Abstract
The current evidence for total disc replacement was assessed by performing a systematic review of the published literature. This search identified two randomised controlled trials (RCTs), two previous systematic reviews, seven prospective cohort studies, eleven retrospective cohort studies and eight case series. The RCTs involved the use of the Charité artificial disc and the Pro-Disc II total disc replacement. All papers analysed were classified according to their level of evidence as defined by the Centre for Evidence Based Medicine, Oxford, UK (www.cebm). For degenerative disc disease at L4/5 or L5/S1, both the clinical outcome and the incidence of major neurological complications following insertion of the Charité artificial disc were found to be equivalent to those observed following a single level anterior lumbar interbody fusion 2 years following surgery. However, only 57% of patients undergoing total disc replacement and 46% of patients undergoing arthrodesis met the four criteria listed for success. The range of flexion/extension was restored and maintained with the Charité artificial disc. The role for two or three level disc replacement in the treatment of degenerative disc disease remains unproven. To date, no study has shown total disc replacement to be superior to spinal fusion in terms of clinical outcome. The long-term benefits of total disc replacement in preventing adjacent level disc degeneration have yet to be realised. Complications of total disc replacement may not be known for many years. There are numerous types of disc prostheses and designs under study or in development. Well designed prospective RCTs are needed before approval and widespread application of this technology.
Keywords: Lumbar vertebrae, Intervertebral disc disease, Total disc replacement, Prostheses, Literature review
Introduction
The management of chronic low back pain remains controversial. Möller and Hedlund, in a randomised controlled trial, demonstrated that the functional outcome was better in the surgically treated group when compared to the exercise group for patients with isthmic spondylolisthesis [33]. Fritzell et al. reported on a randomised controlled multi-centre study for patients with degenerative disc disease [14]. The authors concluded that lumbar fusion in a well-informed and selected group of patients with severe chronic low back pain can diminish pain and decrease disability more efficiently than those with commonly used non-surgical treatment.
Fairbank et al. compared surgical stabilisation to an intensive rehabilitation programme for patients with chronic low back pain in a randomised controlled trial [12]. These authors were unable to show spinal fusion surgery to be any more beneficial than intensive rehabilitation. The following literature compares spinal fusion to total disc replacement for the treatment of symptomatic degenerative disc disease. It is interesting to note that there have been no reported trials comparing total disc replacement to physical therapy or intensive rehabilitation for the treatment of chronic low back pain.
Motion preservation is an intuitively attractive alternative to spinal arthrodesis theoretically preventing adjacent level degeneration. However, the long-term stability, endurance and strength of the prosthesis are unknown for the majority of implants. Significant facet joint osteoarthritis is a contraindication to the procedure and yet, this is difficult to identify in its early stages. The use of total disc replacement may be limited to the treatment of early degenerative disc disease with preservation of disc height thereby eliminating its uses in the majority of patients. Furthermore, the fate of facet joints following a total disc replacement is unknown and facet joint hypertrophy, which accelerates spinal stenosis, may be a potent long-term complication of such a device. Revision procedures will undoubtedly be technically difficult with a significant risk of vascular injury, particularly at the L4/5 level.
There are many types of disc prostheses currently available allowing motion of the degenerative segment. The next generation of disc replacement, which will allow motion and compression across the motion segment, is currently being designed. Well-conducted prospective randomised controlled trials (RCTs) are needed to show safety and efficacy before approval and widespread application of this technology occurs. The long-term benefits and complications of total disc replacement may not be known for many years.
Materials and methods
In order to establish what body of evidence exists to support the use of currently available disc replacements, we conducted a broad search of the literature. As we anticipated the number of prospective RCTs to be small, we included relevant retrospective and prospective cohort studies (non-randomised) to increase the yield of information for ‘best evidence synthesis’.
Search strategy
The literature was searched using some of the most commonly used medical databases:
The Cochrane Library 2006, Issue 1.
Medline (1996 to April 2006).
Embase (1996 to April 2006).
Cinahl (1982 to April 2006).
Pubmed (to April 2006).
The same search strings were used for each database albeit for small modifications for the Cochrane database and Pubmed to take into account different search engines. The search strings and number of ‘hits’ are given in Table 1. Thesaurus mapping was used given the variations of spelling of key terms.
Table 1.
Search strings used in search strategy (as MeSH headings) and respective number of records found
| Search strings | Medline | Cinahl | Embase | Pubmed | Cochrane |
|---|---|---|---|---|---|
| Lumbar disc replacement | 19 | 22 | 23 | 168 | 21 |
| ‘Randomised controlled trial’ or ‘clinical trial’ or ‘cohort series’ or ‘retrospective studies’ | 165,759 | 20,358 | 101,608 | 674,194 | a |
| ‘Lumbar disc’ or ‘intervertebral disc’ or ‘joint prosthesis’ or ‘arthroplasty’ | 11,797 | 5,463 | 30,321 | 33,238 | a |
| Combinations | 48 | 20 | 108 | 29 | 21 |
| Total | 226 |
aSearch terms not used in this database
Results
The combined searches resulted in 226 references. The Pubmed database produced 29 hits whilst Embase, Medline and Cinahl, using the same search strategy, produced 108, 48 and 20 hits, respectively. The Cochrane database revealed 21 hits; however, eight were excluded as they were not relevant to this review. The remainder included the guidelines issued by the National Institute of Clinical Excellence (NICE) and several interim results from the larger multi-centre RCTs.
From these 226 references, 186 were excluded as not relevant to this review on the basis of title and abstract. The remaining 40 papers were evaluated. Breaking these down by type and eliminating duplicate studies, the following list was produced:
Randomised controlled trials2 [4, 10, 11, 15, 29–31, 42–44].
Non-randomised comparative studies0.
Case series/reports and expert opinion8 [8, 21, 25, 27, 28, 32, 35, 36].
Papers obtained were categorised into levels of evidence according to guidelines published by the Centre for Evidence Based Medicine, Oxford, UK (www.cebm.net) (Table 2). Those papers with levels IV and V evidence were rejected from the analysis.
Table 2.
Levels of evidence for therapeutic studies adapted from material published by the Centre for Evidence Based Medicine, Oxford, UK (www.cebm.net)
| Level I | Randomised controlled trials + systematic reviews level I studies |
| Level II | Prospective cohort studies + systematic reviews level II studies |
| Level III | Retrospective cohort studies + systematic reviews level III studies |
| Level IV | Case series |
| Level V | Expert opinion |
Analysis
Retrospective cohort studies (level III evidence)
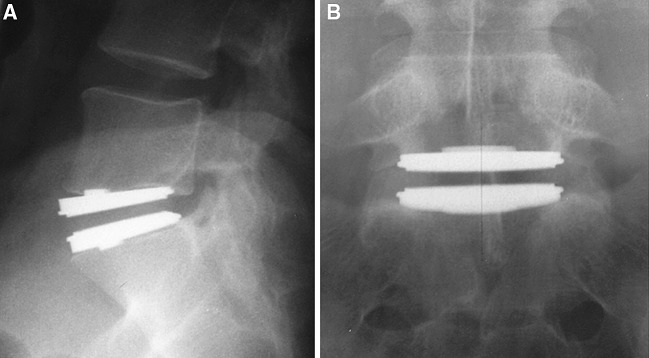
Cinotti et al. [7] reported on 46 patients undergoing artificial disc replacement with Charité SB III disc prosthesis (DePuy Spine, Raynham, MA, USA) with a mean follow-up of 3.2 years (range 2–5 years) (Fig. 1). Twenty-two patients had degenerative disc disease, and 24 patients were post-lumbar discectomy. Thirty-six patients were operated at a single level, and ten patients were operated at two levels. Sixty-three per cent of patients reported satisfactory results. Success rate was highest for those patients undergoing single-level disc replacement, without previous surgery or workers’ compensation. Seven patients with unsatisfactory results subsequently underwent a postero-lateral instrumented fusion; three reported satisfactory results. A further two patients underwent removal of the prosthesis and circumferential fusion. There was no evidence of implant failure, loosening or polyethylene wear. The vertebral motion averaged 9° (range 0–15°) at the operated level with four patients developing spontaneous fusions.
Fig. 1.
Lateral radiograph showing Charité lumbar disc replacement L5/S1
Van Ooij et al. reported on 27 patients with unsatisfactory results or complications following SB Charité disc replacement [40]. There was no information available regarding the total number of patients undergoing total disc replacement over that period. There were 15 women and 12 men; the mean age was 40 (range 30–67 years). Four patients had their implant removed and 11 patients required a second spinal reconstructive salvage procedure. Implant-related complications included anterior subluxation of the prosthesis in two cases, subsidence of the prosthesis in 16 cases and polyethylene wear in one patient. There were two approach-related complications (abdominal wall haematoma and retrograde ejaculation). Further problems arose from disc degeneration at adjacent levels and facet joint arthrosis at the same or other levels.
Tropiano et al. reported on 64 patients undergoing lumbar total disc replacement with the Pro-Disc I (Synthes Inc, Paoli, PA, USA) [38]. Fifty-five patients (86%) were followed up with a mean duration of 8.7 years. The authors reported significant improvements in back pain, radiculopathy, disability and modified Stauffer–Coventry scores. Thirty-three patients reported an excellent result, eight had a good result and 14 had a poor result. The authors found that gender and multi-level surgery did not appear to affect the outcomes. Radiographs showed no evidence of loosening, migration or mechanical failure. There were five approach-related complications including deep venous thrombosis, iliac vein laceration, retrograde ejaculation and incisional hernias.
Tropiano et al. reported on 53 patients who underwent Pro-Disc II lumbar disc replacement [37]. Forty patients had surgery at one level, 11 patients at two levels and two patients at three levels. The mean follow-up time was 1.4 years (range 1–2 years). The mean Visual Analogue Score (VAS) for back pain improved from 7.4 to 1.3, the mean VAS for leg pain improved from 6.7 to 1.9 and the mean Oswestry Disability Index (ODI) improved from 56 to 14 points. There were no implant failures or loosening of implants observed. There was no significant difference in the clinical outcome between the single- or multi-level groups. Satisfactory clinical results were obtained in 90% of patients who had had previous lumbar surgery. Complications including vertebral body fracture, transient radicular pain, implant mal-position and transient retrograde ejaculation occurred in five patients (9%). Three of 53 patients required re-operation. At L5–S1, the flexion/extension range of motion averaged 8° (range 2–12°) at the operated level. At L4-5, the range of motion averaged 10° (8–18°) at the operated level. The mean lumbar lordosis was 56.7° before surgery (range 30–72°) and 61.9° at final follow-up (range 46–72°).
Huang et al. attempted to correlate the range of motion with clinical outcome in 38 patients undergoing one- or two-level total disc replacement with the Pro-Disc I implant [18]. The mean flexion/extension range of motion at the operated level was 4.0 ± 3.9° (range 0–18°). Spearman rank correlation revealed weak to moderate but statistically significant association between the range of motion and outcome for back pain, Oswestry disability questionnaire and modified Stauffer–Coventry scores. Patients with motion of more than 5° had superior outcomes in Oswestry disability questionnaire and Stauffer–Coventry scores.
Huang et al. studied the prevalence of contraindications to total disc replacement in a cohort of lumbar surgical patients [19]. The authors reviewed 100 consecutive patients who had lumbar surgery carried out by one surgeon in 2002. Contraindications to total disc replacement included central or lateral recess stenosis, facet joint arthrosis, spondylolysis or spondylolisthesis, herniated nucleus pulposus with radiculopathy, scoliosis, osteoporosis, post-surgical pseudarthrosis or deficiency of the posterior elements. Patients were divided into fusion or non-fusion groups and the percentage of patients without contraindications to total disc replacement was calculated. Out of 100 patients, 56 underwent spinal fusion and 44 underwent non-fusion surgery. In the fusion group, 56 of 56 patients had contraindications to total disc replacement. In the non-fusion group, 11% (5/44) were considered candidates for total disc replacement. Overall, 5% of patients were considered candidates for total disc replacement. The average number of contraindications to total disc replacement was 2.48 (range 0–5). Predictions that total disc replacement will replace fusion would appear premature based on this study.
Prospective cohort studies (level II evidence)
Mayer et al. reported on 34 consecutive patients treated with the Pro-Disc total disc replacement [26]. Indications included degenerative disc disease (61.8%), degenerative disc disease plus disc herniation (11.8%), post-discectomy (14.7%), adjacent level degeneration (8.8%) and degeneration following nuclear replacement (2.9%). Out of these 34 patients, 26 (76.5%) were available for follow-up at a mean time period of 5.8 months. The mean VAS for low back pain dropped from 6.3 to 2.4 points and the mean ODI dropped from 19.1 to 11.5 points. Three patients suffered complications including an anterior dislocation of the polyethylene inlay, one L5 nerve root irritation and one retrograde ejaculation.
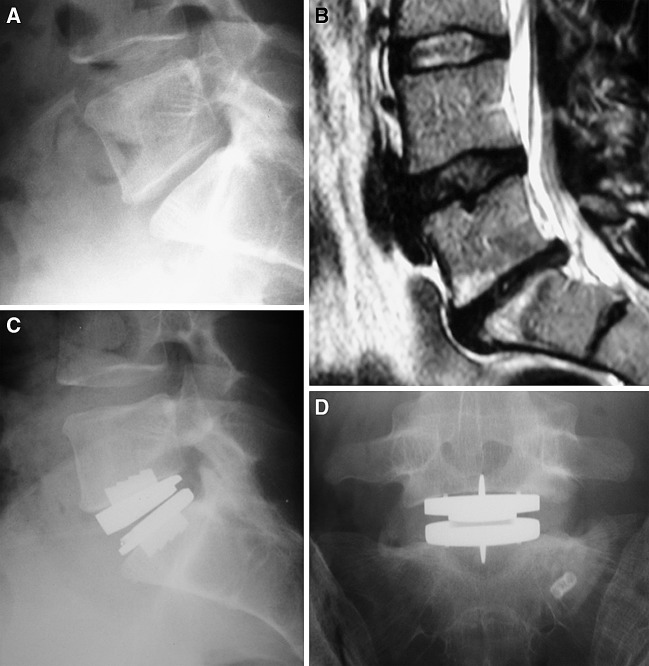
Bertagnoli and Kumar reported on 108 patients undergoing total disc replacement with the Pro-Disc II implant (Fig. 2a–d) [1]. Ninety-four patients underwent surgery at one level, 12 at two levels and two at three levels. Range of follow-up time varied from 3 months to 2 years, with 54 patients (50%) having more than 1-year follow-up. The ODI, VAS and Short-Form 36 (SF-36) questionnaire were used for clinical evaluation; however, these figures were not available in the results section. The authors reported 90.8% of patients achieving an excellent result, 7.4% a good result, 1.8% a fair result and no patients with a poor result. Ten of 108 (9.2%) patients had progression of disc degeneration at an adjacent level following surgery. There were no implant failures and the average range of motion at L5–S1 was 9° (range 2–13°) and at L4-5 was 10° (range 8–15°).
Fig. 2.
a Pre-operative lateral radiograph. Note narrow L5/S1 disc space. b Pre-operative T2 weighted sagittal MRI scan. Note advanced disc degeneration at L5/S1, with loss of hydration at L4/5. Subsequent provocative lumbar discography produced concordant pain at L5/S1 and no pain at L4/5. c Lateral radiograph showing ProDisc II lumbar disc replacement L5/S1. d Antroposterior radiograph with 25° cranial tilt showing ProDisc II lumbar disc replacement L5/S1
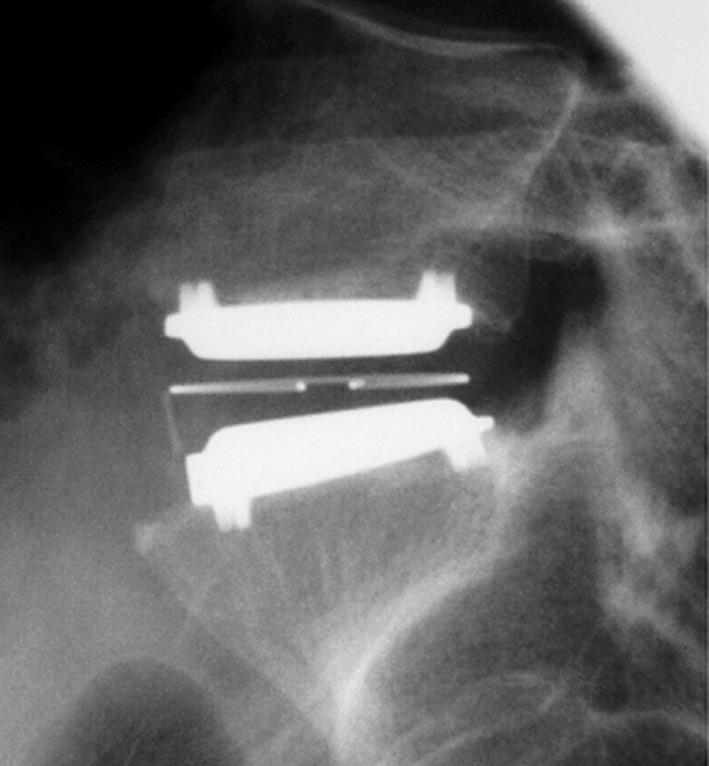
Fraser et al. prospectively evaluated the AcroFlex total disc replacement (DePuy Acromed, Raynham, MA, USA) (Fig. 3a,b) [13]. This implant differed from the low friction devices such as the Charité and the Pro-Disc in that it used an elastomer to replicate the elasticity of the normal human intervertebral disc. The AcroFlex prosthesis consisted of two titanium end plates bound together by a hexane-based polyolefin rubber core. A pilot study was carried out on 28 patients suffering from one- or two-level symptomatic disc degeneration proven on discography. Twenty-four procedures were carried out at a single level (19 at L5–S1 and 5 at L4-5), and four were carried out as double procedures at L4-5 and L5-S1. For the whole group, the ODI improved from 49.3 to 34.4 at 24-month follow-up. The Low Back Outcome Score (LBOS) improved from 17.7 to 33 at 24-month follow-up and there was improvement in five of the eight subscales of the SF-36 general health questionnaire. Complications included nerve root irritation, auto-fusion, partial anterior expulsion, pulmonary embolus and retrograde ejaculation. Of particular concern was the demonstration on fine cut computer tomography of anterior inferior peripheral rubber tears in 36% of patients. Seven patients required revision between 2 and 4 years after disc replacement. In three cases, an anterior revision was performed with removal of the implant, insertion of an interbody fusion device and posterior supplementation with pedicle screws. In two of these three procedures, a tear to the left common iliac vein occurred, which was difficult to repair. In the remaining four, the implant was left in situ and an instrumented postero-lateral fusion was carried out. The discovery of the anterior disruption of the rubber with associated osteolysis led to cessation of the trial and the withdrawal of the AcroFlex total disc replacement from the market. This study illustrates the need for long-term follow-up of all implants in this class.
Fig. 3.
a Lateral radiograph AcroFlex total disc replacement (now discontinued) L5/S1. b Anteroposterior radiograph with 30° cranial tilt. AcroFlex Total Disc Replacement (now discontinued) L5/S1
In 2005 Bertagnoli et al. reported on 25 patients after multi-level disc replacement using the Pro-Disc implant [2]; 10 patients had two-level replacement, and 15 had three-level replacement. The minimum follow-up time was 2 years (range 25–41 months). Sixty-eight per cent had undergone prior posterior spinal decompression at the affected levels. Median Oswestry scores decreased from 65 to 21% in 2 years. The VAS dropped from 8.3 to 2.1 in 2 years. Ninety-six per cent of patients were ‘satisfied’ or ‘completely satisfied’ at 3 months; the percentage dropped to 92% 2 years following surgery. Complications included subsidence, anterior extrusion of a polyethylene component and transient retrograde ejaculation.
Le Huec et al. [22] reported the two-year clinical results of the Maverick (Medtronic Sofamor Danek, Memphis, TN, USA) Lumbar Total Disc Replacement [22]. Sixty-four Maverick devices were implanted between January 2002 and November 2003. The authors noted a degree of improvement equivalent to that obtained with anterior fusion cages using a minimally invasive technique. The ODI improved for 75% patients.
In 2006, Bertagnoli et al. published a study of 20 patients undergoing lumbar disc replacement for adjacent segment degeneration after previous lumbar fusion [3]. The mean ODI dropped from 65.4 to 29.9% at 24 months and the mean VAS for back pain dropped from 7.7 to 3.5 at 2 years. The authors concluded that lumbar disc replacement was an effective treatment modality in this group of patients, but concede that level I evidence is required before this is made a general recommendation.
Systematic review of the literature (level II/III)
Marinus de Kleuver et al. conducted a systematic review of the literature pertaining to total disc replacement for chronic low back pain [9]. The authors employed a ‘Best Evidence Synthesis’ stratifying each study according to the level of Evidence. De Kleuver et al. [9] searched the Cochrane database (2001–2004), MEDLINE (1966–2002) and CINAHL (1982–2001). Studies were ranked from strong to weak according to Shekelle in the following order: RCTs, other controlled trials and non-experimental studies (cohort studies, cross-sectional studies) [34]. The search resulted in 430 references of which nine articles were found to be relevant and fully evaluated. There were no controlled trials comparing intervertebral disc replacement with arthrodesis. The nine articles consisted of six retrospective cohort studies, one cross-sectional study and two prospective cohort studies. Eight studies involved the Charité Total Disc Replacement and one study involved the AcroFlex Total Disc Replacement. A total of 411 patients were represented in these nine articles. The follow-up time was generally short with a mean of 28 months. There was a high rate of secondary arthrodesis observed across all studies. The short-term results (1–68 months) appeared to be comparable to the results of arthrodesis; however, the authors stated that only a very limited number of articles concerning total disc replacement were available. All were non-controlled case series with many methodological flaws. Few of the papers commented on radiological results such as loosening, subsidence, polyethylene wear, maintenance of motion and adjacent level disc degeneration. The authors concluded that despite almost 15 years of clinical application, there was insufficient data to assess the performance of total disc replacement adequately and that total disc replacement should still be considered an experimental procedure.
German and Foley reviewed the literature up to published year 2004 [16]. The search strategy used was not made explicit in the article. The authors discussed papers by device and included interim results of the Food and Drug Administration investigational device exemption studies for the ProDisc II and the SB Charité III implants. The authors concluded that lumbar disc arthroplasty provided similar clinical results to those obtained with interbody fusion at 2 years, but the long-term results of disc arthroplasty remain unknown.
Randomised controlled trials (level I evidence)
Two RCTs comparing total disc replacement with spinal fusion are reported in the literature. The first of these involves the Charité artificial disc [15] and the second involves the ProDisc II total disc replacement [44].
The Food and Drug Administration investigational device exemption multi-centre trial of the Charité artificial disc involved 14 centres across the United States of America [15]. A single participating centre published its early results in two journals [29, 30]. The inclusion criteria for the multi-centre study reported by Geisler et al. listed single-level symptomatic degenerative disc disease either at L4-5 or L5-S1 [15]. Patients were aged between 18 and 60 years. The ODI on entry was greater or equal to 30. The VAS for low back pain was greater or equal to 40 points (on a 100-point scale). All patients had failed conservative measures and had had chronic low back pain for at least 6 months. The randomisation involved a 2:1 schedule in favour of the Charité disc. Two hundred and five patients were randomised to receive the Charité disc and 99 patients were randomised to receive the BAK cage as part of an anterior lumbar interbody fusion using iliac crest autograft. There were no significant intergroup differences at baseline. Neurological status was equivalent between the two groups at 6, 12 and 24 months post-operatively. Major neurological events occurred in 10 (4.9%) of the Charité and 4 (4%) of the BAK fusion group. For the Charité group, the ODI improved from 50.6 preoperatively to 25.8 at 24 months (improvement of 24.8 points). For the BAK fusion group, the ODI improved from 52.1 to 30.1 (an improvement of 22 points). The intergroup change in ODI was not significantly different at 12 or 24 months. The VAS improved from 72 points in the Charité group to 30.6 points at 24 months (improvement of 41.4 points). For the BAK fusion group, the VAS improved from 71.8 to 36.3 points (improvement of 35.5 points). The flexion/extension range of motion in the Charité disc replacement group was a mean of 7.4 ± 5.28° (mean ± standard deviation) whereas in the BAK fusion group, it was 1.1 ± 0.87° (mean ± standard deviation). The authors concluded the Charité intervertebral disc to be a safe and effective treatment for mechanical back pain caused by one-level degenerative disc disease either at L4/5 or L5/S1. The clinical outcomes at 2 years were equivalent to those observed following a single-level anterior interbody fusion using the BAK cage.
Blumenthal et al. reports further on the same cohort of 304 patients [4]. In this paper, the criteria for success include all of the following: greater than 25% improvement in ODI at 24 months, no device failure, no major complications and no neurological deterioration. There was no difference observed between the two groups with respect to operative time, blood loss or level of implantation. The duration of hospital stay was significantly lower in the investigational group (mean of 3.7 days compared to a mean of 4.2 days for the fusion group, p = 0.0039). One hundred and eighty-five patients from 205 (90.2%) patients in the investigational group were followed up to 24 months compared to 82 of 99 (82.8%) patients in the fusion group. At 24 months, the investigational group demonstrated a higher rate of satisfaction (73.7%) compared to the controlled group (53.1% satisfaction, p = 0.0011). All four criteria for clinical success were met in 57.1% of patients in the investigational group and 46.5% in the controlled group (p < 0.0001). For those patients completing 24 months of follow-up, the overall clinical success was reported at 63.6% in the investigational group compared to 56.8% in the controlled group (p = 0.0004). Narcotic usage decreased in both groups at 24 months and there was a 9.2% improvement in employment in the investigational group compared to a 7.4% improvement in the controlled group. The authors conclude that the Charité artificial disc yielded clinical results equivalent to those obtained with anterior lumbar interbody fusion. Significant clinical improvements were observed in the early post-operative period and maintained through the 24-month follow-up period and there were no cases of catastrophic device failure. The Charité artificial disc would appear to be safe and effective for the treatment of single-level lumbar degenerative disc disease either at L4/5 or L5/S1 as an alterative to fusion in properly indicated patients.
Mirza criticises the study for comparing total disc replacement to an operation that has largely been abandoned because surgeons saw it fail frequently firsthand [32]. Few surgeons now perform anterior lumbar interbody fusion with stand-alone cages. Also of concern was the meagre success rate observed in both the artificial disc and the lumbar fusion groups. Disappointingly, only 57% of patients with disc replacement and 46% of those with interbody fusion met all four criteria for success.
McAfee et al. evaluated the radiographic outcomes in both the investigational group and the controlled group [31]. The technical accuracy of the Charité artificial disc replacement was divided into three groups: I, ideal (83%), II, sub-optimal (11%) and III, poor (6%). Those with sub-optimal and poor implant positioning resulted in sub-optimal or poor clinical outcome. The authors reported that total disc replacement with the Charité artificial disc resulted in restoration and maintenance of flexion/extension range of motion 24 months following surgery. The investigational group had significantly better restoration of disc height when compared to the fusion group. The total disc replacement group had significantly less subsidence than the anterior lumbar interbody fusion group. The ideal surgical placement of the Charité artificial disc prosthesis correlated with improved clinical outcomes and improved flexion/extension range of motion compared to poor or sub-optimal surgical placement of the prosthesis.
The second published randomised controlled trial of disc arthroplasty compared the Pro-Disc II with a 360° spinal fusion with both single- and double-level study arms. The study involved 18 sites within the USA and has recently ended [10]. Two sites have reported their preliminary results [10, 11, 42–44]. There are a number of interim results published, with the most recent by Zigler et al. with 54 patients having 1-year follow-up [43]. Significant reductions in ODI and VAS have been reported for both groups. The definitive results from all 18 centres are eagerly awaited.
The National Institute for Clinical Excellence Review
In November 2004, the National Institute for Clinical Excellence published Interventional Procedure Guidance 100 on prosthetic intervertebral disc replacement [20]. The Institute stated that current evidence of the safety and efficacy of prosthetic intervertebral disc replacement appears adequate to support the use of this procedure. However, there is little evidence on outcomes beyond 2–3 years and collection of long-term data is therefore particularly important. The review discusses two studies reporting good or excellent clinical results in 63 (29/46) and 79% (83/105) of patients. The percentage of patients able to return to work was reported to be 67 (31/46) and 87% (91/105), respectively. A third study, however, with 93 patients found no increase in patients returning to work. The same multi-centre study reported on leg pain and found a statistically significant improvement in patients at 12 months compared with baseline. Complication rates in the studies ranged from 16 (8/50) to 45% (9/20). These included implant-related problems such as migration and dislocation. Re-operation rates varied between 3 (3/93) and 24% (12/50). The randomised controlled trial of 304 patients reported major neurological events in 5% (10/205) of patients receiving an artificial disc compared with 4% (99) of patients undergoing spinal fusion [15]. The specialist advisors listed the potential complications as pain, spinal infection, vascular damage and damage to the pre-sacral plexus that may cause retrograde ejaculation in the male.
Conclusions
Total disc replacement with low friction devices incorporating a polyethylene spacer have been used in Europe since 1988 [6]. In 2004, the first RCT comparing the Charité lumbar disc replacement with anterior lumbar interbody fusion demonstrated equivalent clinical outcomes at 2 years [15]. The Charité lumbar disc replacement appears to be safe and effective for the treatment of mechanical low back pain caused by single-level degenerative disc disease at L4/5 or L5/S1 in selected cases. However, the results remain disappointing with only 57% of patients with disc replacement and 46% of those with interbody fusion meeting all four criteria for success [32]. Total disc replacement with the Charité artificial disc results in restoration and maintenance of flexion/extension range of motion 24 months following surgery. Ideal placement of the Charité artificial disc prosthesis appears critical and has been shown to correlate with improved clinical outcome and flexion/extension range of motion [31]. Results of the second randomised controlled trial (Prodisc II versus Fusion) for one- and two-level degenerative disc disease are eagerly awaited.
Results of the elastomeric lumbar total disc replacement (AcroFlex) have been disappointing with implant failure leading to the withdrawal of this device from the market [13]. Future developments with robust compliant artificial discs may address these problems allowing an implant to contribute to shock absorption in the spine.
The long-term benefits of total disc replacement in preventing adjacent level disc degeneration and the role for two- or multi-level disc replacement remain unproven. The role for arthroplasty adjacent to an arthrodesis has yet to be proven. Complications of total disc replacement may not be known for many years. Revision procedures will undoubtedly be difficult with a heightened risk of vascular injury. There are numerous types of disc prostheses and designs under study or in development. Well-designed prospective RCTs will be required before approval and widespread use of this technology.
References
- 1.Bertagnoli R, Kumar S. Indications for full prosthetic disc arthroplasty: a correlation of clinical outcome against a variety of indications. Eur Spine J. 2002;11:S130–S136. doi: 10.1007/s005860100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertagnoli R, Yue J, Shah RV, et al. The treatment of disabling multilevel lumbar discogenic low back pain with total disc arthroplasty utilizing the ProDisc prosthesis. Spine. 2005;30:2192–2199. doi: 10.1097/01.brs.0000181061.43194.18. [DOI] [PubMed] [Google Scholar]
- 3.Bertagnoli R, Yue J, Fenk-Mayer A, et al. Treatment of symptomatic adjacent-segment degeneration after lumbar fusion with total disc arthroplasty by using the ProDisc prosthesis: a prospective study with 2-year minimum follow up. J Neurosurg Spine. 2006;4:91–97. doi: 10.3171/spi.2006.4.2.91. [DOI] [PubMed] [Google Scholar]
- 4.Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomised multi-centre food & drug administration investigational device exemption study of lumbar total disc replacement with the Charité artificial disc versus lumbar fusion. Part I: evaluation of clinical outcomes. Spine. 2005;30:1565–1575. doi: 10.1097/01.brs.0000170587.32676.0e. [DOI] [PubMed] [Google Scholar]
- 5.Buttner-Janz K, Hahn S, Schikora K, et al. Principles for successful application of the Link SB Charité artificial disc. Orthopade. 2002;31:441–453. doi: 10.1007/s00132-001-0297-2. [DOI] [PubMed] [Google Scholar]
- 6.Buttner-Janz K, Schellnack K, Zippel H, et al. Experience and results with the SB Charite lumbar intervertebral endoprosthesis. Z Llin Med. 1988;43:1785–1789. [Google Scholar]
- 7.Cinotti G, David T, Postacchini F. Results of disc prosthesis after a minimum follow-up period of two years. Spine. 1996;21:995–1000. doi: 10.1097/00007632-199604150-00015. [DOI] [PubMed] [Google Scholar]
- 8.David T. Revision of a Charité artificial disc 9.5 years in vivo to a new Charité artificial disc: a case report and explant analysis. Eur Spine J. 2005;14:507–511. doi: 10.1007/s00586-004-0842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleuver M, Oner FC, Jacobs WCH. Total disc replacement for chronic low back pain: background and a systematic review of the literature. Eur Spine J. 2003;12:108–116. doi: 10.1007/s00586-002-0500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delamarter RB, Bae HW, Pradhan BB. Clinical results of ProDisc II lumbar total disc replacement: report from the United States Clinical Trial. Orthop Clin North Am. 2005;36:301–313. doi: 10.1016/j.ocl.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Delamarter RB, David MF, Linda EAK. ProDisc artificial total lumbar disc replacement: introduction and early results from the United States clinical trial. Spine. 2003;28:S167–S175. doi: 10.1097/01.BRS.0000092220.66650.2B. [DOI] [PubMed] [Google Scholar]
- 12.Fairbank J, Frost H, Wilson-MacDonald J, et al. Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: the MRC spine stabilisation trial. Br Med J. 2005;330:1233–1238. doi: 10.1136/bmj.38441.620417.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser RD, Ross ER, Lowery GL, Freeman BJ, et al. Lumbar disc replacement. AcroFlex design and results. Spine J. 2004;4:245S–251S. doi: 10.1016/j.spinee.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Fritzell P, Hägg O, Wessberg P, et al. Volvo award winner in clinical studies: lumbar fusion versus non-surgical treatment for chronic low back pain. A multi-centre randomised controlled trial from the Swedish lumbar spine study group. Spine. 2001;26:2521–2534. doi: 10.1097/00007632-200112010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Geisler FH, Blumenthal SL, Guyer RD, et al. Neurological complications of lumbar artificial disc replacement and comparison of clinical results with those related to lumbar arthrodesis in the literature: results of a multicentre, prospective, randomized investigational device exemption study of Charite intervertebral disc. J Neurosurg (Spine 2) 2004;1:143–154. doi: 10.3171/spi.2004.1.2.0143. [DOI] [PubMed] [Google Scholar]
- 16.German JW, Foley KT. Disc arthroplasty in the management of the painful lumbar motion segement. Spine. 2005;30:S60–S67. doi: 10.1097/01.brs.0000174511.66830.e9. [DOI] [PubMed] [Google Scholar]
- 17.Griffith SL, Shelokov AP, Buttner-Janz K, et al. A multi-centre retrospective study of the clinical results of the link SB Charité intervertebral prosthesis. Spine. 1994;19:1842–1849. doi: 10.1097/00007632-199408150-00009. [DOI] [PubMed] [Google Scholar]
- 18.Huang RC, Girardi FP, Cammisa FP, et al. Correlation between range of motion and outcome after lumbar total disc replacement: 8.6-year follow-up. Spine. 2005;30:1407–1411. doi: 10.1097/01.brs.0000166528.67425.0e. [DOI] [PubMed] [Google Scholar]
- 19.Huang RC, Lim MR, Girardi FP, et al (2004) The prevalence of contraindications to total disc replacement in a cohort of lumbar surgical patients. Spine 29:2538–2541 [DOI] [PubMed]
- 20.Interventional Procedure Guidance 100 (2004) Prosthetic intervertebral disc replacement. National Institute for Clinical Excellence. ISBN: 1-84257-817-0 (www.nice.org.uk)
- 21.Kurtz SM, Peloza J, Sisky R, et al. Analysis of a retrieved polyethylene total disc replacement component. Spine J. 2005;5:344–350. doi: 10.1016/j.spinee.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Le Huec JC, Mathews H, Basso Y, et al. Clinical results of Maverick lumbar total disc replacement: two-year prospective follow-up. Orthop Clin North Am. 2005;36:315–322. doi: 10.1016/j.ocl.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Lemaire JP, Skalli W, Lavaste F, et al. Intervertebral disc prosthesis: results and prospects for the year 2000. Clin Orthop Relat Res. 1997;337:64–76. doi: 10.1097/00003086-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Lemaire JP, Carrier H, et al. Clinical and radiological outcomes with the Charité artificial disc. A 10-year minimum follow-up. J Spinal Disord Tech. 2005;18:353–359. doi: 10.1097/01.bsd.0000172361.07479.6b. [DOI] [PubMed] [Google Scholar]
- 25.Lui J, Ebraheim NA, Haman SP, et al. Effect of the increase in the height of lumbar disc space on facet joint articulation area in sagittal plane. Spine. 2006;31:E198–E202. doi: 10.1097/01.brs.0000206387.67098.a0. [DOI] [PubMed] [Google Scholar]
- 26.Mayer HM, Wiechert K, Korge A, et al. Minimally invasive total disc replacement: surgical technique and preliminary clinical results. Eur Spine J. 2002;11:S124–S130. doi: 10.1007/s00586-002-0446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer HM. Total disc replacement. J Bone Joint Surg [Br] 2005;87-B:1029–1037. doi: 10.1302/0301-620X.87B8.16151. [DOI] [PubMed] [Google Scholar]
- 28.Mathew P, Blackman M, Redla S, et al. Bilateral pedicle fractures following anterior dislocation of the polyethylene inlay of a ProDisc artificial disc replacement: a case report of an unusual complication. Spine. 2005;30:E311–E314. doi: 10.1097/01.brs.0000164135.03844.b6. [DOI] [PubMed] [Google Scholar]
- 29.McAfee PC, Fedder IL, Saiedy S, et al. Experimental design of total disk replacement: experience with a prospective randomized study of the SB Charite. Spine. 2003;28:S153–S162. doi: 10.1097/01.BRS.0000092217.34981.E1. [DOI] [PubMed] [Google Scholar]
- 30.McAfee PC, Fedder IL, Saiedy S, et al. SB Charite disc replacement. Report of 60 prospective randomized cases in a US Centre. J Spinal Disord Tech. 2003;4:424–433. doi: 10.1097/00024720-200308000-00016. [DOI] [PubMed] [Google Scholar]
- 31.McAfee PC, Cunningham B, Holsapple G, et al. A prospective, randomised, multi-centre food and drug administration investigational device exemption study of total lumbar disc replacement with the Charité artificial disc versus lumbar fusion. Part II: evaluation of radiographic outcomes and correlation of surgical technique accuracy with clinical outcomes. Spine. 2005;30:1576–1583. doi: 10.1097/01.brs.0000170561.25636.1c. [DOI] [PubMed] [Google Scholar]
- 32.Mirza SK. Point of view: commentary on the research reports that led to food and drug administration approval of an artificial disc. Spine. 2005;30:1561–1564. doi: 10.1097/01.brs.0000171806.30401.40. [DOI] [PubMed] [Google Scholar]
- 33.Möller H, Hedlund R. Surgery versus conservative management in adult isthmic spondylolisthesis. Spine. 2000;25:1711–1715. doi: 10.1097/00007632-200007010-00016. [DOI] [PubMed] [Google Scholar]
- 34.Shekelle PG, Woolf SH, Eccles M, et al. Clinical guidelines; developing guidelines. BMJ. 1999;318:593–596. doi: 10.1136/bmj.318.7183.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shim CS, Lee S, Maeng DH, et al. Vertical split fracture of the vertebral body following total disc replacement using ProDisc: a report of two cases. J Spinal Disord Tech. 2005;18(5):465–469. doi: 10.1097/01.bsd.0000159035.35365.df. [DOI] [PubMed] [Google Scholar]
- 36.Steiber JR, Donald GD. Early failure of lumbar disc replacement: case report and review of the literature. J Spinal Disord Tech. 2006;19(1):55–60. doi: 10.1097/01.bsd.0000163414.53732.a3. [DOI] [PubMed] [Google Scholar]
- 37.Tropiano P, Huang RC, Girardi FP, et al. Lumbar disc replacement: Preliminary results with ProDisc II after a minimum follow-up period of one year. J Spinal Disord Tech. 2003;4:362–368. doi: 10.1097/00024720-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Tropiano P, Huang RC, Girardi FP, et al. Lumbar total disc replacement. Seven to eleven year follow-up. J Bone Joint Surg Am. 2005;87A:490–496. doi: 10.2106/JBJS.C.01345. [DOI] [PubMed] [Google Scholar]
- 39.Tropiano P, Huang RC, Girardi FP, et al. Lumbar total disc replacement: surgical technique. J Bone Joint Surg Am. 2006;88:50–64. doi: 10.2106/JBJS.E.01066. [DOI] [PubMed] [Google Scholar]
- 40.Ooij A, Oner FC, Verbout AJ. Complications of artificial disc replacement; a report of 27 patients with the SB Charité disc. J Spinal Disord Tech. 2003;16:369–383. doi: 10.1097/00024720-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Zeegers WS, Bohnen LMLG, Laaper M, et al. Artificial disc replacement with the modular type SB Charité III: two-year results in 50 prospectively studied patients. Eur Spine J. 1999;8:210–217. doi: 10.1007/s005860050160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zigler JE. Clinical results with ProDisc: european experience and US Investigation Device Exemption Study. Spine. 2003;28:S163–S166. doi: 10.1097/00007632-200310151-00009. [DOI] [PubMed] [Google Scholar]
- 43.Zigler JE. Lumbar spine arthroplasty using the Pro-Disc II. Spine J. 2004;4:260S–267S. doi: 10.1016/j.spinee.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 44.Zigler JE, Burd TA, Vialle EN, et al. Lumbar spine arthroplasty. Early results using the ProDisc II: a prospective randomized trial of arthroplasty versus fusion. J Spinal Disord Tech. 2003;4:352–361. doi: 10.1097/00024720-200308000-00007. [DOI] [PubMed] [Google Scholar]