Abstract
Disc herniation treated by discectomy results in a significant loss of nucleus material and disc height. Biological restoration through the use of autologous disc chondrocyte transplantation (ADCT) offers a potential to achieve functional integration of disc metabolism and mechanics. Nucleus regeneration using autologous cultured disc-derived chondrocytes has been demonstrated in a canine model and in clinical pilot studies. In 2002 a prospective, controlled, randomized, multicentre study comparing safety and efficacy of ADCT plus discectomy, with discectomy alone was initiated. The clinical goals were to provide long-term pain relief, maintain disc height, and prevent adjacent segment disease. Interim analysis was performed after 2 years; Oswestry (Low Back Pain/disability), Quebec Back Pain Disability Scale, as well as Prolo and VAS Score were used for the evaluation. Disc height was assessed by MRI. A clinically significant reduction of low back pain in the ADCT-treated group was shown by all three pain score systems. The median total Oswestry Score was 2 in the ADCT group compared with 6 in the control group. Decreases in the Disability index in ADCT-treated patients correlated with the reduction of low back pain. Decreases in disc height over time were only found in the control group, and of potential significance, intervertebral discs in adjacent segments appeared to retain hydration when compared to those adjacent to levels that had undergone discectomy without cell intervention.
Keywords: Cell transplantation, Autologous disc chondrocyte transplantation, Discectomy, Oswestry, Disc fluid content
Introduction
Among the most vexing conditions accompanying aging is the assurance that the body will be less able to structure water. Age-associated changes are apparent in the furrows at the corners of our smiles, on the surfaces of skin, and while less obvious cosmetically, equally prevalent in the organs and tissues throughout the body—dehydration is endemic to the human condition. Tissues such as skin are profusely vascular, function with high cell content, and actively secrete a matrix that maintains a capacity for metabolic exchange and retains moisture. In sharp contrast, the intervertebral disc is avascular, aneural, and relies on a cell content of 1% to maintain a structural and functional capacity. Therefore, it is not surprising that disc nutrition, or more accurately altered permeability between the intervertebral disc and the systemic blood supply would be an intuitive focus that might define a cause–effect relationship, or underscore a mechanistic explanation for disc degeneration [23, 24]. Preserving the appearance of youth and maintaining the flexibility and strength in the spine define two different challenges. Although both garner a huge economic investment from the community, degenerative disc disease is among the most disabling and expensive conditions facing modern medicine. The demographics of an aging population make intervention an even more compelling challenge, and have stimulated countless research efforts to effect a means of reducing either incidence or associated morbidity.
Cellular and biochemical changes attributable to degeneration include a decrease in cell density in the disc accompanied by a reduction in synthesis of cartilage-specific extracellular matrix components such as Type II collagen and aggrecan [2]. With the reduced proteoglycan content attending normal disc aging, a loss of water-binding capacity by the matrix is coupled with incompetence for dissipating spinal forces, and an incendiary process of progressive failure follows [3, 13, 14, 16].
Many assessments of intervertebral disc failure have focused on degenerative, morphologic changes in disc tissue morphology that affect the biomechanical performance of the motion segment [22]. In this consideration, mechanical failure is little more than a corollary of matrix structure, which in turn depends on balanced cell metabolism for efficient maintenance of the disc matrix. In the broadest sense of interventional strategy, biologic approaches are guided by advancing confidence that cell stimulation can be genetically tailored to the individual organs including the intervertebral disc [18]. Despite the fact that protein and gene therapy offer a potential capacity to buffer catabolic cytokines and stimulate matrix production, the chronic and progressive nature of disc degeneration would suggest that therapeutic administration needs to address sustained stimulation, or possibly to instil the cells in the disc with specific activity that can be sustained [12, 19].
Given the value of cells to the metabolic health of the disc, an alternative therapeutic strategy would be to replace, regenerate, or augment the intervertebral disc cell population, with a goal of correcting matrix insufficiencies and restoring normal segment biomechanics. A variety of cell transplantation assessments in animal models have addressed the technical challenges incumbent to disc intervention [7–9, 17, 21]. Separate studies stress the importance of notochordal cells in regulating proteoglycan production [4] and for potentially abrogating the inhibition of sensory nerve invasion of the disc [11].
The most compelling and consistent outcomes from the body of work describing cell transplantation has been that cultured intervertebral disc cells remain viable, retain a capacity for proliferation in situ, demonstrate an ability to make appropriate matrix, and undergo expression consistent with the phenotypic demands of the anatomy. Making the transition from an injury and degeneration model in dogs [6] to a therapeutic treatment of disc herniation with cell therapy represents a separate consideration; one that holds several candidate cell lineages [1]. Based on the safety and efficacy demonstrated in an animal model, autologous transplantation represented the least hurdle to the clinic. It afforded the least manipulation of a cell line, imposed little chance of immune rejection, and as a terminally differentiated lineage emphasized integrating disc chondrocytes with the intention of repairing the intervertebral disc.
EuroDisc randomized trial
The use of autologous cells is regulated by the German Drug Law (Arzneimittelgesetz) according to good manufacturing practices, and has been certified according to internationally approved DIN EN ISO 9001 standards. This broad regulatory oversight satisfies the phenotypic expression of the cell line as well as assures that cells meet minimal viability standards.
A pilot trial of 14 patients provided confidence that cells could be delivered safely to a select group of patients with single level, traumatic disc herniation. To broaden the scope and better understand the potential in a larger group of patients, EuroDisc, a prospective, randomized, multicentre clinical trial was initiated to assess the long-term efficacy of autologous disc chondrocyte transplantation (ADCT) in a broader population. The goal was to embrace a representative patient group, examining not only the traumatic, less degenerative disc, but also to include patients with persistent symptoms that had not responded to conservative treatment where an indication for surgical treatment was given. Disc herniation treated by discectomy results in a significant loss of nucleus material and disc height. Biological restoration with interventional cell therapy offers a potential for accentuating disc metabolism with an underlying intent to restore spine mechanics.
Patients having exclusively one level requiring surgical intervention were eligible for participation in the trial; patients requiring treatment at more than one level were excluded from the study. Prior to their participation, all patients were advised of the potential risks and signed a letter of consent. No placebo group was committed to this study; each patient participating in the clinical trial will undergo surgical treatment for their disc prolapse, and the prospective basis of cell transplantation will constitute and separate the active treatment from the control group. Patients were not blinded to their treatment. Randomization was done after the open microdiscectomy. Eligibility was limited to patients between 18 and 60 years of age, with a body mass index below 28. Exclusion criteria for participating in the study included sclerotic changes, oedema, Modic changes of grade II or III, and spondylolisthesis among other accepted criteria such as pregnancy, etc. Twenty-eight patients constitute this report; 12 patients received cell transplantation following discectomy, 16 patients were treated by discectomy alone.
A single puncture with a minimal calibre cannula was used to obtain a precise delivery with minimal trauma to the patient and to the annulus. The technique was developed with respect to literature that has demonstrated a size-specific correlation of annular injury to disc degeneration. A simple, minimally invasive technique was necessary to reduce the wound site trauma and effectively support cell injection without further injury to the annulus. Cells are transplanted approximately 12 weeks following discectomy to assure that the annulus has healed and will contain the cells. Using a pressure–volume test prior to the delivery of any chondrocytes, cells could be placed with confidence that they would be retained at the site of delivery.
One hundred and twelve patients have been enrolled in the EuroDisc Study; the primary criteria follow-up was intended to occur at 1 year, an interim analysis scheduled at 2 years, and the final analysis will be completed at 4 years. The primary clinical evaluation criterion is the Oswestry Low Back Pain Disability Questionnaire. Secondary criteria include the SF-36, PROLO, Quebec Back Pain Disability Scale (QBPD), MRI, and X-ray evaluation. Use of the Oswestry Disability Questionnaire in clinical trials is recommended by the DGOT (Deutsche Gesellschaft für Orthopädie und Traumatologie); demonstrating acceptable test quality and satisfactory test–retest reliability. The QBPD, another self-rating scale, was professionally developed using factor analysis comprising with high internal consistency, high item discriminability, and high test–retest reliability. Finally, the SF-36, an often-used scale to assess patients’ general condition and quality of life, and a VAS will be used to standardize measureable pain.
Interim analysis: critical evaluation
An interim analysis, performed in January of 2006 to assess whether intervention was correlated with positive clinical outcomes, forms the basis for this report. Within the analysis, successive 3-month, 6-month, 12-month, and 24-month assessments are stratified within the continuum of study. The information within this study allows a broad interpretation of the general progress made over 2 years following a clinical intercession with autologous disc chondrocytes. Interim analysis was performed on the first 28 patients who reached 24-months follow-up to the ADCT. These first 28 patients were randomized in three different centres.
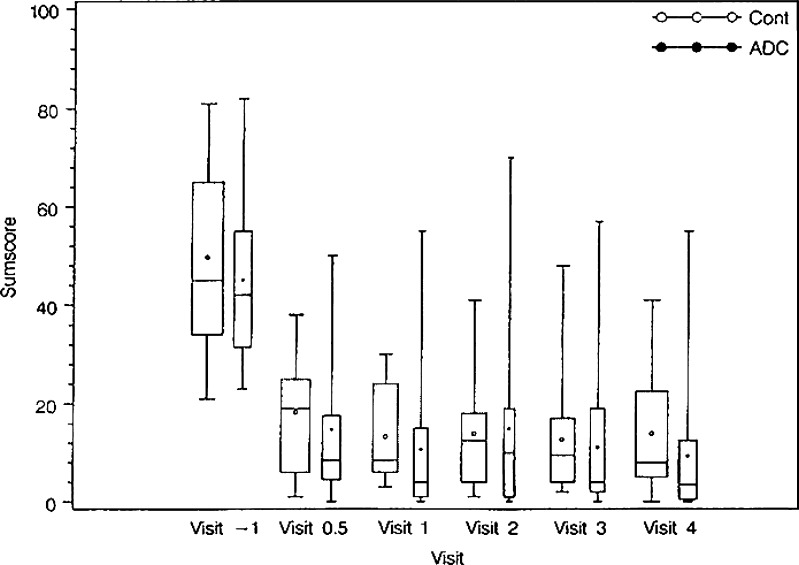
For descriptive analysis of efficacy, the total sumscore as well as the disability index of the Oswestry Low Back Pain Disability Questionnaire (OPDQ) and the total sumscore of the QBPD were taken into account from the initial presurgical presentation through the 2-year follow-up. The outcomes are depicted in Table 1, and graphically displayed in Fig. 1. Based on the mean total sumscore as well as the disability index of the OPDQ, differences in initial presentations between the control group and those receiving autologous cells were not minimal. Surgery as an intervention was a positive experience, and as expected substantially reduced the patient’s disability and pain. The trend in reduction of the total sumscore continued to decrease in the patients whose treatment was supplemented by cell transplantation, while the control group did not sustain continual improvement. At V4, 2 years following the therapeutic intervention with cells, both the total sumscore as well as the disability index of the OPDQ were plainly lower in the ADCT group compared with the control.
Table 1.
Total sumscore and disability index of the OPDQ based on patients who had been followed for 2 years
| N | Mean | SD | Min | Lower quartile | Median | Upper quartile | Max | ||
|---|---|---|---|---|---|---|---|---|---|
| Total sumscore | |||||||||
| Visit -1a | ADCT | 12 | 28.42 | 9.30 | 13.00 | 20.00 | 29.50 | 36.00 | 45.00 |
| Control | 16 | 26.88 | 9.99 | 14.00 | 18.00 | 25.50 | 34.00 | 46.00 | |
| Visit 0.5b | ADCT | 12 | 8.00 | 6.89 | 0.00 | 2.50 | 7.50 | 12.50 | 24.00 |
| Control | 15 | 8.40 | 4.69 | 1.00 | 4.00 | 9.00 | 13.00 | 15.00 | |
| Visit 1c | ADCT | 11 | 6.73 | 8.56 | 0.00 | 0.00 | 5.00 | 12.00 | 28.00 |
| Control | 14 | 7.14 | 6.36 | 0.00 | 1.00 | 5.50 | 13.00 | 19.00 | |
| Visit 2d | ADCT | 10 | 9.10 | 10.72 | 0.00 | 1.00 | 6.50 | 12.00 | 35.00 |
| Control | 14 | 7.79 | 7.42 | 0.00 | 2.00 | 6.50 | 12.00 | 26.00 | |
| Visit 3e | ADCT | 11 | 7.82 | 8.46 | 0.00 | 2.00 | 4.00 | 15.00 | 25.00 |
| Control | 14 | 7.07 | 5.94 | 0.00 | 1.00 | 7.00 | 12.00 | 19.00 | |
| Visit 4f | ADCT | 12 | 6.00 | 8.89 | 0.00 | 0.00 | 2.00 | 8.50 | 29.00 |
| Control | 16 | 7.56 | 6.52 | 0.00 | 2.50 | 6.00 | 13.00 | 19.00 | |
| Disability index (%) | |||||||||
| Visit -1 | ADCT | 12 | 56.83 | 18.60 | 26.00 | 40.00 | 59.00 | 72.00 | 90.00 |
| Control | 16 | 53.75 | 19.97 | 28.00 | 36.00 | 51.00 | 68.00 | 92.00 | |
| Visit 0.5 | ADCT | 12 | 16.06 | 13.73 | 0.00 | 5.33 | 15.00 | 25.00 | 48.00 |
| Control | 15 | 16.80 | 9.37 | 2.00 | 8.00 | 18.00 | 26.00 | 30.00 | |
| Visit 1 | ADCT | 11 | 13.45 | 17.11 | 0.00 | 0.00 | 10.00 | 24.00 | 56.00 |
| Control | 14 | 14.29 | 12.72 | 0.00 | 2.00 | 1100 | 26.00 | 38.00 | |
| Visit 2 | ADCT | 10 | 18.64 | 21.53 | 0.00 | 2.00 | 13.89 | 26.67 | 70.00 |
| Control | 14 | 15.62 | 14.80 | 0.00 | 4.44 | 13.00 | 24.00 | 52.00 | |
| Visit 3 | ADCT | 11 | 15.64 | 16.92 | 0.00 | 4.00 | 8.00 | 30.00 | 50.00 |
| Control | 14 | 14.14 | 11.88 | 0.00 | 2.00 | 14.00 | 24.00 | 38.00 | |
| Visit 4 | ADCT | 12 | 12.00 | 17.79 | 0.00 | 0.00 | 4.00 | 17.00 | 58.00 |
| Control | 16 | 15.19 | 12.99 | 0.00 | 5.50 | 12.00 | 26.00 | 38.00 | |
aSequestrectomy
bADCT/Control
c3 months after ADCT/Control visit 0.5
d6 months after ADCT/Control visit 0.5
e12 months after ADCT/Control visit 0.5
f24 months after ADCT/Control visit 0.5
Fig. 1.
Total sumscore of the OPDQ based on patients with at least 2-years follow-up
Descriptive analyses of the mean total sumscore of the QBPD prior to sequestrectomy, prior to ADCT/control, and 3 months after ADCT/control demonstrated a decrease in mean and median sumscores in both groups. Although the mean and median values for both the ADCT and the control group decreased between 1 year (V3) and 2 years (V4), the assessments for the ADCT group were clearly lower (Table 2). Patient global assessment of pain demonstrated some fluctuation although both groups received substantial relief from the surgical intervention. However, as patients were tracked over the course of the V4, or 2-year follow-up, changes emerged that suggest that the ADCT-treated patients have a lower assessment of their pain (Table 3).
Table 2.
Total sumscore of the QBPD based on patients with at least 2 years follow-up
| N | Mean | SD | Min | Lower quartile | Median | Upper quartile | Max | |
|---|---|---|---|---|---|---|---|---|
| Visit -1a | ||||||||
| ADCT | 12 | 45.08 | 17.60 | 23.00 | 31.50 | 42.00 | 55.00 | 82.00 |
| Control | 16 | 49.69 | 18.69 | 21.00 | 34.00 | 45.00 | 65.00 | 81.00 |
| Visit 0.5b | ||||||||
| ADCT | 12 | 14.75 | 16.07 | 0.00 | 4.50 | 8.50 | 17.50 | 50.00 |
| Control | 15 | 18.27 | 11.04 | 1.00 | 6.00 | 19.00 | 25.00 | 38.00 |
| Visit 1c | ||||||||
| ADCT | 11 | 10.64 | 16.05 | 0.00 | 1.00 | 4.00 | 15.00 | 55.00 |
| Control | 14 | 13.29 | 9.72 | 3.00 | 6.00 | 8.50 | 24.00 | 30.00 |
| Visit 2d | ||||||||
| ADCT | 10 | 15.00 | 20.77 | 0.00 | 1.00 | 10.00 | 19.00 | 70.00 |
| Control | 14 | 13.93 | 11.76 | 1.00 | 4.00 | 12.50 | 18.00 | 41.00 |
| Visit 3e | ||||||||
| ADCT | 11 | 11.09 | 16.71 | 0.00 | 2.00 | 4.00 | 19.00 | 57.00 |
| Control | 14 | 12.71 | 12.55 | 2.00 | 4.00 | 9.50 | 17.00 | 48.00 |
| Visit 4f | ||||||||
| ADCT | 12 | 9.33 | 15.33 | 0.00 | 0.50 | 3.50 | 12.50 | 55.00 |
| Control | 16 | 13.94 | 12.61 | 0.00 | 5.00 | 8.00 | 22.50 | 41.00 |
aSequestrectomy
bADCT/Control
c3 months after ADCT/Control visit 0.5
d6 months after ADCTVControl visit 0.5
e12 months after ADCT/Control visit 0.5
f24 months after ADCT/Control visit 0.5
Table 3.
Global assessment of pain (100 mm VAS) based on patients with at least 2-years follow-up
| N | Mean | SD | Min | Lower quartile | Median | Upper quartile | Max | |
|---|---|---|---|---|---|---|---|---|
| Visit -1a | ||||||||
| ADCT | 11 | 59.45 | 22.76 | 15.00 | 48.00 | 60.00 | 76.00 | 96.99 |
| Control | 16 | 57.31 | 28.51 | 0.00 | 27.00 | 70.00 | 79.50 | 88.98 |
| Visit 0.5b | ||||||||
| ADCT | 12 | 19.17 | 19.37 | 0.00 | 2.50 | 13.00 | 31.50 | 65.00 |
| Control | 15 | 17.20 | 14.70 | 0.00 | 3.00 | 14.00 | 31.00 | 46.00 |
| Visit 1c | ||||||||
| ADCT | 11 | 12.82 | 19.37 | 0.00 | 0.00 | 3.00 | 24.00 | 61.99 |
| Control | 14 | 14.36 | 10.59 | 1.00 | 4.00 | 15.00 | 22.00 | 33.00 |
| Visit 2d | ||||||||
| ADCT | 10 | 21.00 | 22.85 | 0.00 | 8.00 | 16.50 | 23.00 | 78.99 |
| Control | 14 | 14.00 | 16.51 | 1.00 | 2.00 | 5.50 | 19.00 | 51.00 |
| Visit 3e | ||||||||
| ADCT | 11 | 18.00 | 18.73 | 2.00 | 3.00 | 9.00 | 25.00 | 56.00 |
| Control | 14 | 15.07 | 12.16 | 0.00 | 3.00 | 12.00 | 29.00 | 37.00 |
| Visit 4f | ||||||||
| ADCT | 12 | 11.17 | 13.48 | 0.00 | 1.00 | 5.00 | 17.00 | 39.00 |
| Control | 16 | 15.62 | 15.16 | 1.00 | 3.00 | 12.50 | 26.50 | 53.99 |
aSequestreciomy
bADCT/Control
c3 months after ADCT/Control visit 0.5
d6 months after ADCT/Control visit 0.5
e12 months after ADCT/Control visit 0.5
f24 months after ADCT/Control visit 0.5
MRI was used to assess the respective disc height along the course of the analyses from the date of the sequestrectomy until the 2-year follow-up. In addition to the disc height, the content of the liquid component was evaluated as a means of assessing matrix content. Results of the analysis of the intervertebral disc height compared affected (treated with surgery, or with surgery and cells) with non-affected adjacent segments in the same patients, and also measured the relative vertebral heights as a means of assessing patient demographics and morphologic variation (Table 4). Comparison of the mean intervertebral disc heights and the vertebral heights revealed no differences between the groups.
Table 4.
Mean height of vertebrae (mm) at screening visits, and through 2-year follow-up
| N | Affected vertebrae | Non-affected vertebrae | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Min–max | Mean | SD | Min–max | ||
| Visit -1a | |||||||
| ADCT | 12 | 21.6 | 6.31 | 8.50–28.00 | 22.56 | 6.84 | 8.25–30.00 |
| Control | 15 | 21.55 | 7.92 | 7.75–29.00 | 21.32 | 7.56 | 8.75–30.00 |
| Visit 0.5b | |||||||
| ADCT | 12 | 21.10 | 5.98 | 7.50–28.50 | 22.31 | 6.50 | 7.25–29.50 |
| Control | 14 | 23 48 | 4.25 | 10.25–27.50 | 22.84 | 4.06 | 10.25–28.00 |
| Visit 3c | |||||||
| ADCT | 11 | 24.55 | 2.04 | 21.50–28.50 | 25.00 | 2.32 | 21.00–29.00 |
| Control | 13 | 25.19 | 2.77 | 21.00–32.50 | 25.46 | 3.21 | 22.50–34.50 |
| Visit 4d | |||||||
| ADCT | 12 | 23.92 | 2.36 | 20.00–27.50 | 23.92 | 2.91 | 18.00–28.50 |
| Control | 16 | 25.06 | 2.97 | 21.50–3250 | 24.84 | 3.34 | 20.00–32.50 |
aSequestrectomy
bADCT/Control
c12 months after ADCT/Control visit 0.5
d24 months after ADCT/Control visit 0.5
An analysis of fluid content of the intervertebral disc at each visit demonstrated that more than 80% of the affected segments showed decreased hydration 3-months (V1) following surgery (Table 5). In general, the proportion of affected segments with a decreased content of liquid decreased over the course of the trial. Of particular interest was the outcome at 2 years, where the ADCT treated group showed a substantially higher normalization as a group; 41% normal fluid content compared with only 25% normal content in the control group. Perhaps most interesting of all the data to emerge from this study comes from inspecting discs either one or two segments from the treated intervertebral disc. Fluid levels at both of these segments showed a substantially higher percentage of normal fluid content despite the fact that they were away from the surgical intervention site.
Table 5.
Content of liquid (%), affected and non-affected segments
| N | Affected segment | 1. Non-affected segment | 2. Non-affected segment | ||||
|---|---|---|---|---|---|---|---|
| Normal | Decreased | Normal | Decreased | Normal | Decreased | ||
| Visit -1a | |||||||
| ADCT | 12 | 16.67 | 83.33 | 83.33 | 16.67 | 83.33 | 16.67 |
| Control | 15 | 13.33 | 86.67 | 86.67 | 13.33 | 46.67 | 53.33 |
| Visit 0.5b | |||||||
| ADCT | 12 | 25.00 | 75.00 | 81.82c | 18.18c | 50.00 | 50.00 |
| Control | 14 | 0.00 | 100.0 | 78.57 | 21.43 | 28.57 | 71.43 |
| Visit 3d | |||||||
| ADCT | 11 | 27.27 | 72.73 | 90.91 | 9.09 | 63.64 | 36.36 |
| Control | 13 | 23.08 | 76.92 | 76.92 | 23.08 | 53.85 | 46.15 |
| Visit 4e | |||||||
| ADCT | 12 | 41.67 | 58.33 | 91.67 | 8.33 | 66.67 | 33.33 |
| Control | 16 | 25.00 | 75.00 | 86.67f | 13.33f | 56.25 | 43.75 |
aSequsstrectomy
bADCT/Control
cOnly 11 values available
d12 months after ADCT/Control visit 0.5
e24 months after ADCT/Control visit 0.5
fOnly 15 values available
Summary of results
An analysis of the first 28 patients randomized and treated in this study at 2 years demonstrated a clear trend in the decrease in the sumscore as well as the disability index of the OPDQ to be more pronounced in the ADCT group than in the control group. Despite the fact that neither the intervertebral disc height, nor vertebral height differed between the groups, the proportion of affected intervertebral disc showing a decreased content of liquid is distinctly lower (58%) in the ADCT group when compared to the control group (75%), suggesting a positive influence of the ADCT intervention in this regard. A safety analysis (data not shown) demonstrated no adverse risks or outcomes associated with the additional intervention.
Discussion
Interventional surgery for disc herniation is one of the most widely used and effective treatments for back pain that emerges within the broad scope of disc degeneration. Successful removal of impinging tissue offers the individual patient substantial relief for associated pain. However, the reduction of tissue involved in the surgical procedure anatomically compromises the function of the affected disc, and effects a load transfer to adjacent discs. The goal of this clinical trial was to evaluate whether ex vivo expansion of autologous disc chondrocytes and subsequent percutaneous transplantation would positively affect the disc treated, and potentially stabilize the spine in general. The principle outcomes of our study were:
Disc chondrocytes that had been removed as a normal part of discectomy could be expanded in culture under GMP conditions and returned to the patient after the annulus had been allowed to heal for 12 weeks.
Disc chondrocyte transplantation could be delivered by percutaneous technique.
Patients who received ADCT had greater pain reduction at 2 years compared with patients who did not receive cells following their discectomy surgery.
Discs in patients that received cells demonstrated a significant difference as a group in the fluid content of their treated disc when compared to control.
Adjacent intervertebral discs, both at one level or two levels from the intervertebral disc that received the cell therapy also demonstrated a difference in fluid content.
The results of this study are encouraging from several perspectives; first to the fact that the morphologic outcomes mirrored that seen in our pre-clinical animal study [7]; and second that the pain relief seen in the pilot study which served as a basis for this clinical trial was sustained for the course of this 2-year interim analysis. This gives cause to the success of the cell-based intervention.
Critics of ADCT have cited intervention with cells isolated from degenerative matrices as potentially lacking full capacity to restore normal conditions [5]. From this 2-year follow-up, it would appear that cell transplantation supported pain relief in patients receiving them as well as supplemented the morphology of the disc and adjacent discs. Because the disc has such a limited intrinsic capacity for regeneration, and based on the perceived needs for large amounts of proteoglycan and Type II collagen in the regenerating matrix, several possible donors for cell-based therapeutics have been considered. However, neither in vitro methods for inducing the differentiation of stem cells into NP cells, nor the demonstration of clinical application has been accomplished. To date, ADCT remains the only cell lineage that has been clinically tested and shown to be effective for providing long-term pain relief and sustaining disc morphology.
The supplementary support of fluid content at adjacent intervertebral levels by the interventional cell therapy was not anticipated but offers the strongest encouragement for additional study. Although nutritional exchange, or more appropriately the blocking of capacity for metabolic and fluid replacement in the disc has long since been considered an essential cause and effect, a study by Hutton et al. demonstrated that blocking the subchondral plate for 12 months in an animal model did not precipitate gross disc degeneration—the experimental discs as well as the control discs appeared normal in every dog [10]. After the discs were bisected, they were carefully inspected for any visible signs of degeneration. The experimental discs showed no clear signs of disc degeneration and were not distinguishable from the control discs on a gross level. The numerical results from the ELISA showed that in the experimental discs as opposed to the control discs, there were significant increases in proteoglycan content in both the nucleus (P = 0.033) and annulus (P = 0.01) and clear histologic changes in some of the discs. This study holds in stark contrast the theory of progressive starving of the intervertebral disc.
What seems potentially more coupled to the intervention and outcomes that we have seen is removing the onus of recovery that is incumbent in restoring the quality of life. Activity in itself seems to retain the flexibility in the spinal segments, and clearly there are mechanisms for exchange outside of the vertebral play as noted above. In the context of fluid exchange, there has been little work done to demonstrate physical–chemical imbalance in the aging process. Recent data suggests that more than 50% of adults suffer from hypertonic plasma [20] which would predictably effect cell dehydration with consequences relevant to blood chemistries, bioelectric impedance, and in total fluid balance. While studies of erythrocytes have demonstrated that functional cell dehydration offers constitutive induction of Ca(2+) dependent mechanisms, work defining osmotic load, mechanical deformation, and analyses of combinations that might similarly affect disc chondrocytes, particularly of nucleus pulposus cells, remain untested. Preliminary work in isolated chondrocytes indicate that the increase in [Ca2+] within the chondrocyte cell membrane is dependent on high fluid pressure and receptive to stretch-activated channels [15]. Mechanical forces have been known for some time to alter cell membrane ion channel permeability associated with Ca(2+) and other ion fluxes. In addition, the application of dynamic mechanical forces leads to the activation of growth factor and hormone receptors even in the absence of ligand binding. These are some of the mechanisms that have evolved in vertebrates by which cells respond to changes in external forces that lead to changes in tissue structure and function.
This study confirms that cells can be transplanted, that intervention reduces pain at 2 years compared with a control population, and that fluid level remains higher at treated and at adjacent levels in patients receiving therapeutic cell placement. From these promising results, development of carriers that may imbue additional potential, scaffolds that enhance placement, and cell lineages that offer prophylactic options without imposing chondrocyte sampling as a basis for treatment will remain next considerations.
Acknowledgments
Financial and clinical support for this study was underwritten by co.don AG, Teltow, Germany. Additional collaboration was supported by Bergmannstrost, the Atlanta Medical Center, and the Emory Spine Center.
References
- 1.Anderson DG, Albert TJ, Fraser JK, Risbud M, Wuisman P, Meisel HJ, Tannoury C, Shapiro I, Vaccaro AR. Cellular therapy for disc degeneration. Spine. 2005;30(Suppl. 1):S14–S19. doi: 10.1097/01.brs.0000175174.50235.ba. [DOI] [PubMed] [Google Scholar]
- 2.Antoniou J, Steffen T, Nelson F, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, aging, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bibby SRS, Jones DA, Lee RB, Yu J, Urban JPG. The pathophysiology of the intervertebral disc. Joint Bone Spine. 2001;68:537–542. doi: 10.1016/S1297-319X(01)00332-3. [DOI] [PubMed] [Google Scholar]
- 4.Erwin WM, Inman RD. Notochordal cells regulate intervertebral disc chondrocyte proteoglycan production and cell proliferation. Spine. 2006;31(10):1094–1099. doi: 10.1097/01.brs.0000216593.97157.dd. [DOI] [PubMed] [Google Scholar]
- 5.Evans C. Potential biologic therapies for the intervertebral disc. J Bone Joint Surg Am. 2006;88A(Suppl. 2):95–98. doi: 10.2106/JBJS.E.01328. [DOI] [PubMed] [Google Scholar]
- 6.Ganey TM, Meisel HJ. A potential role for cell-based therapeutics in the treatment of intervertebral disc herniation. Eur Spine J. 2002;11(Suppl 2):S206–S214. doi: 10.1007/s00586-002-0494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganey TM, Libera J, Moos V, Alasevic O, Fritsch KG, Meisel HJ, Hutton WC. Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine. 2003;28:2609–2620. doi: 10.1097/01.BRS.0000097891.63063.78. [DOI] [PubMed] [Google Scholar]
- 8.Gruber HE, Hanley EN., Jr Analysis of aging and degeneration of the human intervertebral disc: comparison of surgical specimens with normal controls. Spine. 1998;23:751–757. doi: 10.1097/00007632-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 9.Gruber HE, Johnson TL, Leslie K, Ingram JA, Martin D, et al. Autologous intervertebral disc cell implantation. Spine. 2002;27:1626–1633. doi: 10.1097/00007632-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 10.Hutton WC, Murakami H, Li J, Elmer WA, Yoon ST, Minamide A, Akamaru T, Tomita K. The effect of blocking a nutritional pathway to the intervertebral disc in the dog model. J Spinal Disord Tech. 2004;17(1):53–63. doi: 10.1097/00024720-200402000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Johnson WE, Sivan S, Wright KT, Eisenstein SM, Stephen M, Maroudas A, Roberts S. Human intervertebral disc cells promote nerve growth over substrata of human intervertebral disc aggrecan. Spine. 2006;31(11):1187–1193. doi: 10.1097/01.brs.0000217669.04903.61. [DOI] [PubMed] [Google Scholar]
- 12.Larson JW, III, Levicoff EA, Gilbertson LG, Kang JD. Biological modification of animal models of intervertebral disc degeneration. J Bone Joint Surg Am. 2006;88A(Suppl. 2):83–87. doi: 10.2106/JBJS.F.00043. [DOI] [PubMed] [Google Scholar]
- 13.Lipson SJ, Muir H. 1980 Volvo Award in basic science. Proteoglycans in experimental intervertebral disc degeneration. Spine. 1981;6:194–210. doi: 10.1097/00007632-198105000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Maroudas A, Stockwell RA, Nachemson A, et al. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113–30. [PMC free article] [PubMed] [Google Scholar]
- 15.Mizuno S. A novel method for assessing effects of hydrostatic fluid pressure on intracellular calcium: a study with bovine articular chondrocytes. Am J Physiol Cell Physiol. 2005;288(2):C329–C337. doi: 10.1152/ajpcell.00131.2004. [DOI] [PubMed] [Google Scholar]
- 16.Nerlich AG, Schleicher ED, Boos N. 1997 Volvo Award winner in basic science studies. Immunohistologic markers for age-related changes of human lumbar intervertebral discs. Spine. 1997;22(24):2781–2795. doi: 10.1097/00007632-199712150-00001. [DOI] [PubMed] [Google Scholar]
- 17.Okuma M, Mochida J, Nishimura K, Sakabe K, Seiki K. Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration: an in vitro and in vivo experimental study. J Orthop Res. 2000;18:988–997. doi: 10.1002/jor.1100180620. [DOI] [PubMed] [Google Scholar]
- 18.Phillips FM, An H, Kang JD, Boden SD, Weinstein J. Biologic treatment for intervertebral disc degeneration: summary statement. Spine. 2003;28(Suppl. 15):S99. doi: 10.1097/00007632-200308011-00017. [DOI] [PubMed] [Google Scholar]
- 19.Sobajima S, Kim JS, Gilbertson LG, Kang JD. Gene therapy for degenerative disc disease. Gene Ther. 2004;11:390–401. doi: 10.1038/sj.gt.3302200. [DOI] [PubMed] [Google Scholar]
- 20.Stookey JD. High prevalence of plasma hypertonicity among community-dwelling older adults: results from NHANES III. J Am Diet Assoc. 2005;105(8):1231–1239. doi: 10.1016/j.jada.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Hurtig M, Pilliar RM, Grynpas M, Kandel RA. Characterization of nucleus pulposus-like tissue formed in vitro. J Orthop Res. 2001;19:1078–1084. doi: 10.1016/S0736-0266(01)00056-0. [DOI] [PubMed] [Google Scholar]
- 22.Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IKY, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Urban JP, Holm S, Maroudas A, Nachemson A. Nutrition of the intervertebral disk. An in vivo study of solute transport. Clin Orthop. 1977;129:101–114. [PubMed] [Google Scholar]
- 24.Urban JP, Holm S, Maroudas A, Nachemson A. The nutrition of the intervertebral disc. Effect of fluid flow on solute transport. Clin Orthop. 1982;170:296–306. [PubMed] [Google Scholar]