Abstract
Clinically, a large number of patients have persistent low back pain attributable to intervertebral disc (IVD) degeneration. After the concept of biologically regenerating the degenerated IVD using growth factor injection was first proposed in early 1990, the advancement of molecular technology to produce recombinant proteins, including growth factors, on an industrial scale accelerated research in this field. The purpose of this review is to summarize the most recent findings of the in vitro and in vivo effects of growth factors on the IVD and, further, to discuss the limitations of growth factor therapy and its clinical implications. In vitro data showed that stimulation of matrix synthesis by growth factors alters the balance of homeostasis by shifting cellular metabolism to the anabolic state. In vivo data using small animals has shown the possibility of using growth factors as a “structural modifying therapy”. Based on in vitro and in vivo data previously reported, the clinical application of growth factors by direct injection of protein into the nucleus pulposus or anulus fibrosus was shown to be feasible as a new therapeutic intervention for treatment of disc degeneration. Stimulation of the biological repair process will create a new category of therapy to treat disc degeneration, where no active treatment currently exists, between conservative therapies and more aggressive therapies such as fusion or disc replacement. However, it should be noted that there are several important factors to be taken into consideration. In a relatively advanced degenerative condition, the supply of nutrients is disturbed and stimulation of cellular activity by growth factors may result in an increased demand for nutrients, eventually inducing an adverse event. Further investigations of the optimal environment for growth factor stimulation should be pursued. Growth factor therapy, which has experimental evidence supporting it to be a “structural modifying therapy”, may not be a “symptom modifying therapy” that is able to resolve the symptoms associated with pathologic changes. Therefore, further studies on the effect of growth factor therapy on pain are essential to shed light on its therapeutic usefulness for degenerative disc disease.
Keywords: Intervertebral disc, Growth factor, Regeneration, Repair
Introduction
Low back pain is responsible for enormous human suffering, high health care costs, and significant socioeconomic losses, including days lost from work. Statistics on the impact of low back pain on the individual and society are voluminous [7]. Back conditions result in significant restrictions on activity and are the most common cause of activity limitation in people younger than 45 years, especially in men. Approximately 1% of the US population is chronically disabled because of back pain and an additional one percent is temporarily disabled [67]. Significantly, eight in ten adults will, at sometime in their lives, have back pain that impairs activity [7]. Furthermore, as a result of the increased number of elderly citizens, the burden from low back pain on the individual and on society as a whole is expected to increase dramatically [43]. The current treatment approach for back pain includes conservative approaches (e.g., medication, steroid injection, and physical therapy) that all aim at an amelioration of symptoms such as pain. While the role of the intervertebral disc (IVD) as a primary generator of back pain remains controversial, the main target of many current therapeutic methods for back pain is the degenerated IVD. These treatment methods for disc degeneration have been limited to only a few courses of action, the most common of these being spinal surgery; however, none of them aim at the restoration of the structural integrity of the disc.
Degenerative disc diseases
Although the exact pathogenesis remains unknown, degenerative disc disease is characterized as a pathological condition, associated with genetic background, which is induced mechanically and mediated biologically, often concurrent with age-related changes.
Recent genetic studies, particularly the identical twin studies of Finland [9], have supported the contention that IVD degeneration is a consequence of multiple intrinsic and extrinsic factors. This report concluded that identical twins have similarities in degenerative findings in their discs and suggested that genetic factors might play an important role in inducing disc degeneration [9]. Other data, obtained as a result of recent advances in genetic analysis technologies and molecular biology, have indicated that heredity plays a dominant role. Videman et al. first described that specific genes, vitamin D receptor alleles, were associated with disc degeneration [92]. Since that report, an association has been demonstrated between disc degeneration or disc herniation with polymorphisms in the genes encoding collagen type I [85], collagen type IX [8, 74], aggrecan [33], matrix metalloprotease-3 (MMP-3) [81], interleukin-1β (IL-1β) [73], interleukin-6 (IL-6) [60], and cartilage intermediate layer protein [71].
Biologically, disc cells residing in both the anulus fibrosus (AF) and nucleus pulposus (NP) actively regulate the homeostasis of IVD tissues by maintaining a balance between anabolism and catabolism [46]. The modulation of disc cell metabolism involves a variety of molecules, such as cytokines, enzymes, enzyme inhibitors, and growth factors that act in a paracrine and/or autocrine fashion [46]. The anabolic regulators include polypeptide growth factors and small molecules, such as insulin-like growth factor (IGF), transforming growth factor-β (TGF-β), the bone morphogenetic proteins (BMPs) [66, 84], and the synthetic peptide of link protein [53]. The catabolic process is mediated by various enzymes, such as MMPs [17, 30, 31, 42, 80] and aggrecanases [78] which are both thought to be regulated by pro-inflammatory cytokines [32]. Several investigators, using disc culture systems and disc tissue extracts from surgical samples, have reported the presence of proteolytic enzymes, such as collagenase-1 (MMP-1) and stromelysin-1 (MMP-3) [18, 21, 42, 48, 56, 58, 98], as well as members of a disintegrin-like and metalloprotease with thrombospondin motifs (ADAMTS) family [37, 68] in degenerated human IVDs. In an animal model of disc degeneration, similar changes in mRNA and protein levels of these molecules were also observed [6, 72]. A more recent study suggested that pro-inflammatory cytokines, such as IL-1 or tumor necrosis factor-α (TNF-α), are expressed by human IVD cells [38] and animal degenerated IVDs [2, 11, 27, 65, 97]. Thus, these pro-inflammatory cytokines and proteases may be candidates for the causation of IVD degradation. Alterations in both anabolic and catabolic processes are thought to play key roles in the onset and progression of IVD degeneration, but the biochemical processes that regulate these changes are poorly understood.
Biological approaches for the restoration of disc structural integrity or prevention of disc degeneration
As described above, degenerative disc disease is associated with a complex milieu of intrinsic and extrinsic factors. The anabolic response of IVD cells is an important control mechanism for maintaining matrix homeostasis [46, 47, 82]. One strategy to retard the progression of disc degeneration and to ameliorate the loss of disc structural integrity is to shift the metabolic status from catabolic to actively anabolic by stimulating cells residing in the IVD with growth factors.
Before growth factors can be applied clinically to treat degenerative disc disease, a detailed analysis of their mode of action should be performed. Human IVD cells may react to stimulation by a growth factor in a way that is different from the response of animal cells; this may be especially important in the dose used. Cells found in a disc with an advanced stage of degeneration may not respond to growth factor stimulation. After confirming the effects of growth factors on IVD matrix metabolism, several factors such as indication, mode of delivery, dose, duration, and side effects, all of which affect therapeutic outcome, should be taken into consideration in order to achieve the greatest therapeutic effect. When the clinical use of a growth factor is contemplated, some of these questions can be addressed by carefully performing in vitro and in vivo animal model studies using a validated methodology.
In vitro evidence for the feasibility of growth factor application
Growth factors play an important role in the development of the spine. Interestingly, several growth factors have been found in normal and degenerated IVD tissues; this suggests that IVD cells are capable of expressing and producing growth factors. These include IGF-1 [66, 75], basic fibroblast growth factor (bFGF) [14, 51, 54, 88], BMP-2 [79], BMP-4 [55, 79], growth differentiation factor-5 (GDF-5) [55], platelet-derived growth factor (PDGF) [90], and TGF-β1-3 [35, 50, 63, 69, 75, 89]. A recent study in human degenerated IVDs showed the expression of TGF-β1 and -β2 as well as bFGF [87]. TGF-β receptor II (TGF-βRII), BMPRII, and FGF receptor 3 were also found to a similar extent in both human normal and diseased tissues [39]. Nerlich et al. have reported that TGF-β1 was expressed by nuclear and occasional anular cells spatially associated with fibronectin-synthesizing cells [57].
There is compelling evidence for the feasibility of utilizing growth factors to upregulate matrix metabolism. In general, several investigators have shown in different culture systems that cell proliferation or matrix metabolism has been upregulated when growth factors are added exogenously to tissue or cell cultures.
In 1991, Thompson et al. first described the positive effects of various growth factors, including TGF-β, epidermal growth factor (EGF), and bFGF, on proteoglycan (PG) synthesis by IVD cells, especially in NP cells [84]. Osada et al. have reported that IGF-1 stimulated PG synthesis by bovine NP cells in serum-free conditions in a dose-dependent manner [66].
Yoon et al. have shown that recombinant human BMP-2 (10–1000 ng/ml) increased cell proliferation, PG synthesis, mRNA expression of collagen type II, aggrecan, SOX9, and osteocalcin in monolayer cultures of rat AF cells [86]. Li et al. also reported that BMP-2 significantly increased aggrecan, collagen type II, TGF-β1, and BMP-7 mRNA expression and downregulated versican gene expression [40].
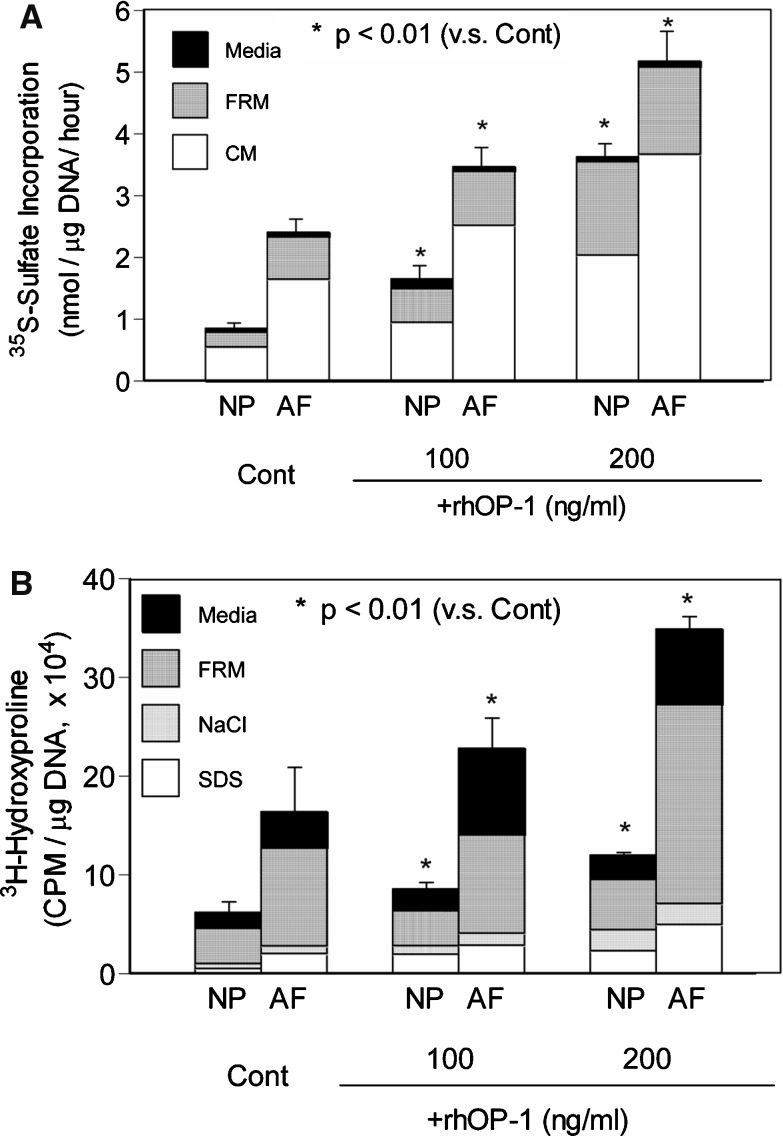
Using rabbit IVD cells, we have also demonstrated that BMP-7, also known as osteogenic protein-1 (OP-1), strongly stimulated the production and formation of PG and collagen and slightly affected cell proliferation [47]. In both NP and AF cells, recombinant human OP-1 (rhOP-1) stimulated the synthesis and accumulation of PG and collagen in a dose-dependent manner (50–200 ng/ml) in the presence of 10% fetal bovine serum (FBS) (Fig. 1). Data from previous cadaveric studies indicated that the capacity of NP and AF cells to synthesize PGs decreases with age [1]. In rabbit IVD cells, the responsiveness of IVD cells to IGF-1 and TGF-β has been shown to decrease with increasing age [62]. However, OP-1 significantly stimulated PG synthesis by all fetal, adult, and old bovine NP and AF cells [49], suggesting that the IVD cells in older animals are very responsive to a growth factor, such as OP-1. To model the pathological state, we treated IVD cells cultured in alginate beads with IL-1 or chondroitinase ABC (C-ABC) to deplete or to damage the PG-rich matrices. After depletion of the extracellular matrix following exposure of IVD cells to IL-1, OP-1 was found to be effective in the replenishment of a matrix rich in PG and collagen [83]. A similar result with OP-1 was reported when the matrix was first depleted by in vitro chemonucleolysis using C-ABC [82].
Fig. 1.
Effect of recombinant human rhOP-1 treatment on collagen synthesis by NP and AF cells cultured in alginate beads (Reprinted from Masuda K et al. (2003) J Orthop Res 21:922–930, with permission from Elsevier). After 7 days in culture, NP and AF cells were cultured for an additional 72 h in DMEM/F12+10% FBS containing various concentrations of rhOP-1. Newly synthesized PGs, formed during the last 4 h of culture in the presence of [35S]-sulfate, were quantified. In both NP and AF cultures, significant, dose-dependent, increases in the rate of synthesis per μg DNA were observed after the addition of rhOP-1 to the medium (a). Newly synthesized collagens, formed during the last 16 h of culture in the presence of L- [2,3,4,5-3H]-proline, were quantified. rhOP-1 stimulated collagen synthesis by both NP cells and AF cells in a dose-dependent manner (b). CM cell-associated matrix, FBS fetal bovine serum, FRM further-removed matrix, NaCl sodium chloride extract, SDS SDS extract
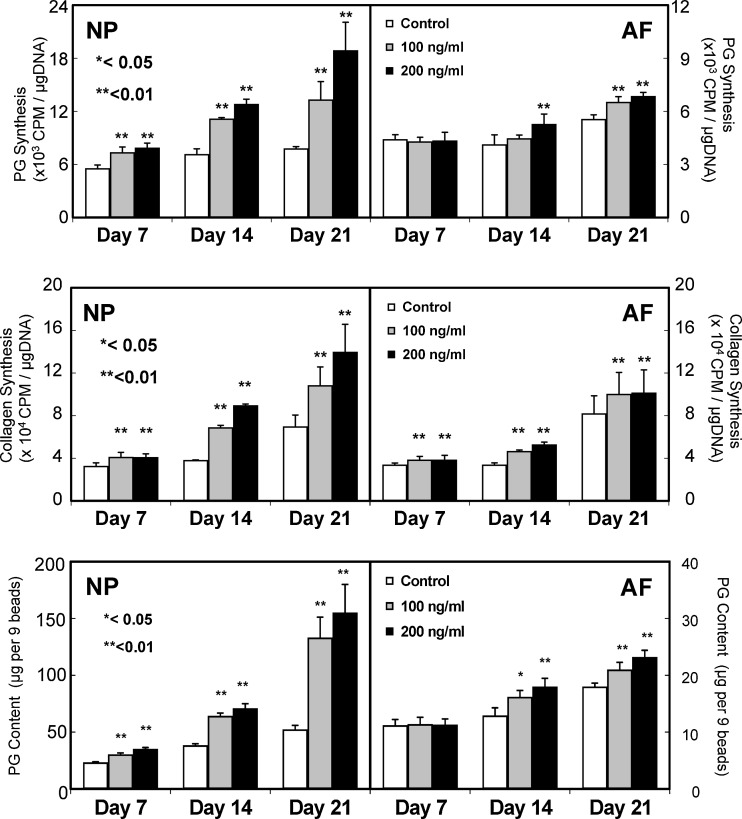
Growth and differentiation factor-5 (GDF-5), another member of the BMP family, was originally found to be a factor responsible for skeletal alterations in brachypodism mice [77]. Li et al. reported that GDF-5-deficient mice were associated with disc degeneration, characterized by a low T2-weighted signal intensity and loss of normal lamellar architecture of the AF and a shrunken, disorganized NP with a decreased PG content. They demonstrated that GDF-5 also stimulated aggrecan and collagen type II expression in mouse IVD cells [41]. Our study also indicated that rhGDF-5 enhanced cell proliferation and matrix synthesis and accumulation by both bovine NP and AF cells with a greater response by NP cells than by AF cells [12, 13] (Fig. 2).
Fig. 2.
Effect of recombinant human growth and differentiation factor-5 (rhGDF-5) on proteoglycan (PG) synthesis, (top), collagen synthesis (middle), and PG content (bottom) by bovine NP and AF cells in vitro (From: Chujo T et al (2006) Trans Orthop Res Soc 14, with permission. Spine: in print). Top: Cells were cultured in DMEM/F12+10% FBS in the presence or absence of 100 and 200 ng/ml of rhGDF-5 for 21 days and then PG synthesis was assessed. NP cells treated with rhGDF-5 showed a greater response than AF cells. A dose-dependent effect of rhGDF-5 was observed in NP cells only at day 21. Middle: The rate of collagen synthesis was also significantly higher in the rhGDF-5 groups (100 and 200 ng/ml) than in the control group at all time points in both cell types. Bottom: On days 7, 14, and 21, the PG content in alginate beads was measured. rhGDF-5, at both concentrations, stimulated PG accumulation in beads containing NP cells at all time points and in beads containing AF cells at the 14- and 21-day time points
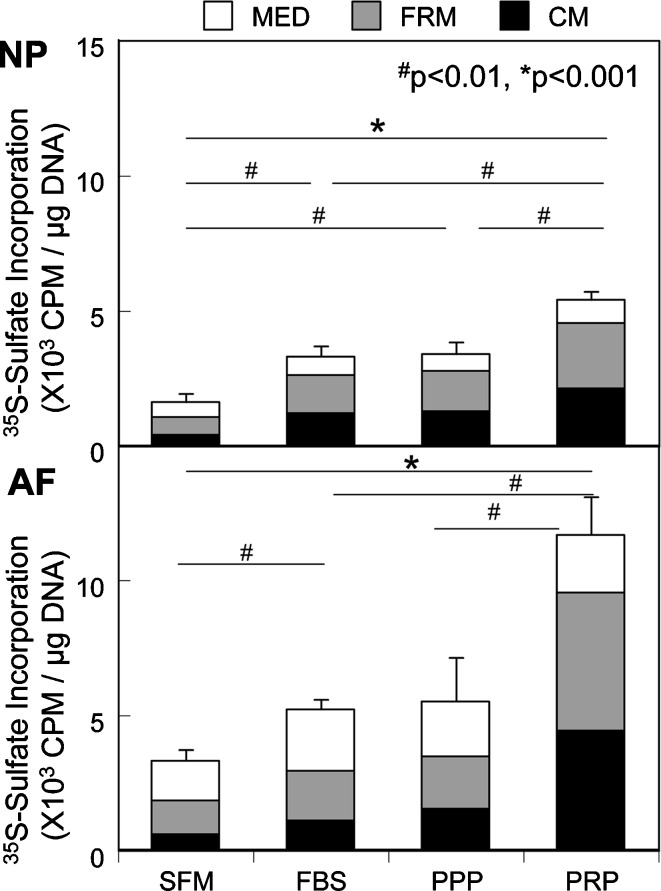
Platelet-rich plasma (PRP) is a plasma fraction containing multiple growth factors concentrated at high levels [15, 36, 61, 96] that can be produced by centrifugal separation of whole blood in the operating room. Recently, it has been found that PRP is an effective stimulator of cell proliferation and PG and collagen synthesis, as well as PG accumulation, by porcine NP and AF cells cultured in alginate beads [3] (Fig. 3).
Fig. 3.
Effect of PRP treatment on proteoglycan (PG) synthesis by NP and AF cells cultured in alginate beads (From: Akeda K et al. (2006) Spine, 31:959–966, with permission). During the last 4 h of the 72-h treatment period, the cells were radiolabeled with 35S-sulfate (20 μCi/ml) and the amount of radiolabeled 35S-PGs in the cell-associated matrix (CM) extract, the further-removed matrix (FRM) fraction and the media were quantified. PG synthesis in the PRP-treated NP and AF cells was significantly higher than that in the platelet-poor plasma (PPP)- and FBS-treated cells. SFM: serum free media
In human cells, Gruber et al. first showed that TGF-β stimulated cell proliferation of human anulus cells after four days of exposure in three-dimensional culture [23]. They also reported that IGF-1 and PDGF significantly reduced the percentage of apoptotic AF cells induced by serum depletion in culture [26].
Wehling showed that the combination of autologous IL-1 receptor antagonist (IL-1ra)/IGF-1/PDGF proteins reduced the percentage of apoptosis and the production of biochemical markers of disc degeneration, such as IL-1 and IL-6 [94]. These results suggested the feasibility for the use of an autologous protein mixture, containing IL-1ra/IGF-1/PDGF, for the treatment of degenerative disc disease [94].
Kim et al. reported that BMP-2 facilitates the expression of the chondrogenic phenotype by human IVD cells [34]. rhBMP-2 increased PG synthesis and upregulated the expression of aggrecan, collagen type I, and collagen type II mRNA, compared to untreated control levels.
Imai et al. reported that OP-1 (100–200 ng/ml) enhanced the in vitro production of PGs by human NP and AF cells cultured in alginate beads in the presence of 10% FBS [29]. OP-1 also enhanced the accumulation of PGs in the matrix. Interestingly, AF cells, which are more fibrochondrocytic, strongly responded to OP-1, suggesting that OP-1 might be beneficial not only for nucleus repair but for anular repair as well [29].
We have also shown that OP-1 treatment increased DNA content, PG synthesis and accumulation, and collagen synthesis in both early and advanced stages of IVD degeneration, but to a greater extent in early stages of disc degeneration [52]. These studies suggested that the application of growth factors may be suitable in the early rather than in the late stages of IVD degeneration.
In vivo evidence for the feasibility of growth factor application
The first study of growth factor injection into the IVD was reported by Walsh et al. in the mouse caudal disc degeneration model induced by static compression [93]. A single injection of GDF-5, but not that of IGF-1, TGF-β, or bFGF, was effective in promoting disc regeneration. Multiple injections (four injections, one per week) of TGF-β showed a stimulatory effect, although multiple injections of IGF-1, GDF-5, and bFGF did not show a significant enhancement of their original effect [93]. The authors suggested that a sustained delivery system or a combined approach with a mechanical or cell-based device was required to achieve a beneficial therapeutic effect.
We have performed an in vivo experiment using the normal rabbit IVD and a single injection of OP-1 (2 μg per disc) to test the feasibility for the therapeutic use of OP-1 in the treatment of degenerative disc disease [4]. A single intradiscal administration of OP-1 in vivo resulted in increased disc height (15%) and PG content of the NP [4].
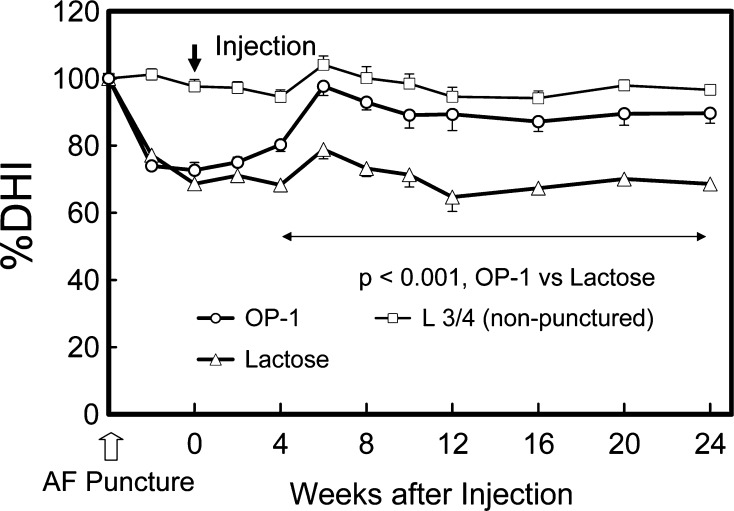
Using a defined-gauge needle puncture of the AF [44], we then conducted a study of radiographic and magnetic resonance imaging (MRI) changes in the rabbit IVD after injection of OP-1 into the NP in the anular-puncture disc degeneration model. Disc degeneration was induced by anular puncture in two noncontiguous discs with an 18G needle. Four weeks later, either 5% lactose (10 μl) or OP-1 (100 μg in 10 μl 5% lactose) was injected into the center of the NP. Six weeks after the OP-1 injection, a restoration of disc height and an increase of signal intensity of NP in T2-MRI were observed and sustained for the entire experimental period, up to 24 weeks (Fig. 4) [45]. Biochemically, the PG content of the NP and AF was significantly higher in the OP-1-injected group than in the control (lactose-injected) group. Histologically, the degeneration grades of the punctured discs in the OP-1-injected group were significantly lower than those of the lactose-injected group. The results of this study demonstrate the feasibility of restoring degenerative rabbit discs by a single injection of OP-1 into the NP. It was noteworthy that the restoration of disc height, which began at 6 weeks, was sustained for up to 24 weeks.
Fig. 4.
Changes in intervertebral disc height index (DHI) after anular puncture and OP-1 injection (with permission, Masuda K et al. (2006) Spine, 31:742–754). The percent DHI (%DHI = [postoperative DHI/preoperative DHI] × 100) was measured at each time point to quantify changes in disc height. By 4 weeks after the OP-1 injection, the mean %DHI of injected discs in the OP-1 group was significantly higher than that in the lactose control group (P<0.001, repeated ANOVA). This significant difference in mean %DHI was maintained during the follow-up period (P<0.001)
In the C-ABC-induced matrix depletion model of disc degeneration in the rabbit, 4 weeks following the injection of C-ABC, an injection of OP-1 (100 μg/disc) restored disc height by 6 weeks that was maintained for up to 12 weeks after the OP-1 injection [28]. These data suggest that OP-1 may be utilized in patients with disc degeneration who had received chemonucleolysis in the past that resulted in some loss of disc height.
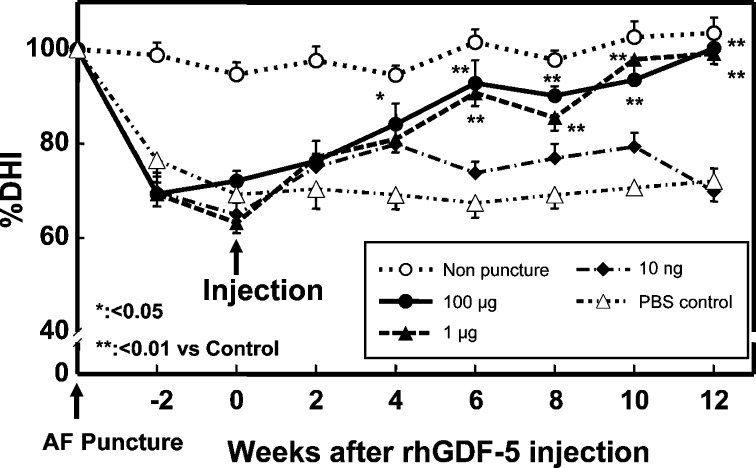
The results of an injection of rhGDF-5 in the rabbit anular-puncture model also confirmed the efficacy of growth factor injection. The discs were punctured by an 18G needle as described in the OP-1 experiment. Four weeks later, the rabbits received an injection of phosphate buffered saline (PBS) or rhGDF-5 (10 ng, 1, and 100 μg) and were followed up for 16 weeks for disc height (Fig. 5), MRI, and histological grades. The injection of rhGDF-5 resulted in a restoration of disc height and improvements in MRI and histological grading scores with statistical significance (P<0.05–0.001) [12, 13].
Fig. 5.
Changes in intervertebral disc height index (DHI) after anular puncture and rhGDF-5 injections (Reprinted from: Chujo T et al. (2005) Spine J. S: S145 with permission from Elsevier, Spine in print). By 4 weeks after the rhGDF-5 injection, the mean %DHI of injected discs in the rhGDF-5 group (100 μg) was significantly higher than in the PBS group (P<0.05). Six weeks after the injection of rhGDF-5, the %DHI was significantly higher, at doses of 1 and 100 μg, in the rhGDF-5 group when compared to the PBS group (control versus rhGDF-5 1 and 100 μg; P<0.01). The effect of a single injection of rhGDF-5 continued to be significant throughout the study
In the three different in vivo experiments mentioned above, we have seen delayed responses in the recovery of disc height by IVD cells in vivo after an injection of growth factors. The mechanism of this phenomenon remains to be determined; however, we postulate the following: First, the half life of the injected growth factor may be longer than we expected. Second, the response of cells to a single exposure of growth factor may continue over a long period of time. Third, the stimulation by growth factor may induce a cascade of events, eventually resulting in the recovery of disc height.
The in vitro and in vivo evidence described above supports the contention that the direct injection of growth factors into the NP or the AF may be clinically effective as a new therapeutic intervention for treatment of IVD degeneration. The acceleration of the biological repair process by the stimulation of the cellular anabolic capacity will create a new category of therapy, where no active treatment currently exists, between conservative therapies and more aggressive therapies such as fusion or disc replacement.
Limitations of growth factor injection therapy
When the biology of the IVD is taken into account, several important limitations should be contemplated in the development of growth factor therapy.
The injected growth factor does not by itself induce an immediate structural and biomechanical alteration in the disc; cells residing in the disc need to be able to respond to the applied growth factor in order for there to be a biological effect. It is well known that the cell number is decreased in discs in an advanced stage of degeneration [24]. To overcome this deficit, the transplantation of healthy functional cells, such as, but not limited to, autologous NP cells, may be required [19, 25, 59, 64]. In addition, cells transfected with a therapeutic gene may be utilized to obtain both a supplementation of cells and the gene therapeutic effect [95].
Nutrition is another crucial factor in the pathogenesis of disc disease [91]. The IVD is the largest avascular tissue in the body; it receives its main supply of nutrients by diffusion from the vertebral bodies through the endplates. Thus, an adequate transport of glucose to the cells and appropriate removal of metabolic wastes, such as lactic acid, are essential for cell survival. In pathologic tissues, the supply of nutrients may be disturbed by sclerosis of the endplate. An IVD that lacks proper nutrition or one that cannot remove metabolic wastes might experience an adverse environment, i.e., acidic conditions, and might degenerate significantly because of the loss of steady-state metabolism of its cells. The increased demand for energy resulting from the stimulation by growth factors or by cell supplementation may affect cell viability under those conditions where nutrient transport is compromised. Further investigations of the optimal environment for growth factor stimulation should be pursued. The effect of growth factors under conditions where levels of nutrition are compromised is an area especially warranting further investigation.
Because of the emerging trend to treat disc degeneration in adults or older individuals by the local injection of growth factors, age-related and degeneration stage-related changes in the response to growth factors are of special interest for future studies [5].
Future clinical indications for the use of growth factor injection therapy and potential side effects
Clinically, a large number of patients have persistent low back pain due to IVD degeneration. Discogenic low back pain is frequently associated with twisting and bending injuries. The axial lumbar pain is often referred to the buttock and posterior thigh, typically worse with sitting and forward-bending activities. The majority of patients respond to rest, activity modification, anti-inflammatory medications, and physical therapy, but some patients have symptoms persisting for many months that require invasive treatment. Currently, the gold-standard surgical treatment is lumbar spinal fusion, but alternatives such as artificial discs and intradiscal electrothermal therapy (IDET) are available. Discography is usually recommended to confirm that suspicious degenerated discs detected by MRI are painful prior to any invasive treatment. For degenerated discs with a mild loss of disc height, IDET may be a minimally invasive treatment option [16]. For nearly completely collapsed discs, it is technically difficult to insert the IDET catheter into the disc space and the outcome is generally inferior. For moderately degenerated discs with healthy facet joints, an artificial disc [10, 20] or other motion preservation treatment may be a reasonable option [22, 70, 76]. For severely degenerated discs with compromised facets and stability of the motion segment, fusion is the only option.
Because selection from the currently available surgical treatments depends on the severity of degeneration of the IVD and the motion segment, it is logical that the effectiveness of biological treatments would be affected by the different stages of IVD degeneration. Biological treatments for IVD degeneration probably will not work for severely degenerated IVDs with significant biomechanical changes of the motion segment, unless they are combined with additional treatments, such as posterior motion preservation procedures. For early to moderately degenerated IVDs, growth factor injection therapy has the potential to replenish the matrix, restore disc height, and possibly relieve the patient’s discogenic low back pain. Perhaps the injection of growth factor could be done at the timing of discography for patient convenience. Obviously, clinical studies are needed in the future to assess efficacy and safety. Also, future studies of growth factor injection will help define specific indications and potential complications.
Because recombinant growth factors and BMPs have been thoroughly tested in humans for bone formation, their safety profile is well known. However, for spinal injection, where there is the risk of intradural penetration, preclinical studies should include intrathecal injection. Additionally, there is the potential for bone formation in the disc space and interbody fusion. This should not result in an adverse outcome for the patient because fusion ought to stabilize the motion segment, as well as relieve the pain. Side effects are dose-dependent; therefore, dose-response studies should also be performed to assess both efficacy and safety.
In summary, in vitro and in vivo animal studies on the use of growth factors to treat IVD degeneration have thus far met with success on structural modification. Growth factor injection therapy for treatment of IVD degeneration has great potential for patients with chronic discogenic low back pain. As with any new technology, thorough preclinical studies should be followed by well-designed clinical studies to accurately assess safety and efficacy.
References
- 1.Adams ME, Billingham MEJ, Muir H. The glycosaminoglycans in menisci in experimental and natural osteoarthritis. Arthritis Rheum. 1983;26:69–76. doi: 10.1002/art.1780260111. [DOI] [PubMed] [Google Scholar]
- 2.Ahn SH, Cho YW, Ahn MW, Jang SH, Sohn YK, Kim HS. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27:911–917. doi: 10.1097/00007632-200205010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Akeda K, An H, Pichika R, Attawia M, Lenz M, Uchida A, Thonar E, Masuda K. Platelet-rich Plasma (PRP) stimulates the extracellular matrix metabolism of porcine nucleus pulposus and annulus fibrosus cells cultured in alginate beads. Spine. 2006;31:959–966. doi: 10.1097/01.brs.0000214942.78119.24. [DOI] [PubMed] [Google Scholar]
- 4.An HS, Takegami K, Kamada H, Nguyen CM, Thonar EJ, Singh K, Andersson GB, Masuda K. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine. 2005;30:25–31. doi: 10.1097/01.brs.0000148002.68656.4d. [DOI] [PubMed] [Google Scholar]
- 5.An HS, Thonar EJ, Masuda K. Biological repair of intervertebral disc. Spine. 2003;28:S86–S92. doi: 10.1097/00007632-200308011-00015. [DOI] [PubMed] [Google Scholar]
- 6.Anderson DG, Izzo MW, Hall DJ, Vaccaro AR, Hilibrand A, Arnold W, Tuan RS, Albert TJ. Comparative gene expression profiling of normal and degenerative discs: analysis of a rabbit annular laceration model. Spine. 2002;27:1291–1296. doi: 10.1097/00007632-200206150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 8.Annunen S, Paassilta P, Lohiniva J, Perala M, Pihlajamaa T, Karppinen J, Tervonen O, Kroger H, Lahde S, Vanharanta H, Ryhanen L, Goring HH, Ott J, Prockop DJ, Ala-Kokko L. An allele of COL9A2 associated with intervertebral disc disease. Science. 1999;285:409–412. doi: 10.1126/science.285.5426.409. [DOI] [PubMed] [Google Scholar]
- 9.Battie MC, Haynor DR, Fisher LD, Gill K, Gibbons LE, Videman T. Similarities in degenerative findings on magnetic resonance images of the lumbar spines of identical twins. J Bone Joint Surg Am. 1995;77:1662–1670. doi: 10.2106/00004623-199511000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Blumenthal S, McAfee PC, Guyer RD, Hochschuler SH, Geisler FH, Holt RT, Garcia R Jr, Regan JJ, Ohnmeiss DD (2005) A prospective, randomized, multicenter food and drug administration investigational device exemptions study of lumbar total disc replacement with the charite artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine 30:1565–1575 [DOI] [PubMed]
- 11.Burke JG, Watson RG, Conhyea D, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Human nucleus pulposus can respond to a pro-inflammatory stimulus. Spine. 2003;28:2685–2693. doi: 10.1097/01.BRS.0000103341.45133.F3. [DOI] [PubMed] [Google Scholar]
- 12.Chujo T, Akeda K, An H, Thonar E, Attawia M, Masuda K. In vitro and in vivo effects of recombinant human growth and differentiation factor-5 on the intervertebral disc. Spine J. 2005;5:S145. doi: 10.1016/j.spinee.2005.05.289. [DOI] [PubMed] [Google Scholar]
- 13.Chujo T, An H, Akeda K, Miyamoto K, Muehleman C, Attawia M, Andersson G, Masuda K (in print) Effects of growth differentiation factor-5 (GDF-5) on the intervertebral disc–in vitro bovine study and in vivo rabbit disc degeneration model study. Spine (in press) [DOI] [PubMed]
- 14.Doita M, Kanatani T, Harada T, Mizuno K. Immunohistologic study of the ruptured intervertebral disc of the lumbar spine. Spine. 1996;21:235–241. doi: 10.1097/00007632-199601150-00015. [DOI] [PubMed] [Google Scholar]
- 15.Dugrillon A, Eichler H, Kern S, Kluter H. Autologous concentrated platelet-rich plasma (cPRP) for local application in bone regeneration. Int J Oral Maxillofac Surg. 2002;31:615–619. doi: 10.1054/ijom.2002.0322. [DOI] [PubMed] [Google Scholar]
- 16.Freeman BJ, Fraser RD, Cain CM, Hall DJ, Chapple DC (2005) A randomized, double-blind, controlled trial: intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine 30:2369–2377 [DOI] [PubMed]
- 17.Fujita K, Nakagawa T, Hirabayashi K, Nagai Y. Neutral proteinases in human intervertebral disc. Role in degeneration and probable origin. Spine. 1993;18:1766–1773. doi: 10.1097/00007632-199310000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Furusawa N, Baba H, Miyoshi N, Maezawa Y, Uchida K, Kokubo Y, Fukuda M. Herniation of cervical intervertebral disc: immunohistochemical examination and measurement of nitric oxide production. Spine. 2001;26:1110–1116. doi: 10.1097/00007632-200105150-00004. [DOI] [PubMed] [Google Scholar]
- 19.Ganey T, Libera J, Moos V, Alasevic O, Fritsch KG, Meisel HJ, Hutton WC. Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine. 2003;28:2609–2620. doi: 10.1097/01.BRS.0000097891.63063.78. [DOI] [PubMed] [Google Scholar]
- 20.Geisler FH, Blumenthal SL, Guyer RD, McAfee PC, Regan JJ, Johnson JP, Mullin B. Neurological complications of lumbar artificial disc replacement and comparison of clinical results with those related to lumbar arthrodesis in the literature: results of a multicenter, prospective, randomized investigational device exemption study of charite intervertebral disc. Invited submission from the joint section meeting on disorders of the Spine and Peripheral nerves, March 2004. J Neurosurg Spine. 2004;1:143–154. doi: 10.3171/spi.2004.1.2.0143. [DOI] [PubMed] [Google Scholar]
- 21.Goupille P, Jayson MI, Valat JP, Freemont AJ. Matrix metalloproteinases: the clue to intervertebral disc degeneration? Spine. 1998;23:1612–1626. doi: 10.1097/00007632-199807150-00021. [DOI] [PubMed] [Google Scholar]
- 22.Grob D, Benini A, Junge A, Mannion AF. Clinical experience with the dynesys semirigid fixation system for the lumbar spine: surgical and patient-oriented outcome in 50 cases after an average of 2 years. Spine. 2005;30:324–331. doi: 10.1097/01.brs.0000152584.46266.25. [DOI] [PubMed] [Google Scholar]
- 23.Gruber HE, Fisher EC, Jr, Desai B, Stasky AA, Hoelscher G, Hanley EN., Jr Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta1. Exp Cell Res. 1997;235:13–21. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- 24.Gruber HE, Hanley EN., Jr Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine. 1998;23:751–757. doi: 10.1097/00007632-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Gruber HE, Johnson TL, Leslie K, Ingram JA, Martin D, Hoelscher G, Banks D, Phieffer L, Coldham G, Hanley EN., Jr Autologous intervertebral disc cell implantation: a model using psammomys obesus, the sand rat. Spine. 2002;27:1626–1633. doi: 10.1097/00007632-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 26.Gruber HE, Norton HJ, Hanley EN., Jr Anti-apoptotic effects of IGF-1 and PDGF on human intervertebral disc cells in vitro. Spine. 2000;25:2153–2157. doi: 10.1097/00007632-200009010-00002. [DOI] [PubMed] [Google Scholar]
- 27.Igarashi T, Kikuchi S, Shubayev V, Myers RR. 2000 Volvo Award winner in basic science studies: exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine. 2000;25:2975–2980. doi: 10.1097/00007632-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 28.Imai Y, An H, Matsumoto T, Nguyen C, Andersson G, Thonar E, Masuda K (2002) Intervertebral disc regeneration with rhOP-1 following C-ABC chemonucleolysis: an in vivo study using the rabbit model the International Society for the study of the Lumbar Spine. In: 29th Annual Meeting Proceeding, Cleveland, OH, p71
- 29.Imai Y, An H, Pichika R, Thonar E, Otten L, Andersson G, Masuda K. Recombinant human osteogenic protein-1 upregulates extracellular matrix metabolism by human annulus fibrosus and nucleus pulposus cells. Trans Orthop Res Soc. 2003;28:1140. doi: 10.1016/S0736-0266(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 30.Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson WF, 3rd, Evans CH. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21:271–277. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 31.Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Evans CH. Herniated cervical intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1995;20:2373–2378. doi: 10.1097/00007632-199511001-00001. [DOI] [PubMed] [Google Scholar]
- 32.Kang JD, Stefanovic-Racic M, McIntyre LA, Georgescu HI, Evans CH. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine. 1997;22:1065–1073. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi Y, Osada R, Kanamori M, Ishihara H, Ohmori K, Matsui H, Kimura T. Association between an aggrecan gene polymorphism and lumbar disc degeneration. Spine. 1999;24:2456–2460. doi: 10.1097/00007632-199912010-00006. [DOI] [PubMed] [Google Scholar]
- 34.Kim DJ, Moon SH, Kim H, Kwon UH, Park MS, Han KJ, Hahn SB, Lee HM. Bone morphogenetic protein-2 Facilitates expression of chondrogenic, not osteogenic, phenotype of human intervertebral disc cells. Spine. 2003;28:2679–2684. doi: 10.1097/01.BRS.0000101445.46487.16. [DOI] [PubMed] [Google Scholar]
- 35.Konttinen YTu, Kemppinen P, Li TF, Waris E, Pihlajamaki H, Sorsa T, Takagi M, Santavirta S, Schultz GS, Humphreys-Beher MG. Transforming and epidermal growth factors in degenerated intervertebral discs. J Bone Joint Surg Br. 1999;81:1058–1063. doi: 10.1302/0301-620X.81B6.9321. [DOI] [PubMed] [Google Scholar]
- 36.Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297–300. doi: 10.1016/S0278-2391(00)90058-2. [DOI] [PubMed] [Google Scholar]
- 37.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 38.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–R745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Maitre CL, Richardson SM, Baird P, Freemont AJ, Hoyland JA. Expression of receptors for putative anabolic growth factors in human intervertebral disc: implications for repair and regeneration of the disc. J Pathol. 2005;207:445–452. doi: 10.1002/path.1862. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Yoon ST, Hutton WC. Effect of bone morphogenetic protein-2 (BMP-2) on matrix production, other BMPs, and BMP receptors in rat intervertebral disc cells. J Spinal Disord Tech. 2004;17:423–428. doi: 10.1097/01.bsd.0000112084.85112.5d. [DOI] [PubMed] [Google Scholar]
- 41.Li X, Leo BM, Beck G, Balian G, Anderson GD. Collagen and proteoglycan abnormalities in the GDF-5-deficient mice and molecular changes when treating disk cells with recombinant growth factor. Spine. 2004;29:2229–2234. doi: 10.1097/01.brs.0000142427.82605.fb. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Roughley PJ, Mort JS. Identification of human intervertebral disc stromelysin and its involvement in matrix degradation. J Orthop Res. 1991;9:568–575. doi: 10.1002/jor.1100090413. [DOI] [PubMed] [Google Scholar]
- 43.Manek NJ, MacGregor AJ. Epidemiology of back disorders: prevalence, risk factors, and prognosis. Curr Opin Rheumatol. 2005;17:134–140. doi: 10.1097/01.bor.0000154215.08986.06. [DOI] [PubMed] [Google Scholar]
- 44.Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, Andersson GB, An HS. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30:5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 45.Masuda K, Imai Y, Okuma M, Muehleman C, Nakagawa K, Akeda K, Thonar E, Andersson G, An H. Osteogenic protein-1 (OP-1) injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit annular puncture model. Spine. 2006;31:742–754. doi: 10.1097/01.brs.0000206358.66412.7b. [DOI] [PubMed] [Google Scholar]
- 46.Masuda K, Oegema TR, Jr, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine. 2004;29:2757–2769. doi: 10.1097/01.brs.0000146048.14946.af. [DOI] [PubMed] [Google Scholar]
- 47.Masuda K, Takegami K, An H, Kumano F, Chiba K, Andersson GB, Schmid T, Thonar E. Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J Orthop Res. 2003;21:922–930. doi: 10.1016/S0736-0266(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 48.Matsui Y, Maeda M, Nakagami W, Iwata H. The involvement of matrix metalloproteinases and inflammation in lumbar disc herniation. Spine. 1998;23:863–868. doi: 10.1097/00007632-199804150-00005. [DOI] [PubMed] [Google Scholar]
- 49.Matsumoto T, An H, Thonar E, Andersson G, Masuda K. Effect of osteogenic orotein-1 on the metabolism of proteoglycan of intervertebral disc cells in aging. Trans Orthop Res Soc. 2002;27:826. [Google Scholar]
- 50.Matsunaga S, Nagano S, Onishi T, Morimoto N, Suzuki S, Komiya S. Age-related changes in expression of transforming growth factor-beta and receptors in cells of intervertebral discs. J Neurosurg. 2003;98:63–67. doi: 10.3171/spi.2003.98.1.0063. [DOI] [PubMed] [Google Scholar]
- 51.Melrose J, Smith S, Little CB, Kitson J, Hwa SY, Ghosh P. Spatial and temporal localization of transforming growth factor-beta, fibroblast growth factor-2, and osteonectin, and identification of cells expressing alpha-smooth muscle actin in the injured anulus fibrosus: implications for extracellular matrix repair. Spine. 2002;27:1756–1764. doi: 10.1097/00007632-200208150-00014. [DOI] [PubMed] [Google Scholar]
- 52.Miyamoto K, Masuda K, Thonar E-M, An H. Differences in the response of human intervertebral disc cells to osteogenic protein-1 at different stages of degeneration. Spine J. 2005;5:137S. doi: 10.1016/j.spinee.2005.05.272. [DOI] [Google Scholar]
- 53.Mwale F, Demers CN, Petit A, Roughley P, Poole AR, Steffen T, Aebi M, Antoniou J. A synthetic peptide of link protein stimulates the biosynthesis of collagens II, IX and proteoglycan by cells of the intervertebral disc. J Cell Biochem. 2003;88:1202–1213. doi: 10.1002/jcb.10479. [DOI] [PubMed] [Google Scholar]
- 54.Nagano T, Yonenobu K, Miyamoto S, Tohyama M, Ono K. Distribution of the basic fibroblast growth factor and its receptor gene expression in normal and degenerated rat intervertebral discs. Spine. 1995;20:1972–1978. doi: 10.1097/00007632-199509150-00002. [DOI] [PubMed] [Google Scholar]
- 55.Nakase T, Ariga K, Miyamoto S, Okuda S, Tomita T, Iwasaki M, Yonenobu K, Yoshikawa H. Distribution of genes for bone morphogenetic protein-4, -6, growth differentiation factor-5, and bone morphogenetic protein receptors in the process of experimental spondylosis in mice. J Neurosurg. 2001;94:68–75. doi: 10.3171/spi.2001.94.1.0068. [DOI] [PubMed] [Google Scholar]
- 56.Nemoto O, Yamagishi M, Yamada H, Kikuchi T, Takaishi H. Matrix metalloproteinase-3 production by human degenerated intervertebral disc. J Spinal Disord. 1997;10:493–498. doi: 10.1097/00002517-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 57.Nerlich AG, Bachmeier BE, Boos N. Expression of fibronectin and TGF-beta1 mRNA and protein suggest altered regulation of extracellular matrix in degenerated disc tissue. Eur Spine J. 2005;14:17–26. doi: 10.1007/s00586-004-0745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishida T. Kinetics of tissue and serum matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1 in intervertebral disc degeneration and disc herniation. Kurume Med J. 1999;46:39–50. doi: 10.2739/kurumemedj.46.39. [DOI] [PubMed] [Google Scholar]
- 59.Nishimura K, Mochida J. Percutaneous reinsertion of the nucleus pulposus. An experimental study. Spine. 1998;23:1531–1538. doi: 10.1097/00007632-199807150-00006. [DOI] [PubMed] [Google Scholar]
- 60.Noponen-Hietala N, Virtanen I, Karttunen R, Schwenke S, Jakkula E, Li H, Merikivi R, Barral S, Ott J, Karppinen J, Ala-Kokko L. Genetic variations in IL-6 associate with intervertebral disc disease characterized by sciatica. Pain. 2005;114:186–194. doi: 10.1016/j.pain.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 61.Okuda K, Kawase T, Momose M, Murata M, Saito Y, Suzuki H, Wolff LF, Yoshie H. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J Periodontol. 2003;74:849–857. doi: 10.1902/jop.2003.74.6.849. [DOI] [PubMed] [Google Scholar]
- 62.Okuda S, Myoui A, Ariga K, Nakase T, Yonenobu K, Yoshikawa H. Mechanisms of age-related decline in insulin-like growth factor-I dependent proteoglycan synthesis in rat intervertebral disc cells. Spine. 2001;26:2421–2426. doi: 10.1097/00007632-200111150-00005. [DOI] [PubMed] [Google Scholar]
- 63.Okuda S, Nakase T, Yonenobu K, Ono K. Age-dependent expression of transforming growth factor-beta1 (TGF-beta1) and its receptors and age-related stimulatory effect of TGF-beta1 on proteoglycan synthesis in rat intervertebral discs. J Muscle Res. 2000;4:151–159. [Google Scholar]
- 64.Okuma M, Mochida J, Nishimura K, Sakabe K, Seiki K. Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration: an in vitro and in vivo experimental study. J Orthop Res. 2000;18:988–997. doi: 10.1002/jor.1100180620. [DOI] [PubMed] [Google Scholar]
- 65.Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine. 1998;23:2538–2544. doi: 10.1097/00007632-199812010-00008. [DOI] [PubMed] [Google Scholar]
- 66.Osada R, Ohshima H, Ishihara H, Yudoh K, Sakai K, Matsui H, Tsuji H. Autocrine/paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insulin-like growth factor-1 on proteoglycan synthesis in bovine intervertebral discs. J Orthop Res. 1996;14:690–699. doi: 10.1002/jor.1100140503. [DOI] [PubMed] [Google Scholar]
- 67.Praemer A, Furner S, Rice DP. Musculoskeletal conditions in the United States. Rosemont: American Academy of Orthopaedic Surgeons; 1999. [Google Scholar]
- 68.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 69.Saal JA, Saal JS, Herzog RJ. The natural history of lumbar intervertebral disc extrusions treated nonoperatively. Spine. 1990;15:683–686. doi: 10.1097/00007632-199007000-00013. [DOI] [PubMed] [Google Scholar]
- 70.Schnake KJ, Schaeren S, Jeanneret B. Dynamic stabilization in addition to decompression for lumbar spinal stenosis with degenerative spondylolisthesis. Spine. 2006;31:442–449. doi: 10.1097/01.brs.0000200092.49001.6e. [DOI] [PubMed] [Google Scholar]
- 71.Seki S, Kawaguchi Y, Chiba K, Mikami Y, Kizawa H, Oya T, Mio F, Mori M, Miyamoto Y, Masuda I, Tsunoda T, Kamata M, Kubo T, Toyama Y, Kimura T, Nakamura Y, Ikegawa S. A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat Genet. 2005;37:607–612. doi: 10.1038/ng1557. [DOI] [PubMed] [Google Scholar]
- 72.Sobajima S, Shimer AL, Chadderdon RC, Kompel JF, Kim JS, Gilbertson LG, Kang JD. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005;5:14–23. doi: 10.1016/j.spinee.2004.05.251. [DOI] [PubMed] [Google Scholar]
- 73.Solovieva S, Kouhia S, Leino-Arjas P, Ala-Kokko L, Luoma K, Raininko R, Saarela J, Riihimaki H. Interleukin 1 polymorphisms and intervertebral disc degeneration. Epidemiology. 2004;15:626–633. doi: 10.1097/01.ede.0000135179.04563.35. [DOI] [PubMed] [Google Scholar]
- 74.Solovieva S, Lohiniva J, Leino-Arjas P, Raininko R, Luoma K, Ala-Kokko L, Riihimaki H. Intervertebral disc degeneration in relation to the COL9A3 and the IL-1ss gene polymorphisms. Eur Spine J. 2006;15:613–619. doi: 10.1007/s00586-005-0988-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Specchia N, Pagnotta A, Toesca A, Greco F. Cytokines and growth factors in the protruded intervertebral disc of the lumbar spine. Eur Spine J. 2002;11:145–151. doi: 10.1007/s00586-001-0361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stoll TM, Dubois G, Schwarzenbach O. The dynamic neutralization system for the spine: a multi-center study of a novel non-fusion system. Eur Spine J. 2002;11(Suppl. 2):S170–S178. doi: 10.1007/s00586-002-0438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee SJ. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature. 1994;368:639–643. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- 78.Sztrolovics R, Alini M, Roughley PJ, Mort JS. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem J. 1997;326:235–241. doi: 10.1042/bj3260235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takae R, Matsunaga S, Origuchi N, Yamamoto T, Morimoto N, Suzuki S, Sakou T. Immunolocalization of bone morphogenetic protein and its receptors in degeneration of intervertebral disc. Spine. 1999;24:1397–1401. doi: 10.1097/00007632-199907150-00002. [DOI] [PubMed] [Google Scholar]
- 80.Takahashi H, Suguro T, Okazima Y, Motegi M, Okada Y, Kakiuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996;21:218–224. doi: 10.1097/00007632-199601150-00011. [DOI] [PubMed] [Google Scholar]
- 81.Takahashi M, Haro H, Wakabayashi Y, Kawa-uchi T, Komori H, Shinomiya K. The association of degeneration of the intervertebral disc with 5a/6a polymorphism in the promoter of the human matrix metalloproteinase-3 gene. J Bone Joint Surg Br. 2001;83:491–495. doi: 10.1302/0301-620X.83B4.11617. [DOI] [PubMed] [Google Scholar]
- 82.Takegami K, An HS, Kumano F, Chiba K, Thonar EJ, Singh K, Masuda K. Osteogenic protein-1 is most effective in stimulating nucleus pulposus and annulus fibrosus cells to repair their matrix after chondroitinase ABC-induced in vitro chemonucleolysis. Spine J. 2005;5:231–238. doi: 10.1016/j.spinee.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 83.Takegami K, Thonar EJ, An HS, Kamada H, Masuda K. Osteogenic protein-1 enhances matrix replenishment by intervertebral disc cells previously exposed to interleukin-1. Spine. 2002;27:1318–1325. doi: 10.1097/00007632-200206150-00014. [DOI] [PubMed] [Google Scholar]
- 84.Thompson JP, Oegema TJ, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 1991;16:253–260. doi: 10.1097/00007632-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 85.Tilkeridis C, Bei T, Garantziotis S, Stratakis CA. Association of a COL1A1 polymorphism with lumbar disc disease in young military recruits. J Med Genet. 2005;42:E44. doi: 10.1136/jmg.2005.033225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tim Yoon S, Su Kim K, Li J, Soo Park J, Akamaru T, Elmer WA, Hutton WC. The effect of bone morphogenetic protein-2 on rat intervertebral disc cells in vitro. Spine. 2003;28:1773–1780. doi: 10.1097/01.BRS.0000083204.44190.34. [DOI] [PubMed] [Google Scholar]
- 87.Tolonen J, Gronblad M, Vanharanta H, Virri J, Guyer RD, Rytomaa T, Karaharju EO. Growth factor expression in degenerated intervertebral disc tissue an immunohistochemical analysis of transforming growth factor beta, fibroblast growth factor and platelet-derived growth factor. Eur Spine J. 2006;15:588–596. doi: 10.1007/s00586-005-0930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tolonen J, Gronblad M, Virri J, Seitsalo S, Rytomaa T, Karaharju E. Basic fibroblast growth factor immunoreactivity in blood vessels and cells of disc herniations. Spine. 1995;20:271–276. doi: 10.1097/00007632-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 89.Tolonen J, Gronblad M, Virri J, Seitsalo S, Rytomaa T, Karaharju E. Transforming growth factor beta receptor induction in herniated intervertebral disc tissue: an immunohistochemical study. Eur Spine J. 2001;10:172–176. doi: 10.1007/s005860000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tolonen J, Gronblad M, Virri J, Seitsalo S, Rytomaa T, Karaharju EO. Platelet-derived growth factor and vascular endothelial growth factor expression in disc herniation tissue: and immunohistochemical study. Eur Spine J. 1997;6:63–69. doi: 10.1007/BF01676576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29:2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 92.Videman T, Leppavuori J, Kaprio J, Battie MC, Gibbons LE, Peltonen L, Koskenvuo M. Intragenic polymorphisms of the vitamin D receptor gene associated with intervertebral disc degeneration. Spine. 1998;23:2477–2485. doi: 10.1097/00007632-199812010-00002. [DOI] [PubMed] [Google Scholar]
- 93.Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine. 2004;29:156–163. doi: 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- 94.Wehling P (2002) Antiapoptotic and antidegenerative effect of an autologous IL-1ra/IGF-1/PDGF combination on human intervertebral disc cells in vivo The International Society for the study of the lumbar spine. In: 29th Annual Meeting Proceeding, Cleveland, OH, p24
- 95.Wehling P, Schulitz KP, Robbins PD, Evans CH, Reinecke JA. Transfer of genes to chondrocytic cells of the lumbar spine. Proposal for a treatment strategy of spinal disorders by local gene therapy. Spine. 1997;22:1092–1097. doi: 10.1097/00007632-199705150-00008. [DOI] [PubMed] [Google Scholar]
- 96.Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30:97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 97.Weiler C, Nerlich AG, Bachmeier BE, Boos N (2005) Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine 30:44–53 [DOI] [PubMed]
- 98.Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE Award competition in basic science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11:308–320. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]