Abstract
The ability of the intervertebral disc to resist compression is dependent on its high proteoglycan concentration. The disc proteoglycans are classified as aggregating or non-aggregating depending on their ability to interact with hyaluronan. The majority of the aggregating proteoglycans are derived from aggrecan, though their glycosaminoglycan substitution pattern has not been determined. In contrast, the origin of the non-aggregating proteoglycans is unclear, though it has been postulated that they are derived from aggrecan by proteolysis. The present work demonstrates that keratan sulfate (KS) in the glycosaminoglycan-binding region of disc aggrecan is confined to the KS-rich domain of the core protein and is not present in association with chondroitin sulfate (CS) in the CS1 and CS2 domains. It also shows that the non-aggregating disc proteoglycans are derived from aggrecan, with the large molecules possessing both the KS-rich and CS1 domains and the smaller molecules being generated from either the KS-rich or CS2 domain. The origin and spectrum of disc proteoglycan heterogeneity is the same in both the annulus fibrosus and nucleus pulposus.
Keywords: Proteoglycan, Aggrecan, Keratan sulfate, Intervertebral disc
Introduction
The intervertebral discs separate the vertebrae of the spine and permit the twisting and bending associated with spine mobility. The discs are composed of two regions that are structurally and functionally distinct [3, 16]—the outer annulus fibrosus (AF) and the central nucleus pulposus (NP). The AF consists of concentric lamellae rich in collagen, whereas the NP is more gelatinous in texture and is rich in proteoglycan. It is this proteoglycan that provides the disc with its osmotic properties and its ability to resist compressive loads [33]. During early adult life the proteoglycan content of the NP declines [1] and is to some extent compensated by an increase of proteoglycan content in the inner AF, which may provide functional compensation [21]. It is unclear whether this change is a true consequence of age, or is associated with disc degeneration which increases throughout life [6, 19].
The disc proteoglycans can be divided into two populations [5, 13]—aggregating proteoglycans, which have the ability to interact with hyaluronan (HA), and non-aggregating proteoglycans. The aggregating proteoglycans are mainly derived from aggrecan [16], though versican, another member of the hyalectan family of proteoglycans, is also present [30]. The non-aggregating proteoglycans contain members of the small leucine-rich repeat proteoglycan (SLRP) family [26, 29], but the majority of this population are larger in size than the SLRPs and have been postulated to be proteolytic degradation products of aggrecan [22]. Such non-aggregating proteoglycans are present in greater abundance than the aggregating proteoglycans [11].
Aggrecan consists of a large core protein that is substituted with multiple chondroitin sulfate (CS) and keratan sulfate (KS) chains [9, 34]. In an amino-terminal to carboxy-terminal orientation, the aggrecan core protein can be divided into seven structural domains [7]—the G1 domain (a globular domain responsible for the interaction with HA), the interglobular domain (IGD), the G2 domain, the KS-rich domain, the CS1 domain, the CS2 domain, and the G3 domain (Fig. 1). The adjacent KS-rich, CS1 and CS2 domains collectively constitute the glycosaminoglycan (GAG)-binding region. At present it is unclear whether KS and CS substitution are confined to unique domains, or whether they can occur throughout the GAG-binding region. KS abundance increases considerably with juvenile development [28], and it has been postulated that in the adult some of the additional KS may be in the CS1 or CS2 domains [16].
Fig. 1.
The structure of human aggrecan. Aggrecan is depicted as a central core protein with keratan sulfate (KS) and chondroitin sulfate (CS) side chains. The core protein possesses three globular domains (G1, G2 and G3), with the G2 and G3 domains being separated by a KS-rich domain and two CS-rich domains (CS1 and CS2)
The purpose of this work was to address some of these deficits by determining where KS substitution occurs on the aggrecan core protein, how the major non-aggregating proteoglycans are related to aggrecan, and whether the proteoglycan heterogeneity in the AF is analogous to that in the NP.
Methods
Tissue source
Intervertebral discs from the lumbar spine were obtained at the time of autopsy from individuals ranging in age from the fetus to the mature adult. With the exception of fetal tissue, discs were divided into AF and NP regions for proteoglycan extraction. Articular cartilage was also obtained from the femoral condyles of a young adult.
Proteoglycan purification
Disc tissue was finely divided with a scalpel into pieces 1–2 mm in each dimension. For younger individuals, tissue from adjacent discs was pooled to maximize yields. The tissue was extracted with ten volumes 4 M guanidinium chloride, in the presence of proteinase inhibitors, at 4°C for 48 h [24]. The filtered extracts were dialyzed to associative conditions, and proteoglycans were separated from other matrix proteins by CsCl density gradient centrifugation, with a starting density of 1.5 g/ml [2]. Proteoglycans were recovered from the fractions having a density greater than 1.55 g/ml (A1-preparations). The A1-preparations were divided into aggregating and non-aggregating proteoglycans by gel filtration through Sepharose CL-2B. The aggregating proteoglycans were recovered at the void volume (A1Vo-preparation) and the non-aggregating proteoglycans were recovered in the included volume as two pools of equal GAG content (A1Vi1 and A1Vi2-preprations). An A1-preparation was also generated in an analogous manner from articular cartilage.
Enzyme treatments
Proteoglycan solutions (1 mg/ml) were digested with trypsin (20 μg/mg proteoglycan) in 50 mM Tris, 150 mM NaCl, 1 mM CaCl2, pH 7.5 [20]. Incubations were overnight at 37°C, and enzyme activity was then inhibited by adding soybean trypsin inhibitor (5 μg/μg trypsin). Subsequent chondroitinase digestion was carried out following dialysis into 100 mM Tris, 100 mM sodium acetate, pH 7.3, using chondroitinase ABC (50 μU/μg proteoglycan) [25]. Alternatively, keratanase digestion was carried out following dialysis into 10 mM sodium acetate, pH 6.0, using keratanase II (5 μU/μg proteoglycan) [25]. Incubation for both glycosidases was overnight at 37°C.
Agarose gel electrophoresis and immunoblotting
Proteoglycan preparations and their trypsin digestion products were analyzed by electrophoresis in 1.2% agarose gels [4]. Between 1 and 20 μg proteoglycan was used for analysis depending on the detection conditions. Routinely, proteoglycans were detected by direct staining of the gels with 0.02% Toluidine blue. In addition, proteoglycans or their fragments possessing either the CS1 domain or KS chains were identified by immunoblotting. The presence of CS or KS substitution was confirmed by pretreatment of the samples with chondroitinase ABC or keratanase II, respectively. For immunolocalization, proteoglycans were transferred to derivatized nitrocellulose membranes by capillary blotting [12]. The nitrocellulose was derivatized by exposure to 1% cetyl pyridinium chloride, which generated a cationic membrane able to interact with the anionic GAG chains of the proteoglycans. Immunodetection utilized either a rabbit polyclonal antibody recognizing the CS1 region of the human aggrecan core protein or a mouse monoclonal antibody recognizing KS [17]. The anti-CS1 antibody was raised to the peptide GRIEWPSTPTVGELGC conjugated to ovalbumin [10]. This was followed by a second step biotinylated antibody recognizing either rabbit or mouse IgG, and subsequently a streptavidin–horseradish peroxidase complex. Finally, a chemiluminesent substrate was added and the reaction product visualized by exposure to X-ray film. Results are presented for a single individual and are representative of all adults studied in the present work.
Results
Proteoglycans were recovered as A1-preparations from the lumbar discs of two third trimester fetuses, two young adults (24 and 27 years), two middle-aged adults (both 56 years), and two elderly adults (76 and 87 years). The neonatal discs were not subdivided into AF and NP, and the A1-preparations accounted for 24 and 40 mg/g tissue (Table 1). The yield increased in the young adult and declined with age in both the NP (98–17 mg/g tissue) and the AF (52–18 mg). The decline was greatest in the NP and resulted in the NP:AF proteoglycan ratio decreasing with age in the adult from 1.9 to 0.7.
Table 1.
Yield of A1-preparations from human intervertebral disc
| Age | Site | Tissue (g) | A1 mg/g tissue | NP:AF |
|---|---|---|---|---|
| F | NP/AF | 0.38 | 24.4 | |
| F | NP/AF | 0.34 | 39.7 | |
| 24 | NP | 1.74 | 98.0 | 1.9 |
| AF | 1.92 | 52.4 | ||
| 27 | NP | 1.83 | 70.5 | 1.5 |
| AF | 1.82 | 48.1 | ||
| 56 | NP | 1.65 | 54.6 | 1.2 |
| AF | 1.80 | 44.1 | ||
| 56 | NP | 1.80 | 35.7 | 1.2 |
| AF | 1.83 | 28.6 | ||
| 76 | NP | 1.51 | 16.8 | 0.9 |
| AF | 1.81 | 18.4 | ||
| 87 | NP | 1.70 | 18.1 | 0.7 |
| AF | 1.71 | 26.8 |
A1 proteoglycan aggregate preparation, AF annulus fibrous, F fetal, NP nucleus pulposus
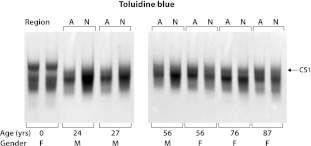
The A1-preparations at each age were analyzed by agarose gel electrophoresis following trypsin digestion (Fig. 2). Trypsin digestion results in cleavage throughout the human aggrecan core protein, with the exception of the CS1 domain which remains intact as it does not contain any lysine or arginine residues [7]. The CS1 domain can be resolved from fragments derived from the CS2 or KS-rich domains by virtue of its larger size and slower electrophoretic mobility. Toluidine blue staining revealed a three-component pattern at all ages, with the adults showing greater heterogeneity than the fetal samples. In all cases, the NP and AF preparations from the adult disc gave a similar pattern indicating a comparable degree of structural heterogeneity. Immunoblotting experiments revealed that the component of slowest electrophoretic mobility represents the CS1 domain (data not shown).
Fig. 2.
Comparison of AF and NP proteoglycans from intervertebral discs of different ages. Proteoglycan A1-preparations were treated with trypsin and then analyzed by agarose gel electrophoresis. Proteoglycan fragments were identified by Toluidine blue staining. The migration position of the CS1 domain is indicated, together with the gender (M or F) and age of the individual from whom tissue was obtained. The annulus fibrosus (A) and nucleus pulposus (N) proteoglycans were analyzed separately for all post-natal individuals
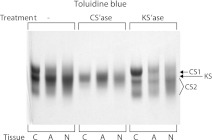
Analysis of adult A1-preparations revealed that the disc proteoglycan fragments derived by trypsin treatment are similar in electrophoretic mobility to those derived from an adult human articular cartilage aggrecan preparation, supporting their origin from disc aggrecan (Fig. 3). Keratanase treatment resulted in the three component pattern becoming more distinct on gel electrophoresis, but with no absolute change in mobility. In contrast, chondroitinase treatment resulted in the generation of a single relatively broad component migrating near the position of the central CS-containing component prior to treatment. Since the only Toluidine blue staining material resistant to chondroitinase treatment is KS, it appears that KS-containing fragments account for the more diffuse staining of the proteoglycan preparations prior to keratanase treatment. These results support the concept that the majority of CS and KS are on separate fragments. By default, the more mobile Toluidine blue staining components after keratanase treatment correspond to fragments derived from the aggrecan CS2 domain.
Fig. 3.
Comparison of adult articular cartilage and intervertebral disc proteoglycans. Proteoglycan A1-preparations from a 22 year old (cartilage) and a 24 year old (disc) were treated with trypsin and then analyzed by agarose gel electrophoresis. Proteoglycan fragments were identified by Toluidine blue staining either directly (−) or following treatment with chondroitinase ABC (CS’ase) or keratanase II (KS’ase). The migration position of the aggrecan KS-rich, CS1 and CS2 domains are indicated. Proteoglycans were isolated from articular cartilage (C), annulus fibrosus (A) and nucleus pulposus (N)
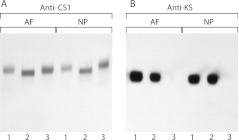
Further proof of the segregation of CS and KS on the trypsin-generated proteoglycan fragments was obtained by immunoblotting following treatment with chondroitinase ABC or keratanase II (Fig. 4). With respect to the CS1-derived fragment, chondroitinase treatment caused a shift in electrophoretic mobility compatible with the diminution in size expected upon removal of the CS chains, whereas keratanase treatment did not affect their electrophoretic mobility. In contrast, the electrophoretic mobility of the KS-containing fragments was unaffected by chondroitinase treatment. Identical results were obtained for proteoglycan derived from either the AF or NP. Thus there appears to be no KS in the CS1 domain of aggrecan and no CS in association with KS.
Fig. 4.
Analysis of GAG substitution pattern on intervertebral disc aggrecan. Proteoglycan A1-preparation from a 24 year old was treated with trypsin and then analyzed by agarose gel electrophoresis. Proteoglycan fragments possessing the aggrecan CS1 domain or KS chains were identified by immunoblotting. They were analyzed directly (1) or following treatment with chondroitinase ABC (2) or keratanase II (3) to determine GAG substitution. Proteoglycans from the annulus fibrosus (AF) and nucleus pulposus (NP) were analyzed separately
To further analyze the adult disc proteoglycans, they were separated into aggregated and non-aggregated components by gel filtration chromatography. The non-aggregated components were divided into molecules of large or small size (AlVi1 and AlVi2, respectively). For the NP, the aggregated and non-aggregated proteoglycans represented 24–31 and 69–76%, respectively, of the total proteoglycan (Table 2). For the AF, the aggregated and non-aggregated proteoglycans represented 41–43 and 57–59%, respectively. Thus non-aggregated proteoglycans were present in greater abundance than the aggregated proteoglycans, and their relative abundance was higher in the NP than the AF. Among the non-aggregated proteoglycans, the proportion of molecules of lower molecular weight was less in the AF than the NP.
Table 2.
Yield of aggregated and non-aggregated disc proteoglycans
| Age (years) | Fraction | NP %A1 | NP %Vi | AF %A1 | AF %Vi |
|---|---|---|---|---|---|
| 24 | Vo | 24.1 | – | 41.5 | – |
| Vi1 | 30.4 | 40 | 39.2 | 67 | |
| Vi2 | 45.5 | 60 | 19.3 | 33 | |
| 56 | Vo | 30.9 | – | 43.2 | – |
| Vi1 | 38.8 | 56 | 41.9 | 74 | |
| Vi2 | 30.3 | 44 | 14.9 | 26 |
A1 proteoglycan aggregate preparation, AF annulus fibrous, NP nucleus pulposus, Vo the aggregated proteoglycans eluting at the void volume on gel filtration, Vi1 the non-aggregated proteoglycans eluting in the first part of the included volume on gel filtration, Vi2 the non-aggregated proteoglycans eluting in the second part of the included volume on gel filtration
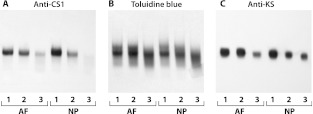
Upon analysis of the trypsin-generated degradation products of the aggregated and non-aggregated proteoglycans, it was apparent that there was similarity in fragment heterogeneity (Fig. 5). The large non-aggregated proteoglycans gave rise to CS1 and KS-containing fragments that were of analogous electrophoretic mobility to those derived from the aggregated proteoglycans. The small non-aggregated proteoglycans also gave rise to similar KS-containing fragments but were deplete in CS1-containing fragments. The Toluidine blue staining pattern indicated that fragments, equivalent in electrophoretic mobility to those derived from the CS2 domain of aggrecan, were present in all three proteoglycan populations.
Fig. 5.
Analysis of aggrecan domains in intervertebral disc non-aggregated proteoglycans. Proteoglycan A1-preparation from a 24 year old was subdivided into aggregated and non-aggregated populations which were then analyzed separately by agarose gel electrophoresis following trypsin digestion. Proteoglycan fragments were detected by Toluidine blue staining (b) or following immunoblotting to identify the aggrecan CS1 domain (a) or KS chains (c). Aggregated proteoglycans (1) and large (2) and small (3) non-aggregated proteoglycans were analyzed separately from annulus fibrosus (AF) and nucleus pulposus (NP)
Discussion
The present data support several concepts regarding the proteoglycans in the human intervertebral disc. First, they verify that the proteoglycan content is maximal in the young adult and declines thereafter. Second, they indicate that the age-related decrease in proteoglycan content in the adult disc occurs to a greater extent in the NP compared to the AF. Third, they indicate that the majority of disc proteoglycans exist in the non-aggregated form, with the proportion of non-aggregated molecules being greater in the NP than the AF. Fourth, they demonstrate that many of the disc non-aggregating proteoglycans are derived from aggrecan. Finally, they demonstrate that disc aggrecan does not possess KS in either its CS1 or CS2 domains.
The observation with respect to KS location is dependent on immunological detection of KS using an antibody whose epitope recognizes an over-sulfated region of the KS chain [14]. It would therefore be possible that some KS could elude detection if it were deplete in sulfation. Such KS chains may also show little ability to interact with Toluidine blue and may evade degradation by keratanase II. There is, however, no evidence to support the existence of such poorly sulfated polylactosamine chains on aggrecan, although short O-linked oligosaccharides analogous to the linkage domain of KS have been described [15]. The location of these oligosaccharides is not known. If one accepts that the KS is not present in the CS1 or CS2 domains, then one must also accept that the increase in KS chain length and number that occurs throughout life [27] is taking place mainly in the KS-rich domain of the aggrecan core protein.
The ability of the CS1 region to be isolated intact following trypsin treatment of aggrecan permits the absence of KS to be verified conclusively. Such is not the case with the CS2 domain, which is fragmented into multiple components. While the present results show that none of the CS-containing fragments from the CS2 domain contains KS, it could be argued that KS is present in this region, but at unique sites that are fortuitously separated by trypsin treatment. The protein sequence of the CS2 domain [7] makes this scenario extremely unlikely.
The present work suggests that the non-aggregating proteoglycans of the disc are derived from aggrecan, presumably by proteolysis, which is known to occur throughout life and is responsible for the heterogeneity of the aggregating proteoglycans [21]. Based on the current observations, it is likely that the larger non-aggregating proteoglycans contain both the KS-rich and CS1 domains of aggrecan. In contrast, the smaller non-aggregating proteoglycans contain either the KS-rich or CS2 domain. It is not clear which proteinases are responsible for generating such fragments. While aggrecanases can separate CS2 fragments from a KS-rich/CS1 fragment, they are not capable of separating the KS-rich domain from the CS1 domain [31, 32]. Such cleavage may, however, be possible by matrix metalloproteinases (MMPs). Both aggrecanase and MMP action are known to occur in the disc in vivo [8, 18].
Metalloproteinase action is also known to occur in articular cartilage throughout life resulting in proteolytic degradation of aggrecan [23]. Yet in this tissue large amounts of non-aggregating aggrecan degradation products do not accumulate, presumably due to their diffusion from the tissue into the synovial fluid. This raises the issue of why such loss does not occur in the disc. The most likely explanation is the large avascular nature of the disc, with the proteoglycan-containing central region being confined above and below by the vertebral end plates and circumferentially by the outer AF. Thus, there is no easy route for diffusion of aggrecan degradation products from the disc irrespective of whether they remain bound to hyaluronan or not. Presumably loss of proteoglycan does occur slowly from the disc (over decades) accounting for its decline in the elderly.
It is likely that as long as the non-aggregating proteoglycans remain in the disc, they may serve a functional role in resisting compression, as they maintain the fixed charge density of the tissue. Thus, the slow rate of progression of degenerative disc disease may not necessarily reflect a slow rate of proteolysis of aggrecan, but rather a slow loss of the aggrecan degradation products. Such limited diffusion could explain why the small non-aggregated proteoglycans are in lower abundance in the AF than the NP, as escape from the inner AF through the outer AF should precede similar escape from the NP. Escape via the vertebral end plates is unlikely from either region.
Finally, one should consider the consequences of the present data on the potential use of KS as a marker of the proteolytic events associated with disc degeneration. Because of the limited distribution of KS to the KS-rich domain of the aggrecan core protein, proteolytic release of KS from the proteoglycan aggregates will require cleavage in the G1, G2 or interglobular domains. Even then, the accumulation of aggrecan degradation products in the disc may prevent their release into the blood. It may require substantial collagen damage in the AF before proteoglycan fragments become available for bioassay, which may occur slowly over decades.
Acknowledgement
This work was supported by research funding from the Shriners of North America.
References
- 1.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc—evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayliss MT, Roughley PJ. The properties of proteoglycan prepared from human articular cartilage by using associative caesium chloride gradients of high and low starting densities. Biochem J. 1985;232:111–117. doi: 10.1042/bj2320111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin M, Ralphs JR. Biology of fibrocartilage cells. Int Rev Cytol. 2004;233:1–45. doi: 10.1016/S0074-7696(04)33001-9. [DOI] [PubMed] [Google Scholar]
- 4.Björnsson S. Size-dependent separation of proteoglycans by electrophoresis in gels of pure agarose. Anal Biochem. 1993;210:292–298. doi: 10.1006/abio.1993.1198. [DOI] [PubMed] [Google Scholar]
- 5.Bushell GR, Ghosh P, Taylor TFK, Akeson WH. Proteoglycan chemistry of intervertebral disks. Clin Orthop. 1977;129:115–123. doi: 10.1097/00003086-197711000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Cassinelli EH, Hall RA, Kang JD. Biochemistry of intervertebral disc degeneration and the potential for gene therapy applications. Spine J. 2001;1:205–214. doi: 10.1016/S1529-9430(01)00021-3. [DOI] [PubMed] [Google Scholar]
- 7.Doege KJ, Sasaki M, Kimura T, Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991;266:894–902. [PubMed] [Google Scholar]
- 8.Goupille P, Jayson MIV, Valat JP, Freemont AJ. Matrix metalloproteinases: the clue to intervertebral disc degeneration? Spine. 1998;23:1612–1626. doi: 10.1097/00007632-199807150-00021. [DOI] [PubMed] [Google Scholar]
- 9.Hascall VC. Proteoglycans: the chondroitin sulfate/keratan sulfate proteoglycan of cartilage. ISI Atlas Sci Biochem. 1988;1:189–198. [Google Scholar]
- 10.Hughes CE, Caterson B, White RJ, Roughley PJ, Mort JS. Monoclonal antibodies recognizing protease-generated neoepitopes from cartilage proteoglycan degradation. Application to studies of human link protein cleavage by stromelysin. J Biol Chem. 1992;267:16011–16014. [PubMed] [Google Scholar]
- 11.Jahnke MR, McDevitt CA. Proteoglycans of the human intervertebral disc. Electrophoretic heterogeneity of the aggregating proteoglycans of the nucleus pulposus. Biochem J. 1988;251:347–356. doi: 10.1042/bj2510347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlsson M, Edfors-Lilja I, Bjornsson S. Binding and detection of glycosaminoglycans immobilized on membranes treated with cationic detergents. Anal Biochem. 2000;286:51–58. doi: 10.1006/abio.2000.4767. [DOI] [PubMed] [Google Scholar]
- 13.McDevitt CA. Proteoglycans of the intervertebral disc. In: Ghosh P, editor. Biology of the intervertebral disc. Boca Raton: CRC Press; 1988. pp. 151–170. [Google Scholar]
- 14.Mehmet H, Scudder P, Tang PW, Hounsell EF, Caterson B, Feizi T. The antigenic determinants recognized by three monoclonal antibodies to keratan sulphate involve sulphated hepta- or larger oligosaccharides of the poly(N-acetyllactosamine) series. Eur J Biochem. 1986;157:385–391. doi: 10.1111/j.1432-1033.1986.tb09680.x. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson B, Luca S, Lohmander S, Hascall VC. Structures of N-linked and O-linked oligosaccharides on proteoglycan monomer isolated from the Swarm rat chondrosarcoma. J Biol Chem. 1982;257:10920–10927. [PubMed] [Google Scholar]
- 16.Oegema TR. Biochemistry of the intervertebral disc. Clin Sports Med. 1993;12:419–439. [PubMed] [Google Scholar]
- 17.Poole AR, Webber C, Reiner A, Roughley PJ. Studies of a monoclonal antibody to skeletal keratan sulphate. Importance of antibody valency. Biochem J. 1989;260:849–856. doi: 10.1042/bj2600849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase—their role in disorders of the human intervertebral disc. Spine. 2000;25:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 19.Roberts S, Urban JPG. Degeneration of intervertebral disc. Arthritis Res Ther. 2003;5:120–130. doi: 10.1186/ar613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roughley PJ. A comparative study of the glycosaminoglycan-peptides obtained after degradation of cartilage proteoglycan by different proteinases, and their use in the characterization of different proteoglycans. Connect Tissue Res. 1978;6:145–153. doi: 10.3109/03008207809152624. [DOI] [PubMed] [Google Scholar]
- 21.Roughley PJ. Biology of intervertebral disc aging and degeneration—involvement of the extracellular matrix. Spine. 2004;29:2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 22.Roughley PJ, Alini M, Antoniou J. The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochem Soc Trans. 2002;30:869–874. doi: 10.1042/BST0300869. [DOI] [PubMed] [Google Scholar]
- 23.Roughley PJ, Mort JS. Ageing and the aggregating proteoglycans of human articular cartilage. Clin Sci. 1986;71:337–344. doi: 10.1042/cs0710337. [DOI] [PubMed] [Google Scholar]
- 24.Roughley PJ, White RJ. Age-related changes in the structure of the proteoglycan subunits from human articular cartilage. J Biol Chem. 1980;255:217–224. [PubMed] [Google Scholar]
- 25.Roughley PJ, White RJ, Cs-Szabó G, Mort JS. Changes with age in the structure of fibromodulin in human articular cartilage. Osteoarthr Cartil. 1996;4:153–161. doi: 10.1016/S1063-4584(96)80011-2. [DOI] [PubMed] [Google Scholar]
- 26.Roughley PJ, White RJ, Magny M-C, Liu J, Pearce RH, Mort JS. Non-proteoglycan forms of biglycan increase with age in human articular cartilage. Biochem J. 1993;295:421–426. doi: 10.1042/bj2950421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santer V, White RJ, Roughley PJ. O-linked oligosaccharides of human articular cartilage proteoglycans. Biochim Biophys Acta. 1982;716:277–282. doi: 10.1016/0304-4165(82)90017-4. [DOI] [PubMed] [Google Scholar]
- 28.Scott JE, Bosworth TR, Cribb AM, Taylor JR. The chemical morphology of age-related changes in human intervertebral disc glycosaminoglycans from cervical, thoracic and lumbar nucleus pulposus and annulus fibrosus. J Anat. 1994;184:73–82. [PMC free article] [PubMed] [Google Scholar]
- 29.Sztrolovics R, Alini M, Mort JS, Roughley PJ. Age-related changes in fibromodulin and lumican in human intervertebral discs. Spine. 1999;24:1765–1771. doi: 10.1097/00007632-199909010-00003. [DOI] [PubMed] [Google Scholar]
- 30.Sztrolovics R, Grover J, CS-Szabo G, Shi SL, Zhang YP, Mort JS, Roughley PJ. The characterization of versican and its message in human articular cartilage and intervertebral disc. J Orthop Res. 2002;20:257–266. doi: 10.1016/S0736-0266(01)00110-3. [DOI] [PubMed] [Google Scholar]
- 31.Tortorella MD, Liu RQ, Burn T, Newton RC, Arner E. Characterization of human aggrecanase 2 (ADAM-TS5): substrate specificity studies and comparison with aggrecanase 1 (ADAM-TS4) Matrix Biol. 2002;21:499–511. doi: 10.1016/S0945-053X(02)00069-0. [DOI] [PubMed] [Google Scholar]
- 32.Tortorella MD, Pratta M, Liu RQ, Austin J, Ross OH, Abbaszade I, Burn T, Arner E. Sites of aggrecan cleavage by recombinant human aggrecanase-1 (ADAMTS-4) J Biol Chem. 2000;275:18566–18573. doi: 10.1074/jbc.M909383199. [DOI] [PubMed] [Google Scholar]
- 33.Urban JPG, Roberts S, Ralphs JR. The nucleus of the intervertebral disc from development to degeneration. Am Zool. 2000;40:53–61. doi: 10.1668/0003-1569(2000)040[0053:TNOTID]2.0.CO;2. [DOI] [Google Scholar]
- 34.Watanabe H, Yamada Y, Kimata K. Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. J Biochem (Tokyo) 1998;124:687–693. doi: 10.1093/oxfordjournals.jbchem.a022166. [DOI] [PubMed] [Google Scholar]