Abstract
The anulus fibrosus (AF) of the intervertebral disc consists of concentric sheets of collagenous matrix that is synthesised during embryogenesis by aligned disc cells. This highly organised structure may be severely disrupted during disc degeneration and/or herniation. Cell scaffolds that incorporate topographical cues as contact guidance have been used successfully to promote the healing of injured tendons. Therefore, we have investigated the effects of topography on disc cell growth. We show that disc cells from the AF and nucleus pulposus (NP) behaved differently in monolayer culture on micro-grooved membranes of polycaprolactone (PCL). Both cell types aligned to and migrated along the membrane’s micro-grooves and ridges, but AF cells were smaller (or less spread), more bipolar and better aligned to the micro-grooves than NP cells. In addition, AF cells were markedly more immunopositive for type I collagen, but less immunopositive for chondroitin-6-sulphated proteoglycans than NP cells. There was no evidence of extracellular matrix (ECM) deposition. Disc cells cultured on non-grooved PCL did not show any preferential alignment at sub-confluence and did not differ in their pattern of immunopositivity to those on grooved PCL. We conclude that substratum topography is effective in aligning disc cell growth and may be useful in tissue engineering for the AF. However, there is a need to optimise cell sources and/or environmental conditions (e.g. mechanical influences) to promote the synthesis of an aligned ECM.
Keywords: Intervertebral disc, Anulus fibrosus, Tissue engineering, Topography, Cell culture
Introduction
The mature human intervertebral disc (IVD) is a complex connective tissue comprised of a fibrous outer layer termed the anulus fibrosus (AF) and a gelatinous core termed the nucleus pulposus (NP). Each of these regions consist of an extracellular matrix (ECM) in which disc cells are sparsely embedded. During embryogenesis, the AF is formed when mesenchymal cells at the periphery of the developing disc become orientated in concentric sheets and subsequently synthesise similarly aligned sheets of collagenous ECM (predominantly type I collagen) (Fig. 1a) [10, 18]. The NP forms from segmented notochordal tissue that is gradually replaced postnatally by a highly hydrated ECM rich in type II collagen and proteoglycans [2]. The fibrous nature of the AF permits bending and flexion of the spine, but also plays an important role in containing the less rigid NP, which functions mainly to absorb and transmit compressive loads [22].
Fig. 1.
Using topography to mimic the structure of the anulus fibrosus. a A schematic diagram of the highly organised anulus fibrosus with several concentric lamellae (or sheets) of collagen fibres running at angles of approximately 60°–80° to the vertical axis. b The topography of the micro-grooved PCL membranes onto which disc cells were seeded
Damage to the AF can occur during IVD degeneration (as radial fissures) and/or disc herniation, when inner areas of the disc may rupture the outer anular lamellae [1, 17]. Although there has been considerable interest in restoring or replacing the NP of degenerated IVDs [3, 8], for at least some patients there will be a likely need to encapsulate any such repair tissue within an outer structure, such as the healthy AF. Herniated disc tissues, including the AF, often are surgically removed as they may impinge on adjacent neural processes to cause disability and pain [14]. However, no remedial disc reconstruction is performed during these surgical procedures and recurrent disc prolapse is seen in 10–20% of all patients treated, most commonly at the original site of injury, i.e. where the anular structure has been disrupted [7, 16]. Thus, there are important clinical needs for treatments that repair, regenerate or replace the AF of the human IVD. An important property of any repair or replacement tissue is that it should mimic the orientated morphology of the native anulus tissue, as this would lead to better integration and increased mechanical strength. Such tissue orientation may be achieved by adopting techniques that promote the alignment of cells that synthesise the repair/replacement tissue.
Grooved substrata have been used to promote the alignment of cell-synthesised repair tissue in damaged tendons, which, like the AF, consist of a highly aligned collagenous ECM. In these previous studies [23], rat flexor tendons were transected and cultured as explants over grooved substratum. Subsequently, epitenon fibroblasts migrated from the cut ends of the tendons, aligned along the grooved substratum between the two cut ends, and deposited similarly aligned bundles of collagen fibrils that formed a fusion of the cut ends. More recently, this type of contact guidance has been shown to promote in vivo tendon repair [5]. To determine if such topographical cues may be used to direct the growth of IVD cells, with a view to developing tissue repair or replacement strategies for the AF, we have investigated the behaviour of disc cells cultured on micro-grooved membranes of the bioresorbable material, polycaprolactone (PCL). Polycaprolactone is an USA Federal Drug Agency-approved material that has been utilised previously in tissue engineering studies, where it has been shown to be biocompatible, biodegradable and supportive of cell attachment and proliferation [20]. In addition, PCL constructs have been applied in vivo in animal studies to augment tissue repair processes for damaged tendons and ligaments, both of which constitute a highly organised and orientated ECM [12, 19].
Materials and methods
Cell culture
Disc cells were obtained from the separated AF and NP of mature bovine coccygeal IVDs (n = 5 cows) by digesting the tissue enzymatically in collagenase/DNase (Sigma, Poole, UK). Isolated cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% foetal bovine serum and antibiotics (Gibco, Paisley, UK) in monolayer for two passages, prior to seeding onto micro-grooved membranes of PCL at a density of 5 × 103 cells/cm2. The micro-grooves of the membranes were 4 μ in depth with a 12.5 μ pitch (Fig. 1b). AF and NP cells were cultured also on non-grooved PCL membranes as a control for the effects of substratum topography. Disc cell-PCL cultures were fed every 3–4 days with fresh medium. Cell viability was determined by staining cultures with fluorescein diacetate (Sigma) and propidium iodide (Sigma) to give a live/dead score, as described previously [9]. At days 7, 14 and 28 post-cell seeding, cultures were harvested by fixation with methanol, glutaraldehyde or formalin prior to histochemical staining, scanning electron microscopy or immunolabelling, respectively.
Immunohistochemistry/histochemistry
Formalin-fixed samples of cell-seeded PCL were immunolabelled for collagen types I, II and III, and for different glycosaminoglycan side-chains of proteoglycans, using antibodies and methods described previously [11]. In brief, the samples were incubated with primary antisera, followed by visualisation of immunoreactivity with a commercial kit (Vectastain Elite ABC, Vector Labs, Peterborough, UK) and 3,3′-diaminobenzidine as chromogen (DAB, Sigma). The extent to which cells were immunolabelled was semi-quantitatively assessed. Immunolabelling of parallel samples with an irrelevant primary control antibody (Dako Ltd., Cambridge, UK) was negative. The filamentous-actin (F-actin) cytoskeleton was visualised using fluorescein-labelled phalloidin (Cambridge Bioscience, Cambridge, UK). Cell morphology was revealed with Jenner/Giemsa staining. Immunolabelling, F-actin labelling and histological staining was viewed using conventional and fluorescent microscopy.
Scanning electron microscopy
Disc cells cultured on PCL were fixed in glutaraldehyde, which was gradually replaced by 0.1 M sodium cacodylate. The harvested cultures were immersed in osmium tetroxide, serially dehydrated in ethanol and subjected to critical point drying in liquid CO2 prior to mounting and gold-coating (1 min, 25 mA). Imaging of the disc cell-PCL membranes was performed using a Hitachi S-4500 field emission scanning electron microscope (Hitachi-High Technologies, Finchampstead, UK).
Cell morphometry
A minimum of 10 images of Jenner/Giemsa stained disc cells on PCL were captured in areas of sub-confluence using a 20 × objective and a digital camera (Nikon CoolPix 950, Nikon UK, Kingston, UK). Image analysis was performed using Scion Image Version Beta 4.0.2 (Scion Corporation, Frederick, MD, USA) to determine; (a) cell area, where the edge of individual cells was clearly determined; (b) the cell length (taking a transect through the centre of the nucleus); (c) the cell width (taking a transect through the centre of the nucleus); (d) the angle formed between the longitudinal axis of the nucleus and that of the PCL micro-grooves (nucleus/PCL angle). The ratio of the cell length to cell width (L/W ratio) was taken as an indication of the extent to which cells were polarised, i.e. how rounded or elongated they were. The acute angle formed between the longitudinal axes of the nucleus with the direction of the micro-grooves was taken to indicate cell alignment to the substrate [6]. Cell populations that are unaffected by the substrate will tend towards a mean nucleus/PCL angle of 45°, whilst populations that do align either with or against the substrate will tend towards angles of 0° or 90°, respectively. All measurements were performed for at least 50 cells from each of the AF and NP cell cultures. These measurements were averaged and the differences between AF and NP or grooved and non-grooved groups were determined using the Mann–Whitney U test, where P values of < 0.05 were considered significant.
Gene expression
Gene expression of type α1 (I) collagen, type α1 (II) collagen and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), using primer sequences and methods previously described [13], was determined for AF and NP cells cultured on PCL membranes at 28 days by a reverse transcriptase polymerase chain reaction (RT-PCR). Total RNA was isolated using Qiagen RNeasy (Qiagen Ltd., Crawley, UK) and cDNA synthesised from the RNA with oligo (dT) primers and Qiagen reverse transcriptase (Qiagen Ltd.). RT-PCR products were separated by electrophoresis in 2% agarose gels (Biomarker Low, Cambio Ltd., Cambridge, UK), along with control products for each gene, and stained with ethidium bromide. Message for GAPDH was used to ascertain that an equivalent amount of cDNA was synthesised from each sample.
Results
Disc cell growth and morphology
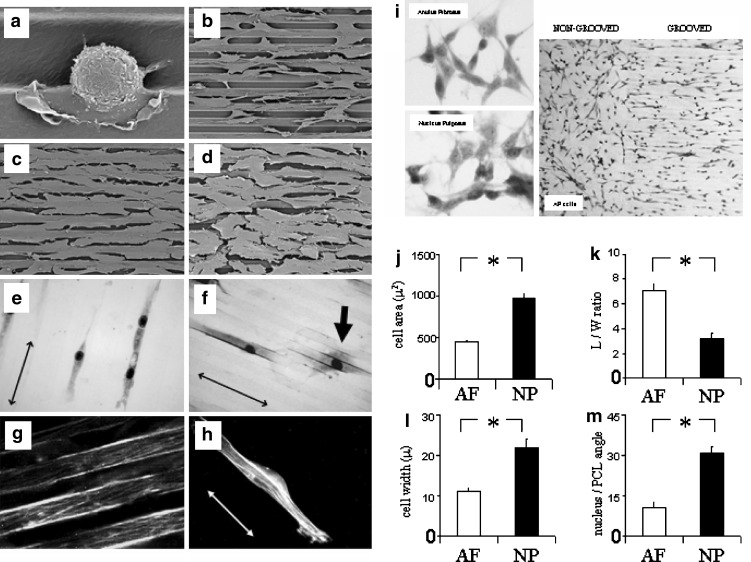
AF and NP cells adhered to the micro-grooved PCL membranes, extended pseudopodia and lamellipodia onto the membranes (as revealed by SEM) and gradually flattened and elongated in the direction of the micro-grooves (Fig. 2a–d). There were no differences in the proportions of AF or NP cells present in the micro-grooves or on the ridges of the PCL membranes by day 7 (and thereafter), although both cell types appeared to preferentially settle and adhere in the membrane’s micro-grooves. Following Jenner/Giemsa staining (Fig. 2e, f) and cell morphometric analysis (Fig. 2j–m), it was apparent that AF cells were significantly smaller (or less spread), more bipolar (i.e. had a greater L/W ratio), and better aligned (i.e. had a lower nucleus/PCL angle) to the direction of the micro-grooves or ridges of the PCL membranes than were NP cells, at least at sub-confluence (such analysis was not possible in areas of confluence). The greater L/W ratio of AF cells in comparison to NP cells was due to AF cells being less wide rather than longer (Fig. 2l). However, for both cell types the L/W ratio was markedly greater than 1, indicating some cell polarity. F-actin stress fibres were present in AF and NP cells, and, in general, these fibres also aligned to the micro-grooves and ridges, i.e. following the morphology of the cells (Fig. 2g, h). At sub-confluence, AF and NP cells cultured on non-grooved PCL membranes (see Fig. 2i) had nucleus/PCL angles (where a horizontal line through each captured image was randomly assigned as the PCL “direction”) of 44.2 ± 1.3 and 44 ± 1.2, respectively. These values were markedly and significantly greater (P < 0.05) than those of AF and NP cells on micro-grooved PCL (Fig. 2m). In non-grooved membrane cultures at full confluence, the AF and NP cells tended to align with each other in swirls.
Fig. 2.
Disc cell morphology on micro-grooved PCL membranes. Representative SEM images are shown of: a an AF cell (1 h post-seeding) which has settled into the micro-groove of the PCL membrane and extended filopodial and lamellipodial attachments onto the substratum; b AF cells that have elongated and aligned to the PCL membrane at day 14; c AF cells; and d NP cells in confluent monolayers that have aligned to the micro-grooves of the PCL membrane at day 28. Images of Jenner/Giemsa stained AF cells (e) and NP (f) cells at sub-confluence are shown. An example of the typical appearance of a more spread and less aligned NP cell is arrowed in (f). g and h shows F-actin stress fibres in AF and NP cells, respectively, which aligned with the PCL substrata. i Examples are shown of the random alignment of AF and NP cells cultured on non-grooved PCL membranes. An example of the alignment of AF cells cultured on a PCL membrane which is both non-grooved and grooved is also shown (right panel). Computer-assisted image analysis demonstrated significant differences between AF and NP cell morphology in terms of: j Cell area; kL/W ratio (an indication of polarity); l Cell width; m The angle formed between the longitudinal axes of the nucleus with the direction of the micro-grooves (an indication of alignment). (↔ shows direction of substrata micro-grooves). Data shown are means ± standard error of the mean. *P < 0.01
By days 14 and 28, AF and NP cells cultured on micro-grooved PCL had reached confluency in many areas to form directed cellular sheets (see Fig. 2c, d), suggesting that both cell types were proliferating and/or migrating on the membranes. This was confirmed using time-lapse video microscopy, with a series of 4–10 h videos collected over the first 14 days of culture (Fig. 3a–f). Of those cells that were migrating (within the time-frame of each video), 85 ± 4% of AF cells and 88 ± 5% of NP cells moved in the same direction as the microgrooves/ridges. Cell proliferation was also apparent as occasional mitotic figures were observed in Jenner/Giemsa stained cultures (Fig. 3g, h). There was no obvious loss of cells from the substratum into the culture medium and at days 7, 14 and 28 more than 95% of adherent AF and NP cells were viable.
Fig. 3.
AF and NP cells proliferate and migrate on micro-grooved PCL. AF and NP cells migrated preferentially along the substratum micro-grooves, as demonstrated by time-lapse video microscopy. a–d Images are shown of AF cells on the same section of PCL membrane at 15 min intervals. The two dotted lines are identically positioned in all of images. The asterisk and hash demark two cells, both of which migrate (in opposite directions) along adjacent micro-grooves passed the dotted lines. Cell division (arrowed) was also observed using video microscopy (e, f) and mitotic figures (arrowed) were seen in harvested Jenner/Giemsa stained cultures (g, h)
Expression of disc matrix molecules
AF and NP cells were differentially immunoreactive for disc matrix molecules, particularly for type I collagen and chondroitin-6-sulphated proteoglycans (Fig. 4a). AF cells were strongly immunoreactive for type I collagen, keratan-sulphated proteoglycan (KS PG) and chondroitin-4-sulphated proteoglycan (C-4-S PG), but weakly immunoreactive for chondroitin-6-sulphated proteoglycan (C-6-S PG). Conversely, NP cells were weakly immunoreactive for type I collagen, appeared similarly immunoreactive to AF cells for KS PG and C-4-S PG, but were more strongly immunoreactive for C-6-S PG. NP cells were weakly immunopositive or negative for type II collagen, whilst AF cells were negative, and both were weakly immunoreactive or negative for type III collagen. The expression of type I collagen by AF and NP cells at day 28 was confirmed by RT-PCR (Fig. 4b); whilst type II collagen expression was not detected in either cell type. The overall pattern of immunoreactivity (Fig. 4c) was consistent in that it was seen in the majority of AF and NP cells at days 7, 14 and 28. There were no marked differences in immunoreactivity seen between disc cells cultured on micro-grooved and non-grooved PCL (see e.g. C-4-S PG immunolocalisation in Fig. 4a). There was no evidence of ECM deposition onto the PCL membranes by either cell type at any time point.
Fig. 4.
Extracellular matrix molecule expression in AF and NP cells on micro-grooved PCL. a Representative images are shown of immunoreactivity for type I collagen and keratan sulphated- (KS PG), chondroitin-4-sulphated- (C-4-S PG), and chondroitin-6-sulphated- (C-6-S PG) proteoglycan molecules in AF cells (left panels) and NP cells (right panels). There was no apparent difference seen in this immunoreactivity between cells cultured on micro-grooved or non-grooved control PCL (e.g. bottom 2 panels). The intracellular immunolocalisation of type I collagen suggests that the pro-collagen form was detected. The C-6-S PG immunoreactivity shown is for monoclonal antibody, 3B3, after chondroitinase ABC pre-treatment. b Total RNA extracted from AF and NP cells cultured on PCL for 28 days was used in RT-PCR reactions for type I collagen. As shown, type I collagen expression was detected in AF and NP samples obtained from three independent bovine sources (i.e. from the AF and NP cells of three cows: C1, C2, C3). RNA extracted from freshly isolated bovine dermal fibroblasts was used as positive control, whilst GAPDH acted as a control for loading. c The immunoreactivity of each matrix component was determined semi-quantitatively, as follows: +++ = cells were strongly immunoreactive; ++ = many cells were strongly immunoreactive, but some were weakly immunoreactive; + = most cells were weakly immunoreactive; +/− = no cells were strongly immunoreactive, many cells were weakly immunoreactive or not immunoreactive; − = no cells were immunoreactive. (The monoclonal antibodies used for each matrix molecule are indicated)
Discussion
We have investigated the use of topography to direct the growth of IVD cells. Passage II cells, originally isolated from the AF or NP of bovine discs, were cultured for extended periods on micro-grooved membranes of bioresorbable PCL. The disc cells adhered to the membranes, became fibroblast-like in morphology, proliferated and migrated to reach confluence. Both cell types aligned and migrated with the membrane topography, but AF cells were smaller (or less spread), more bipolar and better aligned to the micro-grooves than NP cells. There were also differences in the matrix components that the two cell types expressed, with AF cells more strongly immunoreactive for type I collagen, which is normally found in the outer anulus, whilst NP cells were more strongly immunoreactive for chondroitin-6-sulphated proteoglycan.
The different patterns of AF and NP cell growth and matrix molecule immunoreactivity potentially reflect a restricted phenotypic capacity of these cells. This has important implications for the use of disc cell populations in tissue engineering strategies to repair or regenerate the damaged AF and may suggest that AF cells may be better suited for AF repair than NP cells. However, the phenotype of many cartilaginous cells depends on how they are cultured. For example, when grown for extended periods in monolayer culture, articular chondrocytes cease synthesising type II collagen (which they do in vivo) and instead synthesise type I collagen [15, 21]. This change in cell behaviour is associated with the development of a fibroblast-like morphology and formation of a prominent F-actin cytoskeleton (with stress fibres), but it can be reversed by culturing the cells in 3D gels [4]. F-actin stress fibres were seen in both AF and NP cells cultured on the micro-grooved PCL. Furthermore, although the NP cells were less immunopositive for type I collagen than AF cells, they appeared to express similar levels of type I collagen mRNA (using semi-quantitative RT-PCR), which freshly isolated NP cells do not. Clearly, the phenotype of the NP cells was altered during monolayer and it may be that longer periods in monolayer culture (beyond passage II) might increase their capacity to respond to topographical cues or synthesise those matrix molecules (e.g. type I collagen) that are present in the AF.
It has been suggested that the presence of F-actin stress fibres in concentrically aligned cells in the developing AF may have a role to play in the appropriate orientation and deposition of collagen during the formation of the anular lamellae [10]. Despite the presence of aligned stress fibres in both AF and NP cells cultured on the micro-grooved PCL, there was no evidence of matrix deposition at any time. This represents a major limitation of this study in terms of generating an aligned ECM. It may be that 4 weeks in culture was not long enough for substantial matrix synthesis or that cell-synthesised matrix components were lost into the culture medium and not deposited onto the PCL substratum. It is well known that culturing chondrocytic cells in 3D constructs, such as gel-like materials, may stimulate cell differentiation and the synthesis and formation of ECM [4]. Therefore, the development of materials or constructs that align disc cells, but also maintains them in 3D, may prove more efficacious in the generation of an aligned ECM. Alternatively, coating the PCL membranes with other biocompatible materials or stimulatory molecules, or application of an appropriate mechanical stimulation, e.g. tensile and/or shear stress, may also increase the synthesis and deposition of appropriate ECM molecules.
Conclusions
The IVD represents a considerable challenge for workers in connective tissue repair/engineering, not least because of its heterogeneity and complex organisation. Cell scaffolds incorporating topographical guidance have been successful in augmenting healing processes in tendons, which, like the AF, consists of an aligned collagenous matrix. Here, we show that bovine disc cells cultured in monolayer became aligned to a grooved membrane of PCL. However, we show also that cells from the AF and NP differentially responded to these topographical cues and expressed different matrix molecules in vitro. Therefore, in terms of the development of strategies to repair or replace the injured AF, topography may prove useful in guiding disc cell growth, but additional influences, and possibly cell selection, may be required to stimulate the deposition of an orientated tissue of appropriate composition.
Acknowledgment
This study was part-funded by Eurodisc: QLK6-CT-2002-02582. We are grateful to Professor Bruce Caterson for the provision of antibodies and to Helena Evans and Janis Menage for their technical expertise.
References
- 1.Adams MA, Hutton WC. Gradual disc prolapse. Spine. 1985;10:524–531. doi: 10.1097/00007632-198507000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Adams P, Eyre DR, Muir H. Biochemical aspects of development and ageing of human intervertebral discs. Rheumatol Rehabil. 1977;16:22–29. doi: 10.1093/rheumatology/16.1.22. [DOI] [PubMed] [Google Scholar]
- 3.Alini M, Li W, Markovic P, Aebi M, Spiro RC, Roughley PJ. The potential and limitations of a cell-seeded collagen/hyaluronan scaffold to engineer an intervertebral disc-like matrix. Spine. 2003;28:446–454. doi: 10.1097/00007632-200303010-00007. [DOI] [PubMed] [Google Scholar]
- 4.Benya PD, Schaffer JD. Dedifferentiated chondrocytes re-express the differentiated collagen phenotypes when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 5.Curtis ASG, Wilkinson CDW, Crossan J, Broadley C, Darmani H, Johal KK, Jorgensen H, Monaghan W. An in vivo microfabricated scaffold for tendon repair. Eur Cell Mater. 2005;9:50–57. doi: 10.22203/ecm.v009a07. [DOI] [PubMed] [Google Scholar]
- 6.Dalby MJ, Riehle MO, Yarwood SJ, Wilkinson DC, Curtis AS. Nucleus alignment and cell signalling in fibroblasts: response to a micro-grooved topography. Exp Cell Res. 2003;284:274. doi: 10.1016/S0014-4827(02)00053-8. [DOI] [PubMed] [Google Scholar]
- 7.Fritsch EW, Heisel J, Rupp S. The failed back surgery syndrome. Reasons, intraoperative findings, and long-term results: a report of 182 operative treatments. Spine. 1996;21:626–633. doi: 10.1097/00007632-199603010-00017. [DOI] [PubMed] [Google Scholar]
- 8.Gan JC, Ducheyne P, Vresilovic EJ, Shapiro IM. Intervertebral disc tissue engineering II: cultures of nucleus pulposus cells. Clin Orthop. 2003;411:315–324. doi: 10.1097/01.blo.0000063797.98363.d3. [DOI] [PubMed] [Google Scholar]
- 9.Gargiulo BJ, Cragg P, Richardson JB, Ashton BA, Johnson WE. Phenotypic modulation of human articular chondrocytes by bistratene A. Eur Cell Mater. 2002;3:9–18. doi: 10.22203/ecm.v003a02. [DOI] [PubMed] [Google Scholar]
- 10.Hayes AJ, Benjamin M, Ralphs JR. Role of actin stress fibres in the development of the intervertebral disc: cytoskeletal control of extracellular matrix assembly. Dev Dyn. 1999;215:179–189. doi: 10.1002/(SICI)1097-0177(199907)215:3<179::AID-AJA1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 11.Horner HA, Roberts S, Bielby RC, Menage J, Evans H, Urban JP. Cells from different regions of the intervertebral disc: effects of culture system on matrix expression and cell phenotype. Spine. 2002;27:1018–1028. doi: 10.1097/00007632-200205150-00004. [DOI] [PubMed] [Google Scholar]
- 12.Kazimoglu C, Bolukbasi S, Kanatli U, Senkoylu A, Altun NS, Babc C, Yavuz H, Piskin E. A novel biodegradable PCL film for tendon reconstruction: Achilles tendon defect in rats. Int J Artif Organs. 2003;26:804–812. [PubMed] [Google Scholar]
- 13.Kim G, Okumura M, Bosnakovski D, Ishiguro T, Park CH, Kadosawa T, Fujinaga T. Effects of ascorbic acid on the proliferation and biological properties of bovine chondrocytes in alginate beads. Jap J Vet Res. 2003;51:83–94. [PubMed] [Google Scholar]
- 14.Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the local spine under local anesthesia. Orth Clin North Am. 1991;22:181–187. [PubMed] [Google Scholar]
- 15.Mallein-Gerin F, Garrone R, Rest M. Proteoglycan and collagen synthesis are correlated with actin organization in dedifferentiating chondrocytes. Eur J Cell Biol. 1991;56:364–373. [PubMed] [Google Scholar]
- 16.Martin G. Recurrent disc prolapse as a cause of recurrent pain after laminectomy for lumbar disc lesion. N Z Med J. 1980;26:206. [PubMed] [Google Scholar]
- 17.Moore RJ, Vernon-Roberts B, Fraser RD, Osti OL, Schembri M. The origin and fate of herniated lumbar intervertebral disc tissue. Spine. 1996;21:2149–2155. doi: 10.1097/00007632-199609150-00018. [DOI] [PubMed] [Google Scholar]
- 18.Rufai A, Benjamin M, Ralphs JR. The development of fibrocartilage in the rat intervertebral disc. Anat Embryol. 1995;192:53–62. doi: 10.1007/BF00186991. [DOI] [PubMed] [Google Scholar]
- 19.Sato M, Maeda M, Kurosawa H, inoue Y, Yamauchi Y, Iwase H. Reconstruction of the rabbit Achilles tendon with three bioresorbable materials: histological and biomechanical studies. J Orthop Sci. 2000;5:256–267. doi: 10.1007/s007760050161. [DOI] [PubMed] [Google Scholar]
- 20.Schantz JT, Hutmacher DW, Ng KW, Khor HL, Lim MT, Teoh SH. Evaluation of a tissue-engineered membrane-cell construct for guided bone regeneration. Int J Oral Maxillofac Implants. 2002;17:161–174. [PubMed] [Google Scholar]
- 21.Schnabel M, Marlovits S, Eckhoff G, Fichtel I, Gotzen L, Vecsei V, Schlegel J. Dedifferentiation-associated changes in morphology and gene expression by primary human articular chondrocytes in cell culture. Osteoarthr Cartil. 2002;10:62–70. doi: 10.1053/joca.2001.0482. [DOI] [PubMed] [Google Scholar]
- 22.Urban JP, Roberts S. Intervertebral disc. In: Comper WD, editor. Extracellular matrix, vol1 Tissue function. Amsterdam: Harwood Academic; 1996. pp. 203–233. [Google Scholar]
- 23.Wojciak B, Crossan J, Curtis ASG, Wilkinson CDW. Grooved substrata facilitate in vitro healing of completely divided flexor tendons. J Mater Sci Mater Med. 1995;6:266–271. doi: 10.1007/BF00120269. [DOI] [Google Scholar]