Abstract
Disc degeneration is the loss of the normal nucleus pulposus disc matrix to a more fibrotic and less cartilaginous structure. This change in disc micro-anatomy can be associated with pain and deformity, however, prevention and treatment options of disc degeneration are currently limited. Much research is going on to understand intervertebral discs at a molecular/ cellular level in hopes of creating clinically applicable options for treating disc degeneration. This review article will give insight into the current and developing status of treating intervertebral disc degeneration from a molecular standpoint.
Keywords: Disc degeneration, Disc therapy, Intervertebral disc, Disc molecular therapy, Mitogens, Morphogens, Intracellular regulators, Anti-catabolics
Introduction
The normal intervertebral disc consists of a rich extracellular matrix (ECM) that can be divided into the nucleus pulposus (which contains chondrocyte-like cells) and annulus fibrosus (which contains fibroblast-like cells) [1]. ECM provides the mechanical characteristics of the disc while the cells within this vascular fibrocartilaginous structure synthesize and maintain the matrix. Disc degeneration is characterized by loss of the normal nucleus pulposus disc matrix to a more fibrotic and less cartilaginous structure. Disc degeneration is clinically associated with low-back pain and other important disease conditions of the spine [2, 3]. Current treatment options range from pain management to invasive procedures such as spinal fusion and spinal arthroplasty, however, there is no clinically proven biological therapy for disc degeneration. Ongoing research hopes to develop strategies to treat and prevent disc degeneration using biologically active molecules. This review article will focus on the current status of molecular therapy for intervertebral disc.
Biology of intervertebral disc degeneration
The integrity of the intervertebral disc relies on the proper balance between matrix synthesis and degradation. The disc matrix is an elaborate framework of macromolecules that attract and hold water. The major structural components of the macromolecular framework are collagens and proteoglycans.
Collagens provide form and tensile strength while proteoglycans, through interactions with water, give the tissues stiffness, viscoelasticity, and resistance to compression [4]. Table 1 outlines the types of collagenous and non-collagenous proteins found in the disk. Collagenous proteins comprise 70% of the outer annulus dry weight, but only account for 20% of the central nucleus pulposus [4]. On the other hand, the greatest proteoglycan concentration exists in the nucleus pulposus, and proteoglycans have been shown to comprise 50% of the nucleus dry weight in children [4]. The proteoglycan molecule is made of a core protein to which a variable number of glycosaminoglycan units are covalently attached [5]. The most common glycosaminoglycan side chains in discs are chondroitin sulfate and keratan sulfate, with the former predominating in the normal disc and the latter in the degenerated disc [4–6]. Asporin, a member of the small leucine-rich repeat proteoglycan family, is expressed at higher levels in osteoarthritic articular cartilage [7, 8]. Similarly, cartilage intermediate layer protein (CILP) has been shown to increase with age in articular cartilage and enhance in the early stages of human osteoarthritis [9]. Asporin and CILP are still being further investigated and will hopefully give greater insight into degenerative tissue biology.
Table 1.
Intervertebral disc components
| Disc-matrix proteins | Proteoglycans | Collagen | Disc proteinases |
|---|---|---|---|
| Fibronectin | Aggrecan (most abundant) | Fibril-forming collagens | Metalloproteinases (MMPs) |
| Elastin | Versican | I 0–80% | Collagenases (MMPs 1, 8, and 13) |
| CILP | Decorin | II 0–80% | |
| Asporin | Biglycan | III<5% | Gelatinases (MMP 2 and 9) |
| Fibromodulin | V 1–2% | ||
| Lumican | XI 1–2% | Stromelysin (MMP 3) | |
| Perlecan | Short helix collagens | ||
| VI 5–20% | ADAMS | ||
| IX 1–2% | |||
| XII<1% |
It is important to understand disc degeneration from both morphological and molecular levels. Disc morphology changes with age. Degenerative changes have been shown unequivocally in those as young as 11 years [10]. These changes include dehydration, fissures as well as tears of the nucleus, annulus, and endplates. On the molecular level, degenerative changes include decreased diffusion of nutrient and waste products, decreased cell viability, accumulation of apoptosis debris, degradative enzyme activity, accumulation of degraded matrix macromolecules, fatigue failure of the matrix, decreased proteoglycan synthesis, and alteration in collagen distribution [4, 11].
Disc degeneration begins when imbalances occur favoring catabolism and/or the failure to retain matrix proteins over synthesis and/or retention. Decreased disc nutrition is an important contributor to degeneration. Increased disc size and endplate changes, when combined with cell density, lead to decreased nutrition in the center of the nucleus, low pH, and possibly cell death [4, 12]. The most prominent change with degeneration is progressive loss of proteoglycan, water, and collagen II in the disc matrix of the nucleus pulposus. Another notable change is the loss of differentiated chondrocyte phenotypes from the nucleus pulposus resulting in a more fibrotic phenotype. There are qualitative matrix changes that are not well-defined including the breakdown of higher molecular weight proteoglycans and differences in the small leucine-rich proteoglycans. Other changes include collagen crosslinking and organization of the proteoglycan. Alterations in the annulus fibrosus include disorganization of the matrix. These changes take many years to become apparent and result from an imbalance between annular lamella layers and physical defects in the collagenous matrix (Fig. 1).
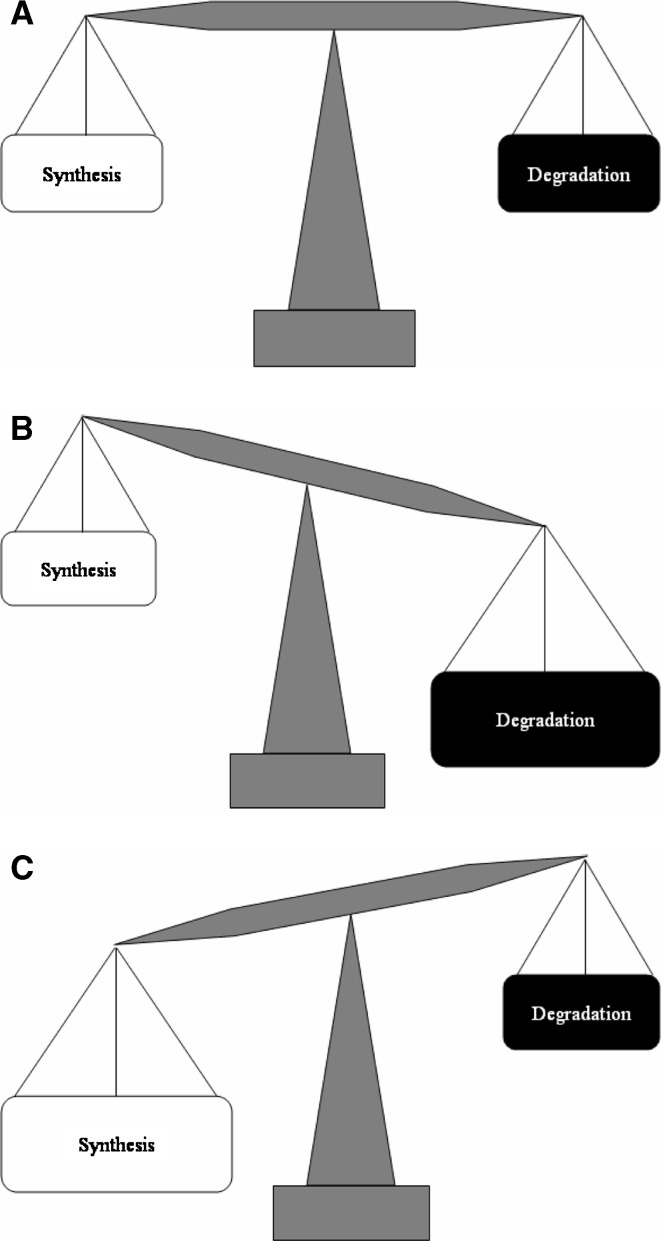
Fig. 1.
Disc-matrix metabolism: balance of synthesis and degradation. a In the homeostatic state, the disc undergoes matrix synthesis and degradation in a balanced manner. b As the disc matrix undergoes a turnover during the course of an individual’s lifetime, any small imbalance between synthesis and degradation can lead to significant changes in overall disc-matrix content. c One of the major goals of molecular therapy of the disc involves modulating this metabolic balance to the more favorable anabolic state. This can be accomplished by increasing the synthesis or decreasing the catabolism
Although inflammatory mediators have been identified in degenerated discs, the pathologic role played by each of these mediators is not well understood. Nitric oxide (NO), interleukin-6 (IL-6), prostaglandin E2 (PGE2), TNF-alpha, fibronectin, and matrix metalloproteinases (MMPs) are a few of the many mediators identified (Table 2) [11, 13–15]. The roles played by these mediators are currently being investigated. The NO, IL-6, and PGE2 appear to be the inhibitory factors of proteoglycan synthesis. These factors are recruited into action by interleukin-1 (IL-1), which also plays a role in the direct breakdown of the proteoglycan matrix. This process of direct breakdown by IL-1 is thought to be mediated by a family of enzymes known as MMPs. IL-1 likely plays a major role in the cascade of inflammatory mediators, but the nature of that role is not well defined [16]. Seguin et al. [14] recently demonstrated that TNF-[alpha] (a proinflammatory cytokine found in herniated nucleus pulposus tissue) at doses of 1–5 ng/ml, induced multiple cellular responses, including: decreased expression of both aggrecan and type II collagen genes; decreases in the accumulation and overall synthesis of aggrecan and collagen; increased expression of MMP-1, MMP-3, MMP-13, ADAM-TS4, and ADAM-TS5; and induction of ADAM-TS dependent proteoglycan degradation. Within 48 h, these cellular responses resulted in 75% loss of proteoglycan content of nucleus pulposus tissue. This loss suggests that TNF-[alpha] plays a role in the process of disc degeneration.
Table 2.
Inflammatory mediators implicated in disc degeneration
| 1. | Matrix metalloproteinases (MMPs) |
| 2. | Nitric oxide (NO) |
| 3. | Interleukin-6 (IL-6) |
| 4. | Prostaglandin E2 (PGE2) |
| 5. | TNF-alpha |
| 6. | Fibronectin |
The balance between synthesis, breakdown, and accumulation of matrix macromolecules determines the quality and integrity of the matrix, and thus the mechanical behavior of the disc itself. The goal of molecular therapy is to prevent or reverse these changes in the disc ECM by altering the degradation–synthesis balance in favor of synthesis.
Molecular therapy strategies for disc disease
Therapeutic strategies under investigation for the biological treatment of disc degeneration include the use of cellular components (mesenchymal stem cells, chondrocytes, culture expanded disc cells, disc allograft, etc.), matrix-derivatives, and molecules influencing disc-cell metabolism and phenotype (Table 3) [17–26]. There are at least four different classes of molecules that are currently being investigated for disc therapy: anti-catabolics, mitogens, morphogens, and intracellular regulators. All of these molecules have some in vitro data, but few have been tested in vivo with an animal model of disc degeneration (Table 4). Each of these categories will be defined and the key literature reviewed in this article.
Table 3.
Molecular therapy strategies for disc disease
| Type of intervention | Examples |
|---|---|
| Cell-based | Whole-disc transplantation |
| Transplantation of cultured disc cells | |
| Chondrocyte transplantation | |
| Mesenchymal stem cells transplantation | |
| Disc cell/scaffold constructs | |
| ECM component-based | ECM—extracellular matrix protein |
| Molecule-based | Anti-catabolic |
| Mitogens | |
| Morphogen | |
| Intracellular regulators |
Table 4.
Molecules under investigation for disc therapy
| Category | Molecule |
|---|---|
| Anti-catabolic | TIMP-1,-2,-3 |
| Anti-TNF-alpha | |
| Anti-MMPs (CPA-926) | |
| Mitogens | IGF-1 |
| PDGF | |
| EGF | |
| FGF | |
| Morphogen | TGF-beta |
| BMP-2 | |
| BMP-7 (OP-1) | |
| BMP-13 (GDF-6 aka CDMP-2) | |
| GDF-5 (CDMP-1) | |
| Intracellular regulators | Link N |
| SMADs | |
| Sox9 | |
| LMP-1 |
Anti-catabolics
Anti-catabolics prevent matrix loss by inhibiting degradative enzymes within the disc. Several families of enzymes are capable of breaking down the various matrix molecules of disc, including cathepsins, aggrecanases, and MMPs [13, 27]. MMPs play an important role in the normal turnover of matrix molecules, but have also been linked with degradation of collagen, aggrecan, versican, and link proteins found in the degenerated disc [27]. The main members of the MMP family are stromelysin (MMP 3), collagenase (MMPs 1, 8, and 13), and gelatinase (MMPs 2 and 9). Stromelysin is found mainly in the nucleus pulposus, and is active in degrading the core protein of proteoglycans. It is the only agent capable of gaining access to the proteolytic cleavage sites, leaving isolated hyaluronate-binding regions, degraded proteoglycan aggregates, and glycosaminoglycan fragments as breakdown products [13, 27–29]. Collagenase and gelatinase are more prevalent in the annulus, and cooperate in the breakdown of collagen. Aggrecan and versican degradation may result from members of a second family of metalloproteinases, ADAMs. Two members of this family (ADAM-TS4 and 5) show a particular avidity for aggrecan and have been termed aggrecanases [11].
Given the degradative properties of MMPs, it is logical that much attention has focused on inhibiting MMPs and thus slowing or stopping disc degeneration. Within the matrix, MMP activity is normally inhibited by tissue-inhibitors-of-MMPs (TIMPs) [30, 31]. Wallach et al. successfully delivered an anti-catabolic gene, TIMP-I, into the cells from degenerated intervertebral discs using an adenoviral vector. They showed an increased expression of TIMP-1 in disc cells and also an increase in the “measured synthesis rate” of proteoglycans. This finding supports catabolic inhibition as a promising avenue of research for the treatment of degenerative disc disease via gene therapy [31]. An esculetin prodrug, CPA-926, which has a better pharmacokinetic profile than esculetin itself has anti-inflammatory and anti-tumorogenic characteristics. It has been shown to prevent degeneration in an osteoarthritic model of cartilage destruction [32]. Based on histological and radiographic evidence, Okuma et al. showed that oral administration of CPA-926 can prevent or delay the onset of disc-height loss using an annulotomy model of disc degeneration in the rabbit [32]. The inhibition of degradative molecules is an important aspect of preventing disc degeneration that has potential for future clinical practice.
Along with the balance of synthesis and degradation, the rate of disc-matrix metabolism may also be important. For example, the overall rate of disc metabolism in younger discs may be much higher than in older discs. In older discs, this may lead to increased accumulation of degraded products in comparison to the newly synthesized molecules. This disparity has been shown to be the case for aggregans when looking at aged discs. Further imbalance results when the demand of nutritional requirement, created by the need for increased metabolic rate, is not met in the degenerative intradiscal cells. Cytokines such as IL-1 and TNF-alpha, as described previously, may also have critical roles in disc metabolism. Molecules such as IL-1Ra and inflixamab, which can block IL-1 and TNF-alpha, respectively, may be useful [33–35]. Further research into anti-catabolic molecules may yield important results.
Mitogens
Growth factors act as anabolic regulators of disc-cell metabolism [36]. Mitogens are defined by their ability to increase the rate of mitosis. These molecules constitute the true “growth factors”, and for the purposes of this review article, we will differentiate mitogenic “growth factors” from the set of molecules that are highly chondrogenic (morphogens) (Fig. 2). Growth factors are typically cytokines, defined by their ability to bind to specific transmembrane receptors resulting in activation of an intercellular signaling cascade. In general, the biological effect of cytokines can be a variety of different effects such as cell proliferation, differentiation, migration, and apoptosis.
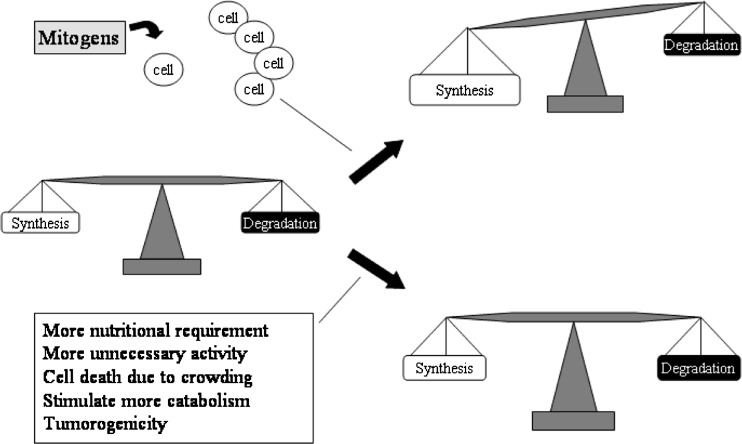
Fig. 2.
Mitogenic molecules: mitogenic molecules are the true growth factors. They increase the cell number without necessarily enhancing cell differentiation. The increase in cell number leads to increase in matrix synthesis. However, this may also increase the nutritional requirement in the disc due to higher needs for keeping the cell alive and perhaps increase the overall catabolism as well as synthesis. Furthermore, high cell density can lead to less nutrition per cell leading to limits on synthetic metabolism. Finally, mitogens may have a potential to promote tumors
For disc cells, mitogenic molecules include insulin-like growth factor-1 (IGF-1), epidermal growth factor (EGF), and fibroblast growth factor (FGF) [37]. Thompson et al. [37] used in vitro experiments with mature canine disc cells to show that mitogenic molecules can increase the rate of mitosis and proteoglycan synthesis to various degrees depending on the region of the disc from which the cells were obtained. In vivo experiments with growth factors using a mouse-tail disc compression model for degeneration by Walsh et al. [38] produced results that were consistent with in vitro experiments by Thompson et al. [37]. Walsh et al. showed that IGF-1 had mild effects (especially in the inner annulus) and FGF had no effect while Thompson et al. found EGF to perform better than other mitogens [37]. Additionally, Okuda et al. demonstrated an age-related decline in proteoglycan synthesis to IGF-1 in rat discs [39]. Based on this information, researchers have speculated that by restoring the IGF-1 in aging discs, matrix synthesis may be increased.
In a related but different potential mechanism of therapy, some growth factors may protect disc cells from apoptosis. Gruber et al. [40] demonstrated a significant reduction in the percentage of apoptotic disc cells after exposure to 50–500 ng/ml IGF-1 or exposure to 100 ng/ml platelet-derived growth factor. These findings expand the understanding of the cell biology of disc cells and show that selected cytokines can retard or prevent programed cell death in vitro. While IGF-1 has some anabolic effects, it may also have an effect on catabolism. IGF-1 has been shown to decrease the levels of active TIMP-2 in tissue-culture experiments, indicating a complex effect on disc-matrix metabolism by IGF-1. The complete understanding of IGF-1 is still evolving and holds potential.
Morphogens
Transforming growth factor-beta (TGF-beta), bone morphogenetic proteins (BMPs), and growth and differentiation factors (GDFs) are chondrogenic morphogens for disc cells. They have mitogenic capability, but are truly characterized by their ability to increase the chondrocyte-specific phenotype of the cell (Fig. 3). These growth factors tend to act as anabolic regulators of disc-cell metabolism and increase the production of collagen II, Sox9, aggrecan, and sulfated-glycosaminoglycans [37]. Chondrogenic morphogens are particularly attractive because they may reverse the fibrotic phenotype of disc cells to the more favorable chondrocytic phenotype found in younger and more “normal” disc. The aging-process changes the expression level and spatial distribution of TGF and BMP molecules and receptors [41, 42]. Okuda et al. supported this notion by demonstrating that the responsiveness of intervertebral cells to IGF-1 and TGF-beta decreases with advancing age in rabbit disc cells [39].
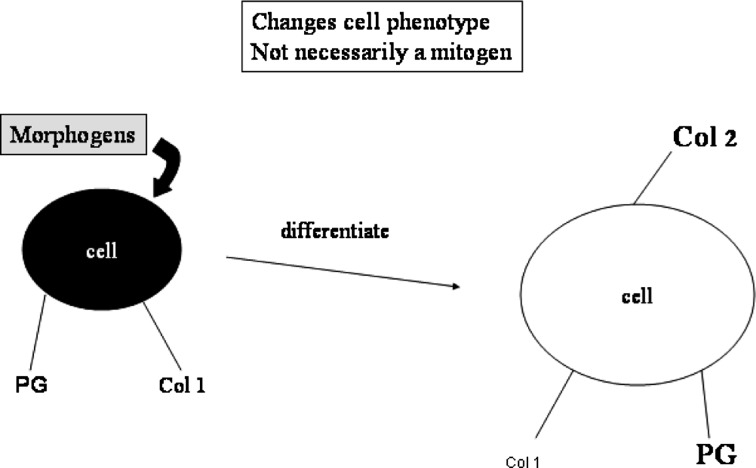
Fig. 3.
Morphogenic molecules: morphogens change the phenotype of the cell as their major mechanism of action without necessarily increasing the cell number. In the disc, morphogens may be used to increase the chondrocyte-like phenotype of the cells and enhance chondrocytic matrix synthesis
Transforming growth factor-beta was one of the first morphogenic molecules studied. Thompson et al. not only reported that TGF-beta was a mitogen, but also showed that it was a highly anabolic molecule leading to significantly increased proteoglycan synthesis by the cell [37]. They further showed that TGF-beta more superiorly increased proteoglycan-synthesis rate in comparison to the growth factors: EGF, IGF-1, PDGF, and FGF [37]. Subsequently, Nishida et al. demonstrated that an adenoviral vector containing the TGF-beta1 gene can be directly injected into immunocompetent rabbit (normal) discs in vivo and lead to expression of TGF-beta1 and increased rate of proteoglycan synthesis [43]. Another report showed that degenerated disc are also capable of responding to TGF-beta1 [44, 45]. This in vitro experiment took cells from degenerated human discs and exposed them to TGF-beta1, revealing increased proteoglycan- and collagen- synthesis rates; suggesting that degenerated disc cells are capable of responding to TGF-beta1 [44, 45]. In vivo experiments in mouse-tail discs indicated that TGF-beta1 had some effect on cell proliferation in the inner annulus, but did not have a measurable effect on the disc height [38]. As demonstrated by the broad interest, TGF-beta1 definitely has potential but its efficacy in vivo degeneration models has not yet been established.
Morphogenetic proteins-2 is another chondrogenic morphogen that has been shown to increase rat disc-cell proteoglycan production and the chondrocytic phenotype of disc cells by Yoon et al. They demonstrated an increased aggrecan and collagen II gene expression, but no change in collagen I gene expression [45, 46]. Because BMP-2 promotes the terminal differentiation of osteoblasts during bone formation, there was an initial concern that BMP-2 may also lead disc cell differentiation along an osteoblastic lineage. However, in vitro experiments with human disc cells demonstrated that BMP-2 enhanced the expression of chondrocytic genes but not osteogenic genes [47]. Further evidence of BMP-2 anabolic effect has been reported using human disc cells obtained during elective surgery in an in vitro model [48]. As of yet, there are no published reports on BMP-2 efficacy for treating disc degeneration in vivo models. It remains to be determined whether cells in a degenerated disc, especially metabolically impaired cells, can respond to growth factors and regenerate a damaged intervertebral disc. Ahn et al. [48] recently reported that BMP-2 and BMP-12 stimulated proteoglycan and collagen synthesis by human nucleus pulposus cells from degenerated discs cultured in monolayer in the absence of serum. They found that a significant and optimal stimulation of proteoglycan synthesis by these cells was achieved at 50 ng/ml of BMP-2 (560%) and BMP-12 (460%). Further studies using aging intervertebral disc cells are anticipated to prove the therapeutic concept that growth factors will be useful to treat degenerated disc disease in the aging population [36].
Morphogenetic proteins-7, also known as OP-1, is another potent disc-cell morphogen [49, 50]. Masuda et al. reported a dose-dependent increase in proteoglycan synthesis and expression of the chondrocytic genes aggrecan and collagen II. This effect was seen in both annulus fibrosus and nucleus pulposus of rabbit disc cells grown in vitro under the influence of BMP-7 [49]. Takegami et al. demonstrated that rabbit disc cells grown in alginate in the presence of the inflammatory cytokine IL-1 leads to loss of proteoglycan and collagen in the alginate as compared to control [17]. However, adding BMP-7 at 200 ng/ml to the IL-1 culture led to increased synthesis of proteoglycan and collagen even when compared to the controls without IL-1. Zhang et al. reported that in vitro cultured bovine disc cells from three distinct spatial zones (outer annulus, inner annulus, and nucleus) all increased cellular proliferation in the presence of BMP-7 [50]. However, only cells from outer annulus and nucleus increased the rate of proteoglycan synthesis in the presence of BMP-7. Recent in vivo experiments with BMP-7 in rabbit models of disc degeneration show that direct intradiscal injection of BMP-7 increases disc height and proteoglycan content in young rabbits [51]. Preliminary data also indicates that intradiscal injection of BMP-7 may be effective in treating an experimental rabbit disc-degeneration model using a small annulotomy [52].
Morphogenetic proteins-13, known as GDF-6 or cartilage-derived morphogenetic protein-2 (CDMP-2) is in the BMP family, but has only 50% homology to BMP-2 in amino acid sequence [53, 54]. BMP-13 increases proteoglycan synthesis rate and chondrocytic phenotype of disc cells, but it is much less potent osteogenic molecule than BMP-2 [54, 55]. For disc cells, the effect of adding both BMP-2 and BMP-13 were additive in increasing proteoglycan production or chondrocytic gene expression [54]. BMP-13 has some effect on tendon healing and therefore is under investigation for annulus fibrosus repair [56, 57].
Growth and differentiation factors-5, also known as CDMP-1, is predominantly found at the stage of precartilaginous mesenchymal condensation and throughout the cartilaginous cores of the developing long bones during embryogenesis [53]. Walsh et al. compared in vivo effects of a single injection and multiple injections of growth factors, such as bFGF (8 ng/disc), GDF-5, IGF-1, or TGF-beta1 in the mouse caudal disc with degeneration induced by static compression [38]. Although the effects of a single growth-factor injection were not apparent within 1 week, the appearance of inner annular fibrochondrocyte clusters was observed in the GDF-5 group. Of the four molecules tested, GDF-5 was the only molecule that increased the disc height as compared to saline controls. Furthermore, there seemed to be an increase in cellular proliferation in the middle and inner annulus as well as transitional zones found on histological sections. However, repeated injection induced an inflammatory response which was thought to be a secondary damage induced by the needles used and not necessarily the GDF-5 since even the saline group had similar inflammatory changes. A gene-therapy approach was used by Wang et al. to demonstrated that GDF-5 delivered by an adenovirus promoted the growth of disc cells cultured in vitro [58]. GDF-5 is being developed commercially for spinal-fusion application. With large quantities of recombinant GDF-5 now available, more in vivo research can be accomplished.
The non-covalent bond between aggrecan and hyaluronan is stabilized by the glycoprotein, Link protein. LinkN is an amino-terminal fragment of link protein that was shown by Mwale et al. to have stimulatory activity on disc cells [59]. Mwale et al. reported that Link N, at concentrations of 10 and 100 ng/ml, stimulated matrix assembly in pellet culture of nucleus pulposus and annulus fibrosus cells by increasing the production and/or accumulation of proteoglycans and collagen, but did not increase cell number in a statistically significant fashion. This suggests that a certain level of degradation products of link protein, which can be generated by MMPs, acts as a “growth factor” in a feedback mechanism. Collagen type II production was found to be increased by 113% in cells derived from the nucleus pulposus and 25% in cells derived from the annulus fibrosus. The mechanism by which Link N induces the specific up-regulation of an important chondrocyte marker (collagen II) without much effect on cell number is not yet clear. However, these findings allow categorization of Link N as a chondrogenic morphogen.
Intracellular regulators
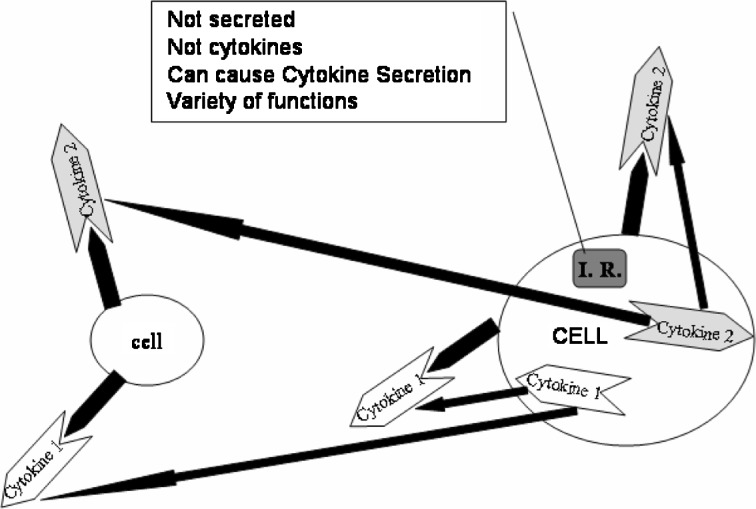
Intracellular regulators have effects similar to those of the molecules previously discussed but are not cytokines. These molecules are distinct because they are not secreted and do not work through transmembrane receptors (Fig. 4). Their major role is to control one or more aspects of cellular differentiation by intracellular activity. Examples of these include: SMADs, Sox 9, and latent membrane protein-1 (LMP-1).
Fig. 4.
Intracellular regulators: intracellular regulatory are not secreted molecules and do not act through a transmembrane receptor. Intracellular regulators can induce the secretion of cytokines to act in autocrine or paracrine fashion or directly up-regulate matrix production
LMP-1 up-regulates the production of BMP-2, BMP-7, and proteoglycans [60]. It is an intracellular molecule that was initially discovered by its positive effect on bone formation and osteoblast differentiation. Its properties have been shown by Yoon et al. to be effective in both short-term monolayer cultures and in longer (3 week) experiments using alginate cultures [60]. By showing that the up-regulation of proteoglycan can be blocked with a specific inhibitor of BMPs (Noggin, San Antonio, TX, USA), Yoon et al. demonstrated that the effect of LMP-1 involved a BMP-dependent mechanism. Subsequent in vivo work with rabbit discs showed that gene therapy with low doses of adenovirus-LMP-1 increased the disc-tissue mRNA levels of BMP-2, BMP-7, and aggrecan. Because LMP-1 stimulates both BMP-2 and BMP-7, this was hypothesized to potentiate the formation of the BMP-2:BMP-7 heterodimers. These heterodimers have been shown to be up to 20 times more effective than homodimers of BMP-2 and BMP-7 in osteogenesis [61].
Another molecule that mediates its effect via BMP-receptor signaling is SMADs [62, 63] Certain SMADs have been predicted to induce effects on disc cells in a similar fashion to BMP-2, such as increasing proteoglycan and collagen II synthesis. However, there are no published papers on the influence of SMADs on disc cells. Sox9 is a chondrocyte marker that is a positive regulator of collagen II mRNA transcription [64, 65]. Paul et al. demonstrated that Sox9 delivered by adenovirus can increase both Sox9 expression and disc-cell collagen II production in vitro experiments [66]. When injected in vivo, the adenovirus-Sox9 construct prevented the histological evidence of degenerative changes in the disc in a rabbit annulus puncture model. Of note, the disc-degeneration model used consisted of a 27-gauge needle puncture, which is a very small injury to the disc and therefore may lead to mild or no disc degeneration. Based on the histology, the authors found that Sox9-treated discs had a more chondrocyte-like phenotype as compared to the control virus-injected discs [66]. The final role of intracellular molecules is yet to be established but its importance is becoming more apparent with studies such as those reviewed above.
Summary
The normal intervertebral disc clinically acts to support and dissipate loads while permitting multi-axial motions of the spine. Its demanding mechanical function is provided by a well-defined microstructural organization and biochemical composition. Intervertebral disc degeneration is a complex process that disrupts this well-defined organization and biochemical balance. One hallmark of intervertebral disc degeneration is the loss of proteoglycan and water in the nucleus pulposus. Because of the central role of proteoglycans in the function of the intervertebral disc, restoration of normal proteoglycan production may be critical. Of the many different biological treatment strategies for intervertebral disc degeneration, the strategy of treating intervertebral disc cells with bioactive molecules has received the most attention. The molecules used to treat disc degeneration have expanded beyond the classical “growth factor”. There are at least four different classes of molecules that may be effective in disc repair. These include anti-catabolics, mitogens, morphogens, and intracellular regulators. While all of these molecules have some in vitro data, few have been tested in vivo with an animal model of disc degeneration. The next phase of experiments will use more realistic animal models of disc degeneration as a bridge to finally attempt human studies. The future holds development of better delivery methods using gene therapy and slow release formulation of therapeutic molecules. With the current foundation of pre-clinical data, the future holds promise for an acceleration in molecular therapy research of the intervertebral disc.
References
- 1.Deyo RA, Tsui-Wu YJ. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine. 1987;12:264–268. doi: 10.1097/00007632-198704000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Pritzker KP. Aging and degeneration in the lumbar intervertebral disc. Orthop Clin North Am. 1977;8:66–77. [PubMed] [Google Scholar]
- 3.Shvartzman L, Weingarten E, Sherry H, Levin S, Persaud A. Cost-effectiveness analysis of extended conservative therapy versus surgical intervention in the management of herniated lumbar intervertebral disc. Spine. 1992;17:176–182. doi: 10.1097/00007632-199202000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Buckwalter JA, Einhorn TA, Simon SR (eds) (2000) Orthopedic basic science: biology and biomechanics of the musculoskeletal system, 2nd edn. American Academy of Orthopedic Surgeons, Rosemont, pp548–555
- 5.Lipson SJ, Muir H. 1980 Volvo award in basic science: proteoglycans in experimental intervertebral disc degeneration. Spine. 1981;6:194–210. doi: 10.1097/00007632-198105000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res. 1987;5:198–205. doi: 10.1002/jor.1100050206. [DOI] [PubMed] [Google Scholar]
- 7.Henry SP, Takanosu M, Boyd TC, Mayne PM, Eberspaecher H, Zhou W, Crombrugghe B, Hook M, Mayne R. Expression pattern and gene characterization of asporin. a newly discovered member of the leucine-rich repeat protein family (Epub 2001 Jan 10) J Biol Chem. 2001;276(15):12212–12221. doi: 10.1074/jbc.M011290200. [DOI] [PubMed] [Google Scholar]
- 8.Lorenzo P, Aspberg A, Onnerfjord P, Bayliss MT, Neame PJ, Heinegard D. Identification and characterization of asporin. a novel member of the leucine-rich repeat protein family closely related to decorin and biglycan. J Biol Chem. 2001;276(15):12201–12211. doi: 10.1074/jbc.M010932200. [DOI] [PubMed] [Google Scholar]
- 9.Lorenzo P, Bayliss MT, Heinegard D. A novel cartilage protein (CILP) present in the mid-zone of human articular cartilage increases with age. J Biol Chem. 1998;273(36):23463–23468. doi: 10.1074/jbc.273.36.23463. [DOI] [PubMed] [Google Scholar]
- 10.Boos N, Weissbachs S, Rohrbach H, et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo award. Spine. 2002;27:2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Guiot BH, Fessler RG. Molecular biology of degenerative disc disease. Neurosurgery. 2000;47(5):1034–1040. doi: 10.1097/00006123-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Roughley Parameters that influence change in nucleus pulposus composition. Spine. 2004;29(23):2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Roughley PJ, Mort JS. Identification of human intervertebral disc stromelysin and its involvement in matrix degradation. J Orthop Res. 1991;9:568–575. doi: 10.1002/jor.1100090413. [DOI] [PubMed] [Google Scholar]
- 14.Seguin CA, Pilliar RM, Roughley PJ, Kandel RA. Tumor necrosis factor [alpha] modulates matrix production and catabolism in nucleus pulposus tissue. Spine. 2005;30(17):1940–1948. doi: 10.1097/01.brs.0000176188.40263.f9. [DOI] [PubMed] [Google Scholar]
- 15.Anderson DG, Li X, Balian G. A fibronectin fragment alters the metabolism by rabbit intervertebral disc cells in vitro. Spine. 2005;30(11):1242–1246. doi: 10.1097/01.brs.0000164097.47091.4c. [DOI] [PubMed] [Google Scholar]
- 16.Kang JD, Stefanovic-Racic M, McIntyre LA, Georgescu HI, Evans CH. Toward a biochemical understanding of human intervertebral disc degeneration and herniation: contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine. 1997;22:1065–1073. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 17.Takegami K, Thonar EJ, An HS, Kamada H, Masuda K. Osteogenic protein-1 enhances matrix replenishment by intervertebral disc cells previously exposed to interleukin-1. Spine. 2002;27(12):1318–1325. doi: 10.1097/00007632-200206150-00014. [DOI] [PubMed] [Google Scholar]
- 18.Sakai D, Mochida J, Yamamoto Y, et al. Transplantation of mesenchymal stem cells embedded in atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials. 2003;24(20):3531–3541. doi: 10.1016/S0142-9612(03)00222-9. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe K, Mochida J, Nomura T, Okuma M, Sakabe K, Seiki K. Effect of reinsertion of activated nucleus pulposus on disc degeneration: an experimental study on various types of collagen in degenerative discs. Connect Tissue Res. 2003;44(2):104–108. doi: 10.1080/713713657. [DOI] [PubMed] [Google Scholar]
- 20.Brisby H, Tao H, Ma DD, Diwan AD. Cell therapy for disc degeneration—potentials and pitfalls. Orthop Clin North Am. 2004;35(1):85–93. doi: 10.1016/S0030-5898(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 21.Gruber HE, Hanley EN., Jr Biologic strategies for the therapy of intervertebral disc degeneration. Expert Opin Biol Ther. 2003;3(8):1209–1214. doi: 10.1517/14712598.3.8.1209. [DOI] [PubMed] [Google Scholar]
- 22.Alini M, Roughley PJ, Antoniou J, Stoll T, Aebi M. A biological approach to treating disc degeneration: not for today, but maybe for tomorrow. Eur Spine J. 2002;11(Suppl 2):S215–S220. doi: 10.1007/s00586-002-0485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato M, Asazuma T, Ishihara M, et al. An experimental study of the regeneration of the intervertebral disc with an allograft of cultured annulus fibrosus cells using a tissue-engineering method. Spine. 2003;28(6):548–553. doi: 10.1097/00007632-200303150-00007. [DOI] [PubMed] [Google Scholar]
- 24.Luk KD, Ruan DK, Chow DH, Leong JC. Intervertebral disc autografting in a bipedal animal model. Clin Orthop Relat Res. 1997;337:13–26. doi: 10.1097/00003086-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Luk KD, Ruan DK, Lu DS, Fei ZQ. Fresh frozen intervertebral disc allografting in a bipedal animal model. Spine. 2003;28(9):864–869. doi: 10.1097/00007632-200305010-00005. [DOI] [PubMed] [Google Scholar]
- 26.Pfeiffer M, Boudriot U, Pfeiffer D, Ishaque N, Goetz W, Wilke A. Intradiscal application of hyaluronic acid in the non-human primate lumbar spine: radiological results. Eur Spine J. 2003;12(1):76–83. doi: 10.1007/s00586-002-0478-7. [DOI] [PubMed] [Google Scholar]
- 27.Roberts S, Caterson B, Menage J et al (2000) Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine 25:3005–3013:491–495 [DOI] [PubMed]
- 28.Takahashi H, Suguro T, Okazima Y, et al. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996;21:218–224. doi: 10.1097/00007632-199601150-00011. [DOI] [PubMed] [Google Scholar]
- 29.Fujita K, Nakagawa T, Hirabayashi K, et al. Neutral proteinases in human intervertebral disc: role in degeneration and probable origin. Spine. 1993;18:1766–1773. doi: 10.1097/00007632-199310000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274(31):21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 31.Wallach CJ, Sobajima S, Watanabe Y, et al. Gene transfer of the catabolic inhibitor TIMP-1 increases measured proteoglycans in cells from degenerated human intervertebral discs. Spine. 2003;28(20):2331–2337. doi: 10.1097/01.BRS.0000085303.67942.94. [DOI] [PubMed] [Google Scholar]
- 32.Okuma M, An HS, Nakagawa K, Akeda K, Muehleman C, Masuda K (2005) Oral administration of esculetin prodrug inhibits intervertebral disc degeneration in the rabbit annular needle puncture model. Orthopaedic research society meeting, p 370
- 33.Muller-Ladner U, Roberts CR, Franklin BN, et al. Human IL-1Ra gene transfer into human synovial fibroblasts is chondroprotective. J Immunol. 1997;158(7):3492–3498. [PubMed] [Google Scholar]
- 34.Bandara G, Mueller GM, Galea-Lauri J, et al. Intraarticular expression of biologically active interleukin 1-receptor-antagonist protein by ex vivo gene transfer. Proc Natl Acad Sci USA. 1993;90(22):10764–10768. doi: 10.1073/pnas.90.22.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olmarker K, Rydevik B. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity: possible implications for future pharmacologic treatment strategies of sciatica. Spine. 2001;26(8):863–869. doi: 10.1097/00007632-200104150-00007. [DOI] [PubMed] [Google Scholar]
- 36.Masuda K, Oegema TR, Jr, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine. 2004;29(23):2757–2769. doi: 10.1097/01.brs.0000146048.14946.af. [DOI] [PubMed] [Google Scholar]
- 37.Thompson JP, Oegema TR, Jr, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 1991;16(3):253–260. doi: 10.1097/00007632-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine. 2004;29(2):156–163. doi: 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- 39.Okuda S, Myoui A, Ariga K, Nakase T, Yonenobu K, Yoshikawa H. Mechanisms of age-related decline in insulin-like growth factor-I dependent proteoglycan synthesis in rat intervertebral disc cells. Spine. 2001;26(22):2421–2426. doi: 10.1097/00007632-200111150-00005. [DOI] [PubMed] [Google Scholar]
- 40.Gruber HE, Norton HJ, Hanley EN., Jr Anti-apoptotic effects of IGF-1 and PDGF on human intervertebral disc cells in vitro. Spine. 2000;25(17):2153–2157. doi: 10.1097/00007632-200009010-00002. [DOI] [PubMed] [Google Scholar]
- 41.Matsunaga S, Nagano S, Onishi T, Morimoto N, Suzuki S, Komiya S. Age-related changes in expression of transforming growth factor-beta and receptors in cells of intervertebral discs. J Neurosurg Spine. 2003;98(1):63–67. doi: 10.3171/spi.2003.98.1.0063. [DOI] [PubMed] [Google Scholar]
- 42.Takae R, Matsunaga S, Origuchi N, et al. Immunolocalization of bone morphogenetic protein and its receptors in degeneration of intervertebral disc. Spine. 1999;24(14):1397–1401. doi: 10.1097/00007632-199907150-00002. [DOI] [PubMed] [Google Scholar]
- 43.Nishida K, Kang JD, Gilbertson LG, et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine. 1999;24(23):2419–2425. doi: 10.1097/00007632-199912010-00002. [DOI] [PubMed] [Google Scholar]
- 44.Yung LJ, Hall R, Pelinkovic D, et al. New use of a three-dimensional pellet culture system for human intervertebral disc cells: initial characterization and potential use for tissue engineering. Spine. 2001;26(21):2316–2322. doi: 10.1097/00007632-200111010-00005. [DOI] [PubMed] [Google Scholar]
- 45.Tan Y, Hu Y, Tan J. Extracellular matrix synthesis and ultrastructural changes of degenerative disc cells transfected by Ad/CMV-hTGF-beta 1. Chin Med J (Engl) 2003;116(9):1399–1403. [PubMed] [Google Scholar]
- 46.Yoon ST, Kim K, Li J, et al. The effect of bone morphogenetic protein-2 on rat interveretebral disc cells in vitro. Spine. 2003;28(16):1773–1780. doi: 10.1097/01.BRS.0000083204.44190.34. [DOI] [PubMed] [Google Scholar]
- 47.Kim DJ, Moon SH, Kim H, et al. Bone morphogenetic protein-2 facilitates expression of chondrogenic, not osteogenic, phenotype of human intervertebral disc cells. Spine. 2003;28(24):2679–2684. doi: 10.1097/01.BRS.0000101445.46487.16. [DOI] [PubMed] [Google Scholar]
- 48.Ahn S-H, Teng P-N, Niyibizi C et al (2002) The effects of BMP-12 and BMP-2 on proteoglycan and collagen synthesis in nucleus pulposus cells from human degenerated discs. ISSLS 29th annual meeting proceeding, p 49
- 49.Masuda K, Takegami K, An H, et al. Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J Orthop Res. 2003;21(5):922–930. doi: 10.1016/S0736-0266(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, An HS, Song S, et al. Growth factor osteogenic protein-1: differing effects on cells from three distinct zones in the bovine intervertebral disc. Am J Phys Med Rehabil. 2004;83(7):515–521. doi: 10.1097/01.PHM.0000130031.64343.59. [DOI] [PubMed] [Google Scholar]
- 51.An HS, Takegami K, Kamada H, et al. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine. 2005;30(1):25–31. doi: 10.1097/01.brs.0000148002.68656.4d. [DOI] [PubMed] [Google Scholar]
- 52.Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an annulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30(1):5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 53.Chang SC, Hoang B, Thomas JT, et al. Cartilage-derived morphogenetic proteins. New members of the transforming growth factor-beta superfamily predominantly expressed in long bones during human embryonic development. J Biol Chem. 1994;269(45):28227–28234. [PubMed] [Google Scholar]
- 54.Li J, Kim KS, Park JS, Elmer WA, Hutton WC, Yoon ST. BMP-2 and CDMP-2: stimulation of chondrocyte production of proteoglycan. J Orthop Sci. 2003;8(6):829–835. doi: 10.1007/s00776-003-0719-6. [DOI] [PubMed] [Google Scholar]
- 55.Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He TC (2003) Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am 85-A(8):1544–1552. Erratum in: J Bone Joint Surg Am (2004) 86-A(1):141 [DOI] [PubMed]
- 56.Mehta V, Kang Q, Luo J, He TC, Haydon RC, Mass DP. Characterization of adenovirus-mediated gene transfer in rabbit flexor tendons. J Hand Surg [Am] 2005;30(1):136–141. doi: 10.1016/j.jhsa.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 57.Helm GA, Li JZ, Alden TD, Hudson SB, Beres EJ, Cunningham M, Mikkelsen MM, Pittman DD, Kerns KM, Kallmes DF. A light and electron microscopic study of ectopic tendon and ligament formation induced by bone morphogenetic protein-13 adenoviral gene therapy. J Neurosurg. 2001;95(2):298–307. doi: 10.3171/jns.2001.95.2.0298. [DOI] [PubMed] [Google Scholar]
- 58.Wang H, Kroeber M, Hanke M, et al. Release of active and depot GDF-5 after adenovirus-mediated overexpression stimulates rabbit and human intervertebral disc cells. J Mol Med. 2004;82(2):126–134. doi: 10.1007/s00109-003-0507-y. [DOI] [PubMed] [Google Scholar]
- 59.Mwale F, Demers CN, Petit A, et al. A synthetic peptide of link protein stimulates the biosynthesis of collagens II, IX and proteoglycan by cells of the intervertebral disc. J Cell Biochem. 2003;88(6):1202–1213. doi: 10.1002/jcb.10479. [DOI] [PubMed] [Google Scholar]
- 60.Yoon ST, Park JS, Kim KS, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine. 2004;29(23):2603–2611. doi: 10.1097/01.brs.0000146103.94600.85. [DOI] [PubMed] [Google Scholar]
- 61.Israel DI, Nove J, Kerns KM, et al. Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors. 1996;13(3–4):291–300. doi: 10.3109/08977199609003229. [DOI] [PubMed] [Google Scholar]
- 62.Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16(3):291–299. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 63.Hatakeyama Y, Nguyen J, Wang X, Nuckolls GH, Shum L. Smad signaling in mesenchymal and chondroprogenitor cells. J Bone Joint Surg Am. 2003;85-A(Suppl 3):13–18. doi: 10.2106/00004623-200300003-00004. [DOI] [PubMed] [Google Scholar]
- 64.Li Y, Tew SR, Russell AM, Gonzalez KR, Hardingham TE, Hawkins RE. Transduction of passaged human articular chondrocytes with adenoviral, retroviral, and lentiviral vectors and the effects of enhanced expression of SOX9. Tissue Eng. 2004;10(3–4):575–584. doi: 10.1089/107632704323061933. [DOI] [PubMed] [Google Scholar]
- 65.Aigner T, Gebhard PM, Schmid E, Bau B, Harley V, Poschl E. SOX9 expression does not correlate with type II collagen expression in adult articular chondrocytes. Matrix Biol. 2003;22(4):363–372. doi: 10.1016/S0945-053X(03)00049-0. [DOI] [PubMed] [Google Scholar]
- 66.Paul R, Haydon RC, Cheng H, et al. Potential use of sox9 gene therapy for intervertebral degenerative disc disease. Spine. 2003;28(8):755–763. doi: 10.1097/00007632-200304150-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]