection at the origin, and the slope of the resultant tangent was used as an estimate of kcat/Km.
| Construct | kcat/Km |
|---|---|
| m-1 s-1 | |
| Wild type | 3200 |
| R60A | 39 |
| C93A | 290 |
| C93S | 220 |
| Y157F | 55 |
Abrogation of cofactor formation by substitution of a phenylalanine for Tyr157, whose phenol group is postulated to play an integral role in the catalytic process (16–18), reduced the catalytic efficiency of the enzyme by almost 2 orders of magnitude (Table 2). The Y157F enzyme still showed evidence of substrate inhibition at supraphysiological levels of cysteine (Fig. 5D). Interestingly, mutation of Arg60, which is hypothesized to play an important role in substrate coordination (16–18), reduced the catalytic efficiency of the enzyme to a level lower than that seen for the Y157F mutant (Table 2). This reduction, however, appeared to be due principally to a substantially increased Km value for cysteine (Fig. 5E). Mutation of His86 to an alanine resulted in no detectable CDO activity, as would be predicted given the essential iron binding function of this residue (data not shown).
In the process of evaluating reaction linearity with time, we also observed that the cross-linked cofactor appeared to affect the catalytic half-life of the enzyme. As shown in Fig. 5F, WT CDO remained catalytically active in vitro approximately three times longer than C93S CDO.
Overall, these data indicate that the formation of the cross-linked cofactor is not strictly essential for catalytic activity. Its presence, however, dramatically increases catalytic efficiency by more than a factor of 10 at physiologically relevant cysteine concentrations and makes the enzyme more refractory to catalytic inactivation in vitro.
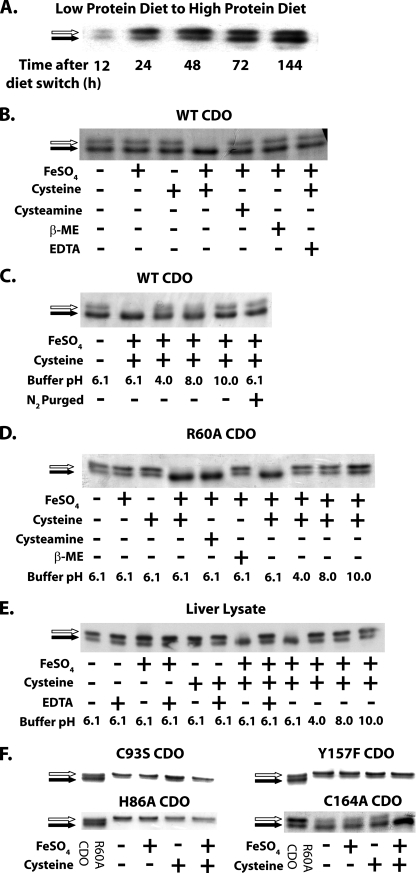
Cofactor Biosynthesis Depends upon Substrate and Catalytic Turnover—Several previous studies have demonstrated that the lower cofactor-containing band of hepatic CDO protein is highly expressed when rats are fed diets that are rich in cysteine or cysteine precursors (6, 23). We further examined the course of this expression in vivo by switching rats that had been sustained for 14 days on a low protein diet (i.e. low sulfur amino acid-containing) to a high protein diet (i.e. high sulfur amino acid-containing). Within 24 h of the diet switch, there was a large initial increase in the non-cofactor-containing upper band. Following the initial increase of the upper band, there was a slow increase in the cofactor-containing lower band, which continued to accumulate for up to 6 days after the diet change (Fig. 6A).
FIGURE 6.
A, Western blot of rat liver lysates illustrates the increase in CDO concentration and the gradual increase in cross-linked cofactor formation (increase in proportion of total CDO and the lower band) between 24 and 144 h after rats were switched from a low to a high protein diet. B, Coomassiestained gel showing the in vitro effect of ferrous iron (FeSO4, final concentration = 0.3 mm), cysteine (final concentration = 10 mm), cysteamine (final concentration = 10 mm), β-mercaptoethanol (final concentration = 10 mm), and EDTA (final concentration = 1 mm) on cofactor formation in recombinant WT CDO protein. C, cofactor formation in recombinant WT CDO following alteration of reaction buffer pH or following reduction of oxygen levels through N2 purging. D, cofactor formation in recombinant R60A CDO protein upon incubation with various substrate analogs or in buffers of different pH values. Final concentrations of compounds are as follows: FeSO4, 0.3 mm; cysteine, 50 mm; cysteamine, 50 mm; β-mercaptoethanol (β-ME), 50 mm. E, Western blot illustrating in vitro cofactor formation in CDO from liver lysate of a rat fed a low protein diet. The final concentrations of compounds used were the same as those employed in B. F, effect of ferrous iron and cysteine on cofactor formation in the mutant CDO proteins H86A, C93S, Y157F, and C164A, which served as controls for catalytically active or inactive proteins that could or could not form the thioether cofactor. The final concentrations of iron and cysteine are the same as those for B. All of the cofactor formation assays shown in B–F were performed for 10 min at 37 °C. Cofactor-deficient CDO is indicated by a white arrow, and cofactor-containing CDO is indicated by a black arrow. The buffering systems used in the reactions are as follows (organized by pH): pH 4.0, 50 mm MES buffer; pH 6.1, 50 mm MES; pH 8.0, 50 mm Tris-Cl; pH 10.0, 50 mm borate buffer. Immunoblots (A and E) and Coomassie-stained gels (B–D and F) show representative results from experiments that were independently replicated 2–3 times.
The whole animal observations suggested that cofactor formation in hepatic CDO was somehow dependent upon prolonged exposure to high levels of cysteine. We therefore hypothesized that the cofactor could be synthesized as a by-product of the binding of cysteine to the active site. In addition to explaining the effect of cysteine on cofactor formation in whole animals, this hypothesis could also explain the previously mentioned effects of the R60A mutant on cofactor formation in purified recombinant CDO. Compared with WT CDO, the R60A mutant exhibited a significantly reduced affinity for substrate and had an increased relative ratio of noncofactor to cofactor-containing species.
When incubated with 10 mm cysteine and 0.3 mm ferrous iron in vitro under activity assay conditions, recombinant WT and R60A CDO protein as well as CDO from rat liver lysate could be entirely converted into the cofactor-containing isoform within 10 min (Fig. 6, B, D, and E). Excluding either component from the in vitro reaction system prevented the formation of the cofactor. Cofactor formation was also prevented when oxygen was excluded from the reaction system by purging all solutions with N2 gas (Fig. 6C).
Additional experiments were conducted to evaluate the dependence of cofactor formation upon catalytic activity. Recombinant CDO protein exhibits catalytic activity only within a narrow pH range (∼5.5–7.0) (21). When the reaction system pH was altered to a value either above or below this range, cofactor formation was significantly impaired (Fig. 6, C–E). The dioxygenation reaction catalyzed by WT CDO is highly specific to cysteine, and structurally similar organic thiols such as cysteamine or β-mercaptoethanol are unable to be catalytically processed (19, 24, 25). In the case of WT CDO or liver lysate, cofactor formation also showed a high degree of specificity for cysteine; cysteamine or β-mercaptoethanol was unable to induce cofactor formation (Fig. 6B). R60A CDO, on the other hand, was able to use either cysteine or cysteamine for cofactor formation (Fig. 6C). We hypothesize that this could be due to relaxed substrate discrimination at the active site caused by mutation of Arg60. No corresponding band shift was observed in C93S and Y157F, which lacked the residues necessary to form the cofactor (Fig. 6E). The catalytically inactive H86A was also not able to form the cofactor-containing species (Fig. 6E), whereas the catalytically active C164A was able to form a cross-linked cofactor (Fig. 6E).
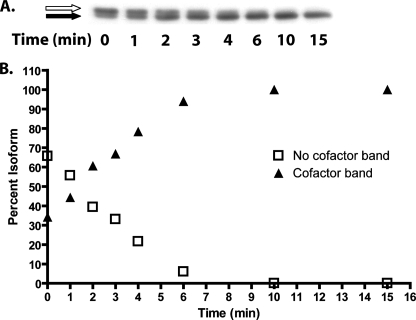
To gain a better understanding of the time course of in vitro cofactor biosynthesis for WT CDO, rat liver lysate from an animal fed a low protein diet was incubated with cysteine and iron under standard assay conditions, and cofactor formation was observed (Fig. 7A). Under these conditions, 10% of the non-cofactor-containing protein was converted to cofactor-containing protein every minute (Fig. 7B). If we assume that the catalytic turnover rate of the non-cofactor containing WT CDO is closely approximated by the turnover rate of the C93S mutant (kcat = 2.3 s–1), then according to the time course data it took ∼800 catalytic cycles to synthesize the cofactor in 50% of the non-cofactor-containing protein pool.
FIGURE 7.
A time course evaluation of cofactor formation in vitro. A, representative Western blot showing cofactor formation in CDO from rat liver lysate that was incubated with cysteine at a final concentration of 10 mm and FeSO4 at a final concentration of 0.3 mm for 0–15 min. Similar data were obtained from a total of three experiments. Cofactor-deficient CDO is indicated by a white arrow, and cofactor-containing CDO is indicated by a black arrow. B, densitometric analysis of the bands in A, expressed as a percent of total.
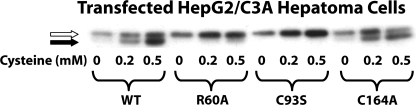
We further tested the dependence of cofactor formation upon substrate turnover in living cells. HepG2/C3A hepatoma cells, which do not express endogenous CDO protein, were transfected with expression constructs that would enable them to produce WT, R60A, C93S, or C164A CDO protein. Regardless of the CDO construct used, the non-cofactor-containing species was present at a low level in cells cultured in cysteinefree medium (Fig. 8). The addition of cysteine to the medium resulted in a progressive increase in this species because of an increase in the half-life of the protein (7). The appearance of the cofactor-containing isoform of CDO was readily apparent in cells transfected with either WT CDO or C164A CDO when these cells were exposed to cysteine. Moreover, the total amount of the cofactor species increased in direct concert with medium cysteine concentration. The R60A CDO construct, however, showed an impairment of in vivo cofactor synthesis, most likely because of the high Km value exhibited by this mutant CDO. Faint amounts of the cofactor form of R60A CDO were detected only when cells were incubated with a high concentration of cysteine. In contrast, no concentration of cysteine was able to induce cofactor formation in cells transfected with C93S.
FIGURE 8.
A Western blot illustrating the effect of cysteine concentration on CDO cofactor formation in vivo. Lysates used in this blot were from HepG2/C3A cells that were transfected with expression plasmids containing the open reading frames of WT, R60A, C93S, or C164A CDO and then incubated with the indicated concentrations of cysteine for 24 h. A total of 30 μg of protein was loaded per lane. Cofactor-deficient CDO is indicated by a white arrow, and cofactor-containing CDO is indicated by a black arrow. The data shown here are representative of results that were replicated in three separate experiments.
In summary, our data from both in vitro and in vivo experiments clearly support the hypothesis that cofactor formation is dependent upon binding of substrate and the occurrence of catalytic turnover. The data also indicated that the process is somewhat inefficient, requiring multiple catalytic cycles to occur.
DISCUSSION
The synthesis of the cross-linked cofactor of CDO has two unusual characteristics that set it apart from other cofactor-containing enzymes. The first characteristic is that cofactor formation is dependent upon the presence of the physiological substrate of the enzyme. The second is that it also requires the enzyme to be catalytically competent. Because cofactor formation showed a strict dependence upon substrate and that the presence of the cofactor greatly enhanced the catalytic efficiency of the enzyme, we propose that cofactor formation constitutes a novel post-translational regulatory mechanism for the control of CDO activity in response to substrate levels in vivo.
In other enzyme systems, for which biogenesis of the protein-derived cofactor has been well characterized, formation of the cofactor is often a self-processing event intrinsic to the enzyme and is separate both temporally and functionally from the biologically relevant catalytic activity of the enzyme. Moreover, cofactor formation must occur before the enzyme becomes catalytically competent. Galactose oxidase, the archetypal enzyme for the study of cross-linked amino acid cofactors, contains a Cys-Tyr cofactor that is structurally analogous to the cofactor found in CDO. In the case of galactose oxidase, however, its cofactor is formed spontaneously and with a rapid time course following exposure of homogeneously purified enzyme to molecular oxygen and copper(I) (13, 14). Biogenesis of the topoquinone cofactor found in copper-dependent amine oxidases has also been reported to occur in a similar fashion, with the hydroxylation/oxidation of a catalytically essential tyrosine residue rapidly occurring simply in response to incubation of the apoenzyme precursor with copper(II) and molecular oxygen (26, 27).
Like the aforementioned enzymes, the formation of the cross-linked cofactor in CDO is a self-processing event that requires no third-party proteins. CDO, however, appears to depart from other amino acid cofactor-containing enzymes in two notable ways. The first major departure is that the Cys-Tyr cofactor in CDO is not essential to the catalytic formation of cysteinesulfinic acid from cysteine, and therefore cofactor synthesis clearly does not need to temporally precede catalytic competency of CDO. More specifically, it was observed that ablation of cofactor formation by mutation of Cys93 resulted in an enzyme that still had substantial amounts of catalytic activity. It is important to note, however, that once the cofactor was produced, the catalytic efficiency of the CDO was increased by an order of magnitude at physiologically relevant concentrations of cysteine.
The strong effect of cofactor formation on CDO activity differs from what was previously reported by Ye et al. (18) for human CDO. They reported that mutation of Cys93 to an alanine or serine reduced activity by only ∼50%, and mutation of Tyr157 to a phenylalanine reduced activity to only 5% of WT CDO. In their study, however, specific activities for these mutants were assessed only at extremely high cysteine levels (100 mm), and full kinetic profiles were not done. This is particularly germane in light of our repeated observation that recombinant WT CDO protein exhibits marked substrate inhibition at high levels of cysteine (19, 21). Moreover, we found in this study that the Cys93 mutants possessed substantially less substrate inhibition than WT, which would also significantly diminish the apparent difference in activity between these mutants and WT CDO when assessed at supraphysiological levels of cysteine. Another possible reason for the discrepancy in results between the two studies is that Ye et al. (18) did not add exogenous ferrous iron to their activity assays. This is an important methodological consideration in light of studies showing that recombinant CDO protein loses substantial amounts of iron over the course of purification, and as a consequence, exogenous ferrous iron must be added to saturate the enzyme and obtain maximal activity (19, 21, 28).
Precisely how the Cys-Tyr cofactor improves the catalytic efficiency and catalytic half-life of CDO is not clear and deserves additional study. One future study to help resolve this issue would be to obtain a crystal structure of a mammalian CDO that is lacking the thioether linkage between Cys93 and Tyr157, either by using a mutant such as C93S or by preparing, expressing, and purifying WT protein under anaerobic and/or iron-free conditions.
The second major departure of CDO from other amino acid cofactor-containing enzymes is that it was unable to synthesize its amino acid cofactor when incubated with its ferrous iron cofactor and oxygen alone. An additional requirement was the presence of its substrate cysteine. The need for cysteine did not appear to be a simple reductant effect. The thiols cysteamine and β-mercaptoethanol, which are structurally very similar to cysteine, were unable to facilitate cofactor formation for WT CDO in vitro. Non-thiol reducing agents such as ascorbate and tris(2-carboxyethyl)phosphine were also unable to induce cofactor formation (data not shown). Moreover, the requirement for cysteine appeared to be contingent upon its binding at the active site along with iron and upon actual catalytic turnover of the cysteine. Decreasing the affinity of CDO for cysteine, as occurred with the R60A mutation, resulted in the need for higher concentrations of cysteine to induce cofactor formation in vitro and in vivo. Catalytic inactivation of the enzyme by mutation of His86 to an alanine, which abolished iron binding, completely prevented cofactor formation. We also suspect that the requirement of cysteine for cofactor formation was the principal reason why much of the WT CDO expressed in mammalian tissues was found as the non-cofactor containing species, whereas WT CDO expressed in E. coli as a recombinant protein was found primarily as the cofactor-containing isoform. Measurements of the concentration of cysteine in the E. coli BL21(DE3) strain used for the expression of recombinant CDO revealed that cysteine levels were ∼ 1 mm following induction of protein expression, which is about an order of magnitude higher than the cysteine levels found in rat liver in vivo (20). The higher cysteine concentration in E. coli would have greatly facilitated cofactor biogenesis in the recombinant protein.
One explanation for the dependence of cofactor formation upon the presence of cysteine could relate to the substrate binding order of CDO. Pierce et al. (29) recently reported that, in the absence of cysteine, the iron metallocenter of CDO is essentially unreactive to NO (used as a surrogate probe for O2). Binding of NO, however, is greatly facilitated when CDO is incubated in the presence of cysteine. The obligate reactant binding order that is implied from their results (i.e. cysteine first binds to the active site and creates an environment that is subsequently favorable for O2 binding) could generate a situation in which the oxygen-requiring process of cofactor biogenesis cannot occur unless cysteine is coordinated at the active site. Nevertheless, we observed that the rate of cofactor formation was considerably slower than the catalytic turnover rate of the non-cofactor containing enzyme; in the amount of time that it took for the cofactor to form in 50% of the protein, each non-cofactor-containing molecule of CDO had gone through ∼800 catalytic cycles. This suggests that the efficiency of cofactor formation may be limited by some other factor apart from oxygen binding at the active site.
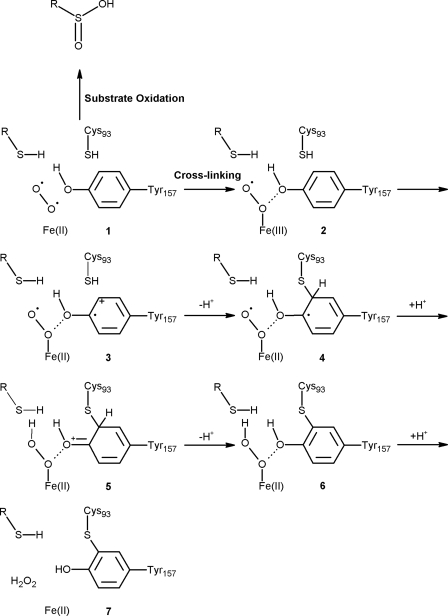
A mechanistic proposal for the formation of the Cys-Tyr cross-link of CDO is outlined in Fig. 9. The observation that the cross-linking reaction is both cysteine- and oxygen-dependent suggests that the enzyme substrate ternary complex 1 partitions between substrate oxidation and cross-linking (50% cross-linking after 800 catalytic cycles). In the cross-linking reaction, iron oxidation followed by addition of cysteine 93 to the tyrosyl radical 3 gives 4. An electron transfer from the modified tyrosine in 4 to the iron superoxide generates 5, which then tautomerizes to yield the cross-linked species 6. Hydrogen peroxide dissociation gives the catalytically enhanced cross-linked enzyme 7.
FIGURE 9.
A mechanistic proposal for the formation of cross-linked amino acid cofactor of CDO. The letter “R” in this schema designates the carbon backbone of a free cysteine molecule. The details of this mechanism are described in the text.
Previous to this work, the only known mechanism by which cysteine levels could regulate the biological activity of CDO was through the modulation of CDO protein turnover rate by ubiquitination and degradation (6, 7). Indeed, the regulation of CDO protein half-life by substrate constitutes an important mechanism whereby mammals are able to rapidly maintain intracellular cysteine homeostasis in the face of wide variations in dietary cysteine intake (30). According to this regulatory model, CDO protein is quickly ubiquitinated and degraded when cysteine levels are low. This spares otherwise scarce intracellular cysteine from irreversible oxidative catabolism and conserves it for other essential metabolic pathways such as synthesis of protein, glutathione, hydrogen sulfide, and coenzyme A. On the other hand, as cysteine levels rise, the rate of CDO ubiquitination decreases and the protein half-life is increased, permitting more of the enzyme to remain functionally viable and to clear cysteine from the intracellular pool before it reaches cytotoxic levels. Given the collective observations on CDO cofactor biogenesis, we propose that the unusual substrate dependence of cofactor formation represents an additional level of post-translational regulation of the enzyme that also contributes to homeostatic maintenance of intracellular cysteine levels. When intracellular levels of cysteine are low, the Cys-Tyr cofactor is not likely to form before the enzyme is ubiquitinated and degraded. This prevents the enzyme from achieving full catalytic efficiency and hence effectively aids the cell in conserving cysteine. In contrast, the Cys-Tyr cofactor forms in vivo only when intracellular levels of cysteine are high (up to ∼0.2 mm for an animal on a high protein diet) for a prolonged period of time. Once formed, the cofactor substantially increases both the catalytic efficiency and the catalytic stability of the enzyme and thus facilitates the clearance of excess cysteine.
The positive effect of cysteine on CDO activity is a novel form of feed-forward regulation of enzyme activity by substrate. Other well known examples of substrate-mediated feed-forward activation generally involve molecules that achieve their regulatory effects by a combination of homoallosteric and heteroallosteric means. Two notable examples are the feed-forward activation of phosphofructokinase-1 (PFK-1) by fructose 6-phosphate and the activation of pyruvate dehydrogenase by pyruvate. Hill coefficient studies have shown that the catalytic efficiency of a given active site in PFK-1 is increased by the binding of fructose 6-phosphate to remote binding sites, thus constituting a homoallosteric phenomenon (31). Heteroallosterically, fructose 6-phosphate can also increase PFK-1 catalytic efficiency by first being phosphorylated by phosphofructokinase-2 to yield fructose 2,6,-bisphosphate, a metabolite that is then able to serve as a potent allosteric activator of PFK-1. High levels of pyruvate indirectly increase pyruvate dehydrogenase (PD) activity by indirectly altering the phosphorylation state of the enzyme. Phosphorylation of PD is catalyzed by PD kinase, generally under conditions of high intracellular ATP/ADP, NADH/NAD+, and/or acetyl-CoA/CoA ratios, and results in inactivation of the PD complex. At high concentrations, however, pyruvate is capable of allosterically inhibiting PD kinase and thus reducing the rate at which PD is phosphorylated (32). Our data suggest, however, that there is no distant allosteric site on CDO for cysteine to bind and induce cofactor formation. Furthermore, cysteine does not need to be converted to another heteroallosterically active metabolite or to recruit a third party enzyme to exert its activational effect on CDO catalytic efficiency. Instead, cofactor formation is mediated by the binding of cysteine directly to the active site and its promotion of catalysis.
The discovery that substrate levels regulate cofactor formation may help to explain the unusual phylogenetic distribution of the Cys-Tyr cofactor in the CDO protein family. A previous phylogenetic analysis has shown that the cross-linked cofactor is strictly conserved in all eukaryotes known to possess a CDO homolog (metazoa and fungi) but is absent from most bacterial CDO homologs (19). It is possible that the eukaryotes, which have a very limited capacity to synthesize cysteine de novo and are subject to large fluctuations in dietary/environmental cysteine availability, are under a stronger selective pressure than bacteria to maintain the Cys-Tyr cofactor as an additional mechanism for regulating intracellular cysteine levels.
In summary, our data show that the formation of the Cys-Tyr cross-linked cofactor in CDO is a process that is distinctly different from that of other known amino acid cofactor-containing enzymes because of its strict dependence upon cysteine, the physiological substrate of the enzyme. It is also different by virtue of the fact that the cofactor is not required for catalytic activity, although its presence significantly enhances catalytic efficiency by severalfold. Furthermore, cysteine-dependent cofactor formation appears to be a physiologically important mechanism for the regulation of CDO activity, and hence intracellular cysteine levels, in vivo.
Supplementary Material
Acknowledgments
We are greatly indebted to Dr. Tadhg Begley (Cornell University) for the work on proposing a mechanistic model for CDO cofactor synthesis. We also thank Dr. P. Andrew Karplus (Oregon State University) for many helpful scientific discussions and Chad Simmons (Arizona State University) for assistance in generating structural images of CDO.
This work was supported in part by NIDDK Grant PHS DK056649 from the National Institutes of Health (to M. H. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Footnotes
The abbreviations used are: CDO, cysteine dioxygenase; WT, wild type; PDB, Protein Data Bank; MALDI-TOF, matrix-assisted laser desorption ionization time-of-flight; MS, Mass spectrometry; MS/MS, tandem MS; nanoLC, nano-liquid chromatography; DTNB, dithio-bis(2-nitrobenzoic acid); IDA, information-dependent acquisition; ACN, acetonitrile; MES, 4-morpholineethanesulfonic acid; PD, pyruvate dehydrogenase.
References
- 1.Davidson, V. L. (2007) Biochemistry 46 5283–5292 [DOI] [PubMed] [Google Scholar]
- 2.Okeley, N. M., and van der Donk, W. A. (2000) Chem. Biol. 7 R159–R171 [DOI] [PubMed] [Google Scholar]
- 3.Dominy, J. E., Jr., Hwang, J., and Stipanuk, M. H. (2007) Am. J. Physiol. 293 E62–E69 [DOI] [PubMed] [Google Scholar]
- 4.Bagley, P. J., and Stipanuk, M. H. (1995) J. Nutr. 125 933–940 [DOI] [PubMed] [Google Scholar]
- 5.Bagley, P. J., and Stipanuk, M. H. (1994) J. Nutr. 124 2410–2421 [DOI] [PubMed] [Google Scholar]
- 6.Dominy, J. E., Jr., Hirschberger, L. L., Coloso, R. M., and Stipanuk, M. H. (2006) Biochem. J. 394 267–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stipanuk, M. H., Hirschberger, L. L., Londono, M. P., Cresenzi, C. L., and Yu, A. F. (2004) Am. J. Physiol. 286 E439–E448 [DOI] [PubMed] [Google Scholar]
- 8.Ito, N., Phillips, S. E., Stevens, C., Ogel, Z. B., McPherson, M. J., Keen, J. N., Yadav, K. D., and Knowles, P. F. (1991) Nature 350 87–90 [DOI] [PubMed] [Google Scholar]
- 9.Whittaker, M. M., Kersten, P. J., Cullen, D., and Whittaker, J. W. (1999) J. Biol. Chem. 274 36226–36232 [DOI] [PubMed] [Google Scholar]
- 10.Whittaker, M. M., Kersten, P. J., Nakamura, N., Sanders-Loehr, J., Schweizer, E. S., and Whittaker, J. W. (1996) J. Biol. Chem. 271 681–687 [DOI] [PubMed] [Google Scholar]
- 11.Schnell, R., Sandalova, T., Hellman, U., Lindqvist, Y., and Schneider, G. (2005) J. Biol. Chem. 280 27319–27328 [DOI] [PubMed] [Google Scholar]
- 12.Klinman, J. P. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 14766–14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whittaker, M. M., and Whittaker, J. W. (2003) J. Biol. Chem. 278 22090–22101 [DOI] [PubMed] [Google Scholar]
- 14.Rogers, M. S., Baron, A. J., McPherson, M. J., Knowles, P. F., and Dooley, D. M. (2000) J. Am. Chem. Soc. 122 990–991 [Google Scholar]
- 15.Stipanuk, M. H., Londono, M., Hirschberger, L. L., Hickey, C., Thiel, D. J., and Wang, L. (2004) Amino Acids (Vienna) 26 99–106 [DOI] [PubMed] [Google Scholar]
- 16.Simmons, C. R., Liu, Q., Huang, Q., Hao, Q., Begley, T. P., Karplus, P. A., and Stipanuk, M. H. (2006) J. Biol. Chem. 281 18723–18733 [DOI] [PubMed] [Google Scholar]
- 17.McCoy, J. G., Bailey, L. J., Bitto, E., Bingman, C. A., Aceti, D. J., Fox, B. G., and Phillips, G. N., Jr. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 3084–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye, S., Wu, X., Wei, L., Tang, D., Sun, P., Bartlam, M., and Rao, Z. (2007) J. Biol. Chem. 282 3391–3402 [DOI] [PubMed] [Google Scholar]
- 19.Dominy, J. E., Jr., Simmons, C. R., Karplus, P. A., Gehring, A. M., and Stipanuk, M. H. (2006) J. Bacteriol. 188 5561–5569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, J. I., Londono, M., Hirschberger, L. L., and Stipanuk, M. H. (2004) J. Nutr. Biochem. 15 112–122 [DOI] [PubMed] [Google Scholar]
- 21.Simmons, C. R., Hirschberger, L. L., Machi, M. S., and Stipanuk, M. H. (2006) Protein Expression Purif. 47 74–81 [DOI] [PubMed] [Google Scholar]
- 22.Shevchenko, A., Wilm, M., Vorm, O., and Mann, M. (1996) Anal. Chem. 68 850–858 [DOI] [PubMed] [Google Scholar]
- 23.Bella, D. L., Hahn, C., and Stipanuk, M. H. (1999) Am. J. Physiol. 277 E144–E153 [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi, K., Hosokawa, Y., Kohashi, N., Kori, Y., Sakakibara, S., and Ueda, I. (1978) J. Biochem. (Tokyo) 83 479–491 [DOI] [PubMed] [Google Scholar]
- 25.Chai, S. C., Bruyere, J. R., and Maroney, M. J. (2006) J. Biol. Chem. 281 15774–15779 [DOI] [PubMed] [Google Scholar]
- 26.Ruggiero, C. E., Smith, J. A., Tanizawa, K., and Dooley, D. M. (1997) Biochemistry 36 1953–1959 [DOI] [PubMed] [Google Scholar]
- 27.Dooley, D. M. (1999) J. Biol. Inorg. Chem. 4 1–11 [DOI] [PubMed] [Google Scholar]
- 28.Chai, S. C., Jerkins, A. A., Banik, J. J., Shalev, I., Pinkham, J. L., Uden, P. C., and Maroney, M. J. (2005) J. Biol. Chem. 280 9865–9869 [DOI] [PubMed] [Google Scholar]
- 29.Pierce, B. S., Gardner, J. D., Bailey, L. J., Brunold, T. C., and Fox, B. G. (2007) Biochemistry 46 8569–8578 [DOI] [PubMed] [Google Scholar]
- 30.Stipanuk, M. H., Dominy, J. E., Jr., Lee, J. I., and Coloso, R. M. (2006) J. Nutr. 136 S1652–S1659 [DOI] [PubMed] [Google Scholar]
- 31.Deville-Bonne, D., Bourgain, F., and Garel, J. R. (1991) Biochemistry 30 5750–5754 [DOI] [PubMed] [Google Scholar]
- 32.Pratt, M. L., and Roche, T. E. (1979) J. Biol. Chem. 254 7191–7196 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.