Abstract
Smith and Nephew (Endoscopy division, Andover, MA, USA) have estimated that 60,000 Intra-Discal Electrothermal Therapy (IDET) procedures have been performed world wide up to June 2005. Despite the large number of procedures performed, a critical appraisal of the evidence of efficacy of IDET has not appeared in the literature. This paper reviews the current evidence of clinical efficacy for IDET obtained via a systematic review of the literature. Studies were included if they used at least one of four specified primary outcome measures; pain intensity as assessed by a visual analogue score (VAS), global measurement of overall improvement, back specific functional status such as Oswestry disability Index (ODI) and return to work. Levels of evidence were assigned according to the hierarchy described by the Oxford Centre for Evidence-Based Medicine (www.cebm.net). Papers addressing possible mechanisms of action of IDET were not considered as the focus of the literature review was clinical effectiveness. Eleven prospective cohort studies (level II evidence) were reported on a total of 256 patients with a mean follow-up of 17.1 months (range 12–28 months). The mean improvement in the VAS for back pain was 3.4 points (range 1.4–6.5) and the mean improvement in ODI was 5.2 points (range 4.0–6.4). A total of 379 patients were reported in five retrospective studies (level III evidence). Between 13 and 23% of patients subsequently underwent surgery for low back pain within the study period. Two randomised controlled trials of IDET have been reported in the literature. The first randomised 64 patients (37 to IDET, 27 to Sham). The advantage for IDET patients amounted to 1.3 points on the VAS and seven points on the ODI. The second study randomised 57 subjects (38 to IDET, 19 to Sham) and showed no benefit from IDET over placebo. The evidence for efficacy of IDET remains weak and has not passed the standard of scientific proof.
Keywords: Intradiscal electrothermal therapy, Clinical outcome, Evidence-based medicine
Introduction
Saal and Saal [24] introduced a new method for the treatment of chronic discogenic low back pain using intra-discal electrothermal therapy (IDET). The technique involved placement of a navigable intradiscal catheter (Fig. 1) with a temperature controlled thermal resistive heating coil to a final position at the inner posterior annulus (Figs. 2a, b). The standard heating protocol raised the catheter tip temperature from 65 to 90°C over 12.5 min. The temperature was maintained at 90°C for 4 min. According to Saal and Saal this created annular temperatures of 60–65°C [24].
Fig. 1.
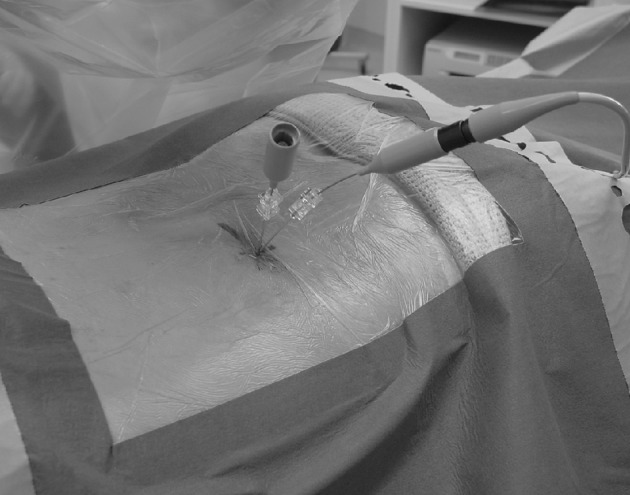
Clinical photograph. Patient prone position. Note 17-guage introducer needles positioned in L4/5 and L5/S1 disc. IDET catheters have been placed with the L4/5 IDET catheter connected to the generator
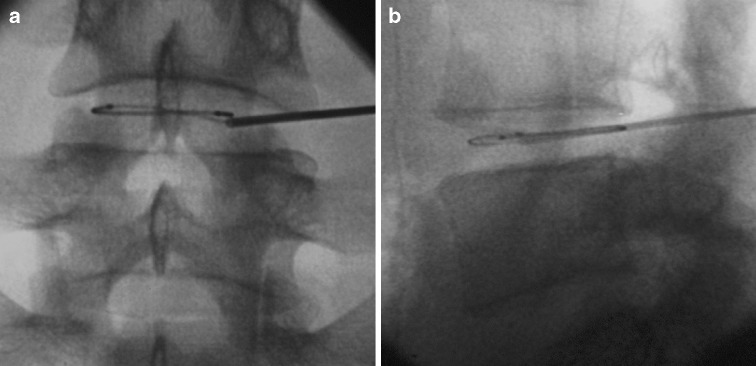
Fig. 2.
a Antero-posterior and b lateral radiograph showing final position of IDET catheter in the L4/5 disc
The authors proposed the mechanism of action of IDET to be a combination of thermo-coagulation of native nociceptors and in-grown un-myelinated nerve fibres plus annular collagen shrinkage stabilising annular fissures [24]. The post-procedural care allowed patients to walk and perform low intensity leg stretches for the first month, to resume stabilisation floor exercises by the end of the second month, and to increase the intensity of exercise at the end of the third month.
Since the food and drug administration (FDA) clearance of the procedure in March 1998, more than 40,000 IDET procedures have been performed on patients throughout the world up to 2003 [28]. Smith and Nephew (Endoscopy division, Andover, MA, USA) have estimated that 60,000 IDET procedures have been performed world wide up to June 2005. The average cost per disc is $8,000 including charges for the facility, equipment and surgeon [24]. Despite the large number of procedures performed, a critical appraisal of the evidence of efficacy has not been published in the literature.
Materials and methods
A broad search of the literature was carried out using the Cochrane Library database 2006, Medline 1996 to January 2006 and PubMed to January 2006. The search strings included ‘IDET’, ‘clinical outcome’ and ‘complications’. A total of 57 records were identified. The abstracts were reviewed and where relevant the full paper was obtained. Prospective randomised controlled trials, retrospective and prospective cohort studies were included to increase the yield of information to enable a ‘best evidence synthesis’. Studies were only included if they used at least one of four specified primary outcome measures; pain intensity as assessed by a visual analogue score (VAS), global measurement of overall improvement, back specific functional status such as Oswestry disability Index (ODI) and return to work. Levels of evidence were assigned according to the hierarchy described by the Oxford Centre for Evidence-Based Medicine (www.cebm.net) [22]. Papers dealing with the possible mechanism of action of IDET were rejected as the focus of the literature review was to assess the clinical effectiveness of IDET.
Prospective cohort studies (level of evidence IIb)
Early studies were promising with 80% of patients reporting a reduction of at least two points on the VAS for low back pain and 72% reporting an improvement in sitting tolerance and reduction in analgesic requirement [24]. Improvements in physical function and bodily pain subsets of the Short-Form 36 (SF-36) were also noted. Patients working prior to the procedure returned to work within 5 days of the procedure.
Saal and Saal [25] subsequently reported the one-year outcome of an expanded cohort of 62 patients undergoing IDET. Between November 1997 and October 1998, 62 from 1,116 patients treated by the authors did not improve adequately after a minimum of 6 months of conservative care. All 62 patients were offered long-term pain management, fusion surgery or IDET. All 62 chose to undergo IDET. The mean age was 41 (range 21–58). Thirty-nine patients were private payers and 23 were receiving worker’s compensation. The mean duration of pre-operative symptoms was 60 months (range 10 months to 17 years). Twenty of 39 private-paying patients were working; none of the 23 worker’s compensation patients were working. Thirty patients were treated at one disc level and 32 patients at two or more disc levels. The VAS improved a mean of 3.0 points for the whole group. For the single-level patients the mean improvement in VAS was 3.4 points, but for the multi-level patients the mean improvement was only 2.6 points. Twelve of 62 patients did not show any improvement in VAS. For the whole group, the physical function score of the SF-36 improved 20 points (23.6 points for the single-level group and 17 points for the multi-level group). For the bodily pain score of the SF-36, the mean improvement for the whole group was 17.4 points (16.8 points for the single level group and 18.0 points for the multi-level group). Six of 62 patients did not improve in either physical function or bodily pain subscales of the SF-36. Three of 62 patients underwent an epidural injection during the first 8 weeks following the procedure. Two of 62 (3.2%) patients underwent fusion surgery 1 year following IDET. There was no significant difference in clinical outcome between private paying and worker’s compensation patients. For patients with decreased disc height (more than 30% loss of disc height) who had treatment at multiple levels, the outcome was less favourable than those with multi-level treatment who had preserved disc height.
Saal and Saal [26] subsequently reported on the 24-month outcome in the same cohort. The mean VAS dropped from 6.57 to 3.41 (improvement of 3.16 points). The sitting time increased on average by 52.7 min. The mean physical function score of the SF-36 improved 31.33 points and the mean bodily pain score improved 21.87 points. Eighty-one per cent of patients showed at least a 7.0-point improvement in physical function and 78% improved at least 7.0-point improvement in bodily pain. Clinical improvement continued over 2 years and was comparable in one, two or three-level treated disease.
Derby et al. [8] reported 32 consecutive cases of IDET. All patients were initially assessed by an orthopaedic surgeon and told either they were not suitable for spine surgery or were offered surgery and declined. Outcome measures included the Roland–Morris disability questionnaire, the VAS, a patient satisfaction index and a questionnaire related to activities of daily living. The mean age of participants was 42 years, with only four workers’ compensation cases. Seven patients had previous surgery. Derby treated both discrete annular fissures and global disc degeneration. The mean improvement in VAS was 1.84 (SD ± 2.38). The mean improvement in Roland–Morris was 4.03 (SD ± 4.82). There was no significant change in outcome measures at 6 and 12 months. Overall, 62.5% had a favourable outcome, 25% no change and 12.5% had a non-favourable outcome. One patient underwent a spine fusion due to persistent discogenic back pain.
Karasek and Bogduk [16] reported 12-month follow-up of a controlled trial of IDET for back pain due to internal disc disruption. From 110 patients undergoing CT discography, 53 satisfied the criteria for internal disc disruption at one or two levels. Authority to undergo IDET was sought from the insurance carriers of these patients. Authority was granted in 36 and denied in 17. The 36 patients constituted the index treatment group and underwent IDET followed by rehabilitation. The 17 patients constituted a ‘convenience sample control group’ and underwent rehabilitation. Outcome measures included the VAS, return to work, use of opioid analgesics and ODI in some patients. The control group were followed for 3 months: the median VAS was eight (range 5–8) before rehabilitation and eight (range 7–8) at 3 months. The IDET group had a median VAS of eight (range 7–9) before treatment reducing to three (range 1–7) at 12 months. Some patients returned to work and reduced their opioid intake.
Bogduk and Karasek [3] subsequently reported on the 24-month follow-up in the IDET group. Fifty-four per cent of patients reduced their pain by half, with one in five patients achieving complete relief of pain. The authors concluded that IDET relieved discogenic pain, had a success rate of between 20 and 60% and was superior to physical rehabilitation. The use of patients who had been denied treatment as a ‘control group’ raises serious methodological flaws in this paper.
Gerszten et al. [13] studied 27 patients following IDET . Eight had private insurance and nine had received workers’ compensation. Sixteen patients underwent IDET at one level and 11 patients underwent IDET at two or more levels. The mean duration of symptoms was 38 months and the follow-up was 12 months. Outcome measures included quality of life as assessed by the SF-36 and disability as measured by the ODI. The physical function score of the SF-36 improved from a baseline of 32 to 47 points at final follow-up. The bodily pain score of the SF-36 improved from 27 to 38 at final follow-up. The ODI improved from 34 at baseline to 30 points at final follow-up. The authors noted that at 1 year 45% of patients reported a significant improvement on the SF-36 survey and that 75% of patients had improvement of their symptoms following IDET. Pain was not measured in this study. The authors found no relationship between outcome and the number of levels treated, the duration of symptoms or workers’ compensation status.
Spruit and Jacobs [29] reported on pain and function after IDET for symptomatic lumbar disc degeneration in a cohort of 20 patients. The mean VAS improved by 1.4 points (p = 0.046), but the individual scores showed great variation. The ODI did not improve significantly. The SF-36 showed improvement, but only for the subscales vitality (p = 0.023) and bodily pain (p = 0.047). The authors concluded that IDET was not effective in reducing pain and improving functional performance.
Lutz et al. [18] treated 33 patients with chronic constant lumbar discogenic pain of more than 6 months with IDET. The mean age was 40 years and the mean duration of symptoms was 46 months. The mean follow-up period was 15 months. The mean VAS improved from 7.5 to 3.9 (p < 0.001) and a mean improvement in the Roland–Morris disability questionnaire of 7.3 points was noted (p < 0.001). With regard to patient satisfaction, 75.7% reported that they would undergo the same procedure for the same outcome. Complete pain relief was achieved in 24% of the patients and partial pain relief in 46% of the patients.
Park et al. [20] conducted a prospective study of 25 patients undergoing IDET. All subjects had concordant pain on discography at a single level. Those with 50% or more disc height loss and those with outstanding worker’s compensation cases were excluded. One year after IDET, 21 of 25 patients complained of lingering back pain. The mean VAS dropped from 7.3 to 4.9 points. Eight patients (32%) reported more pain following the IDET procedure, 14 patients (56%) reported less pain and three patients (12%) reported no change. Twelve patients (48%) were satisfied with IDET, 11 patients (44%) were dissatisfied and two (8%) were undecided. Within 1 year of IDET, 5 of the 25 patients (20%) had undergone spinal fusion, one for iatrogenic discitis.
Kapural et al. [15] carried out a non-randomised comparison of IDET with Intradiscal Radio-Frequency Ablation (RFA). The authors reported the 12 month outcome for each cohort (21 patients in each group). For the IDET group, the mean VAS dropped from 7.9 to 1.4 compared to 6.6 to 4.4 for the RFA group. Larger improvements in the Pain Disability Index were noted in the IDET group.
Table 1 shows a summary of the clinical outcome following IDET for these prospective cohort studies. From a total of 256 patients with a mean follow-up of 17.1 months (range 12–28 months), the mean improvement in the VAS was 3.4 points (range 1.4–6.5) and the mean improvement in ODI 5.2 points (range 4.0–6.4).
Table 1.
Summary of clinical outcome following IDET: prospective cohort studies (level of evidence IIb)
| Authors | Study design | Subjects (loss to FU) | Follow-up (range) | Outcomes measured | Results (Δ) |
|---|---|---|---|---|---|
| Saal and Saal [24] | Prospective cohort | 25 | 7 months | VAS score | 7.3→3.6 (3.7) |
| SF-36 (PF) | 40.1→55.2 (15.1) | ||||
| SF-36 (BP) | 28.5→42.2 (13.7) | ||||
| Saal and Saal [25] | Prospective cohort | 62 | 16 months | VAS score | 6.6→3.7 (2.9) |
| SF-36 (PF) | 39→59 (10) | ||||
| SF-36 (BP) | 29→46.2 (17.2) | ||||
| Saal and Saal [26] | Prospective cohort | 62 (4) | 28 months (24–35) | VAS score | 6.57→3.41 (3.2) |
| SF-36 (PF) | 40.5→71.8 (31.3) | ||||
| SF-36 (BP) | 29.8→51.7 (21.9) | ||||
| Derby et al. [8] | Prospective cohort | 32 | 12 months | VAS score | (−1.84, SD 2.38) |
| Roland–Morris | (−4.03, SD 4.82) | ||||
| Karasek and Bogduk [16] | Prospective quasi-controlled | n = 53 | 12 months | VAS (median) | IDET: 8→3 (5) |
| n = 36 IDET | Control: 8→8 (0) | ||||
| n = 17 controls | Success rate | IDET: 20–60% | |||
| Bogduk and Karasek [3] | Prospective quasi-controlled | n = 53 (5) | 24 months | VAS (median) | IDET: 8→3 (5) |
| n = 36 IDET | Control: 8→7.5 (0.5) | ||||
| n = 17 controls | Success rate | IDET: 54% reduce pain by half | |||
| Gerszten et al. [13] | Prospective cohort | 27 | 12 months | ODI | 34→30 (4.0) |
| SF-36 (PF) | 32→47 (15) | ||||
| SF-36 (BP) | 27→38 (11) | ||||
| Spruit and Jacobs [29] | Prospective cohort | 20 (1) | 12 months | VAS | 6.54→5.06 (1.48) |
| ODI | 43.1→36.7 (6.4) | ||||
| Lutz et al. [18] | Prospective cohort | 33 | 15 months | VAS | 7.5→3.9 (3.6) |
Retrospective cohort studies (level of evidence IIIb)
Freedman et al. [11] reported on his experience with IDET for the management of chronic discogenic low back pain in active-duty soldiers. Forty-one active-duty soldiers underwent IDET for chronic discogenic low back pain unresponsive to non-operative therapy. During the study period, 36 of 41 patients underwent a single trial of IDET and the remaining five underwent two trials of IDET. At the time of analysis 31 from 36 patients had reached final follow-up (mean 29.7 months, range 24–46). Success was defined as a 50% decrease in pain from baseline. The success rate was 47% (17 of 36) at 6 months and 16% (5 of 31 patients) at latest follow-up. Fifty-two per cent of patients had a two-point or greater decrease in the VAS for pain. Nineteen of 31 soldiers (61%) remained on active-duty at a minimum of 24 months after IDET. Seven of 31 soldiers (23%) went onto spinal surgery within 24 months of failed IDET. The authors conclude that IDET is not a substitute for spinal fusion in the treatment of chronic discogenic pain. They consider it at best an antecedent rather than an alternative to spinal fusion.
Cohen et al. [5] carried out a retrospective analysis on 79 patients undergoing IDET for discogenic low back pain. Forty-eight per cent of patients reported more than 50% pain relief at 6 months. The authors divided the cohort into those in whom a positive outcome occurred (n = 38, 48%) and those in whom a negative outcome occurred (n = 41, 52%) For those with a positive outcome the VAS dropped from 5.9 to 2.1, a mean change of 3.8 points and for those patients with a negative outcome the VAS dropped from 6.2 to 5.1, an improvement of only 1.1 points. The complication rate was 10% including transient radicular pain and one case of ipsilateral foot drop. Only one of ten obese patients had successful IDET. The authors suggest that obesity should be considered a relative contraindication to IDET.
Lee et al. [17] studied 62 patients recruited from an academic affiliated private physiatric practice. Fifty-one patients were available with a minimum follow-up of 24 months. The mean age was 41.4 years (18–60 years) and the average duration of symptoms was 46 months (range 6–180 months). Clinical improvement was defined as a change of more than two points on the pain scale and the Roland–Morris scale. There was a statistically significant improvement in lower back pain scores, Roland–Morris scores and lower extremity pain scores. On the North American Spine Society (NASS) patient satisfaction index, 63% (32/51) responded positively and would undergo the procedure again. Seven patients (14%) underwent additional therapeutic procedures during the follow-up period. Two from 51 patients underwent a spinal fusion.
Davis et al. [7] carried out a retrospective study with independent evaluation of patient outcomes 1 year following IDET. The outcome assessment consisted of a telephone interview and completion of a self-administered questionnaire. The mean age was 40 years (range 25–64 years). Responses were received from 44 of 60 (73%) patients. Six patients had undergone lumbar surgery within 1 year of IDET. Their outcomes were excluded from analysis. Ninety-seven per cent of patients continued to have back pain; 29% reported more pain post-IDET, 39% had less pain and 29% reported no change. Fifty per cent were dissatisfied with IDET, 37% were satisfied and 13% were undecided. The authors concluded at one-year post-IDET, half the patients remained dissatisfied with their outcome and the estimated proportion of patients undergoing fusion was predicted to be 15% at 1 year and 30% at 2 years.
Webster et al. [30] investigated the outcome of workers’ compensation claimants post-IDET. The authors identified 142 cases from 23 states treated by 97 different healthcare providers. The mean follow-up was 22 months. Ninety-six (68%) of the cases did not meet one or more of the published inclusion criteria. Fifty-three of 142 cases (37%) had at least one lumbar injection and 32 of 142 cases (23%) had lumbar surgery after IDET. The authors concluded that the procedure may be less effective when performed by a variety of providers compared to the initial case series performed by single providers.
A total of 379 patients were reported in these five retrospective studies (level of evidence IIIb) [22] between 2003 and 2004 (Table 2). The mean follow-up was 20.7 months (range 6–29.7). Between 13 and 23% of patients subsequently underwent surgery for low back pain within the designated study period.
Table 2.
Summary of clinical outcome following IDET: retrospective cohort studies (level of evidence IIIb)
| Authors | Study design | Subjects (loss to FU) | Follow-up (range) | Outcomes measured | Results (Δ) |
|---|---|---|---|---|---|
| Freedman et al. [11] | Retrospective cohort | 36(5) | 29.7 months (24–46) | VAS | 52% had > 2.0 improvement |
| Success | 5/31 (16%) | ||||
| Surgery | 23% underwent surgery within the FU period | ||||
| Cohen et al. [5] | Retrospective cohort | 79 | 6 months | VAS | Positive outcome 5.9→2.1 (3.8) |
| Negative outcome 6.2→5.1 (1.1) | |||||
| Success | 48% reduce pain by half | ||||
| Lee et al. [17] | Retrospective cohort | 62 (11) | 34 months (6–47) | VAS | >2.0 points |
| Satisfaction | 63% satisfied and would have procedure again | ||||
| Davis et al. [7] | Retrospective cohort | 60 (16) | 12 months | Satisfaction | 50% dissatisfied |
| 37% satisfied | |||||
| 13% undecided | |||||
| Employment | 16 employed pre-IDET | ||||
| Status | 11 employed post IDET | ||||
| Surgery | 6/44 (13.6%) underwent surgery within the FU period | ||||
| Webster et al. [30] | Retrospective cohort | 142 | 22 months | Narcotic usage | Unchanged following IDET |
| Surgery | 32/142 (22.5%) underwent surgery within FU period |
Randomised controlled trials (level of evidence Ib)
Three randomised controlled trials of percutaneous intradiscal thermo-coagulation for chronic discogenic low back pain have been published. The first reports on percutaneous intradiscal radio-frequency thermo-coagulation (PIRFT) (Radionics, Burlington, MA, USA) and heats the disc up to 70°C for 90 s [2]. The remaining two studies use IDET (Smith and Nephew, Andover Mass, MA, USA) [12, 21]. IDET uses a thermal resistive coil to deliver heat to the disc, raising the temperature of the catheter tip to 90°C over 12.5 min and maintaining for 4 min. In that respect the two techniques are different and it not appropriate to compare the study presented by Barendse et al. [2] with that of Freeman et al. [12] and Pauza et al. [21].
Barendse et al. conducted a randomised controlled trial of percutaneous PIRFT for chronic discogenic back pain [2]. Outcome measures included VAS, the global perceived effect by the patient, ODI, the Dartmouth COOP functional health assessment chart and a Quality of Life questionnaire. Questionnaires were completed before treatment and 8 weeks following treatment. The radio-frequency lesion of the disc was performed using a radio-frequency probe (Radionics, Burlington, MA, USA). Patients were randomised to receive a 90-s 70°C lesion if allocated to the ‘lesion’ group or no lesion if allocated to the ‘sham’ group. From a total of 287 patients with chronic non-specific low back pain, 28 were recruited according to the inclusion and exclusion criteria. Success was defined as a reduction of at least two points on the VAS and a 50% improvement on the global perceived effect. Fifteen patients were allocated to the sham group, 13 were allocated to the lesion group. Eight weeks following treatment there were two treatment successes in the ‘sham group’ and one in the ‘lesion group’. There were no statistically significant differences in the secondary outcome measures between both groups. Eight weeks after treatment VAS, global perceived effect and ODI showed no significant differences between the two groups. The authors concluded that PIRFT was not effective in reducing chronic discogenic low back pain.
Pauza et al. [21] reported a randomised placebo-controlled trial of IDET for the treatment of chronic discogenic low back pain. Inclusion criteria listed age between 18 and 65 years, low back pain more than leg pain for at least 6 months, failure to improve after 6 weeks of non-operative care (including anti-inflammatory and analgesic medication and physical therapy and/or a home directed exercise programme), low back pain exacerbated by sitting or standing, Beck depression index of less than 20 points, less than 20% loss of disc height on plain radiographs and the presence of a posterior tear of the annulus fibrosus on CT discography. Exclusion criteria listed previous lumbar fusion, spondylolisthesis, spinal stenosis, scoliosis, disc herniation of more than 4 mm, workers compensation, injury litigation and disability remuneration. Also excluded were those with diffuse changes on CT discography.
All patients received conscious light sedation and placement of the 17-guage introducer needle down to the outer aspect of the annulus fibrosus. At this point, the randomisation schedule was revealed to the principle investigator. Randomisation employed a 3:2 ratio (3 IDET : 2 sham). For those randomised to active treatment the intradiscal catheter was positioned to provide complete coverage of the posterior annulus and the standard heating protocol was followed. After treatment was completed the electrode was withdrawn and 1 ml of 0.75% bupivicaine was injected into the disc. For those undergoing sham treatment, the introducer needle remained in position and the patient was exposed to a fluoroscope monitor showing passage of an intradiscal catheter and manufactured generator noises for the full 16.5 min to mimic an active treatment. Both groups underwent a monitored post-operative rehabilitation involving a lumbar corset for 6 weeks followed by a lumbar stabilisation programme for a further 6 weeks. Outcomes assessed included VAS, SF-36 and ODI, prior to treatment and 6 months after treatment.
Publicising the study attracted enquiries from 4,253 people. From 1,360 individuals who were prepared to submit to randomisation, 260 (19.1%) were found potentially eligible after clinical examination and 64 became eligible after discography (4.7%). Thirty-seven were allocated to IDET and 27 to sham treatment. After treatment, eight patients (12.5%) violated the prescribed protocol mandating rejection from the analysis leaving a total of 56 patients: 32 from the IDET group: 24 from the sham group.
Both groups exhibited significant improvement in the VAS, but improvements in the IDET group were significantly greater than the sham group (p = 0.045). For patients in the IDET group, the mean VAS dropped from 6.6 to 4.2, mean improvement 2.4, SD 2.3) and the ODI dropped 31 to 20 (mean 11 points, SD 11). For patients in the sham group, the mean VAS dropped from 6.5 to 5.4, mean improvement 1.1, SD 2.6) and the mean ODI dropped from 33 to 28 (mean four points, SD 12). Taking this into context, the ‘advantage’ for IDET patients over Sham patients was 1.3 points on the VAS (p = 0.045) and seven points on the ODI (p = 0.05). There were no significant differences in the SF-36 subsets bodily pain or physical function between the two groups. However ‘mean scores’ can hide individual scores. IDET was not a universally successful treatment in this study; some 50% of patients did not benefit appreciably or at all. Only 40% of patients treated with IDET achieved greater than 50% relief of pain.
Freeman et al. [12] conducted a prospective, randomised, double-blind, placebo-controlled trial with crossover offered to the placebo subjects when un-blinding occurred at 6 months. A total of 57 subjects were enrolled without inducement according to the inclusion and exclusion criteria listed below. Subjects were selected from consecutive patients from the routine practices of three consultant spinal surgeons in a large city. All subjects had chronic discogenic low back pain, marked functional disability, evidence of degenerative disc disease on magnetic resonance scan and subsequently failed conservative management. To successfully enrol all subjects had one or two-level symptomatic disc degeneration as determined by provocative lumbar discography followed by post-discography computed tomography to delineate the internal disc disruption. The study adopted a 2:1 (IDET: Placebo) randomisation schedule. From the total of 57 subjects, 38 were randomised to IDET and 19 to placebo (sham treatment). All procedures were carried out under light neuroleptic anaesthesia and local anaesthesia. A 17-gauge introducer needle was used employing a standard posterolateral approach to the symptomatic disc under multi-plane fluoroscopic guidance. The intradiscal catheter was navigated to cover at least 75% of the posterior (interpedicular) annulus or at least 75% of the annular tear as defined by the post-discography CT scan. Once a satisfactory position was obtained in the antero-posterior, lateral and Ferguson views the catheter was connected to a lead and passed to an independent technicians. The technician then opened a sealed envelope to ascertain the randomisation schedule and covertly either connected the catheter to the generator (active IDET group) or did not (sham placebo group). Critically both surgeon and subject were blinded to this step. The generator was switched on and the standard heating protocol commenced at 65°C rising over 12.5 min to 90°C and held for 4 min. All subjects followed a common rehabilitation programme including Pilates-based exercises.
Subjects were reviewed at 6 weeks and 6 months by an independent third party to minimize investigator bias. Outcome measures recorded at baseline and 6 months included the VAS for back pain, the Low Back Pain Outcome Score (LBOS), the ODI, the SF-36 General Health questionnaire (Australian version), the Zung Depression Index (ZDI), the Modified Somatic Perception Questionnaire (MSPQ), sitting tolerance, work tolerance, medication and the presence of any neurological deficit.
Successful outcome was defined as one demonstrating the following: No neurological deficit resulting from the procedure, an improvement in the LBOS of seven or more points, and an improvement in the SF-36 subscales of bodily pain and physical functioning of greater than one standard deviation from the mean. The mean clinically important difference in secondary outcome measures was set at 2.0 points for the VAS (back pain), 10 points for the ODI and 8.0 points for the ZDI. Using the 2:1 allocation for active and control treatment in order to have 80% power the study required 50 patients to be treated with IDET and 25 with the sham treatment. Enrolment of subjects was slower than anticipated with only 57 patients enrolled after 25 months. Following advice from the ethics committee, the study was halted and an independent statistical analysis carried out.
The 2:1 (IDET: Placebo) randomisation produced two groups (38 IDET: 19 Placebo) were well matched LBOS, ODI, SF-36, Zung Depression Index and MSPQ scores. After treatment, two subjects (both from the IDET group) from 57 (3.5%) violated the prescribed protocol mandating their rejection from analysis. One was considered a technical failure and was withdrawn from the study at the six-week follow-up. The second subject experienced increased low back pain, withdrew from the study at 3 months and subsequently underwent a spinal fusion. No subject in either treatment arm met the joint criteria for ‘success’. Hence the specified primary analysis showed no difference between the treatments. Secondary outcomes were compared at baseline and 6 months. These included comparisons of change at 6 months in LBOS, ODI, Zung Depression Inventory, MSPQ and SF-36 scores. The mean LBOS for the IDET group was 39.51 at baseline and 38.31 at 6 months. The mean LBOS for the placebo group was 36.71 at baseline and 37.45 at 6 months. The mean ODI for the IDET group was 41.42 at baseline and 39.77 at 6 months. The mean ODI for the placebo group was 40.74 at baseline and 41.58 at 6 months.
The following subgroups were analysed: Males only, those with ‘adequate treatment of the tear’ as assessed by Dr. Saal, those with psychological impairment, those not taking narcotic medication at baseline, those not taking eight or more Panadeine Forte tabs per day at baseline, those with single level treatment for an annulus tear without global degeneration, those with single level treatment for an annulus tear without global degeneration, taking no analgesics at baseline and with ‘adequate treatment of tear’ as assessed by Dr. Saal. These detailed secondary analyses showed no statistically significant or clinicallyimportant differences in the measured study outcomes for either treatment. This was true irrespective of whether the comparison was further adjusted for the baseline measure. A further stratified analysis by surgeons conducting the IDET procedure showed no significant difference in secondary endpoints between treatment arms for any surgeon. There were no serious adverse events in either arm of the study. Transient radiculopathy (< 6 weeks) was reported in four subjects who underwent IDET and in one subject who underwent the sham procedure.
Pauza et al. [21] concluded that IDET is ‘an effective treatment for discogenic low back pain’. However there was modest overall benefit and some patients did not benefit at all. On the other hand Freeman et al. [12] showed no significant benefit from IDET over placebo. How can two similarly sized randomised controlled trials show such different results? There are important differences between the two studies such as the inclusion criteria, severity of patient disease, how the sham procedures were performed, the blinding procedure, and how success and the mean clinically important differences were defined. These are highlighted in Table 3. Pauza et al. [21] may well have shown statistical significance between their two groups, but Freeman et al. would argue that these differences are not clinically important [12].
Table 3.
Comparison of Freeman et al. [12] and Pauza et al. [21] (Adapted from Freeman BJC, Fraser RD, Cain CMJ et al. (2005) A randomized double-blind controlled trial: intra-discal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine 30(21): 2369–2377, with permission)
| Characteristic | Freeman et al. [12] | Pauza et al. [21] | ||
|---|---|---|---|---|
| Start date of trial | November 1999 | September 2000 | ||
| Finish date of trial | December 2001 | April 2002 | ||
| Study total (n) | 57 | 64 | ||
| Withdrawn or loss to follow-up | 2 (3.5%) | 8 (12.5%) | ||
| Mean age (years) | IDET (37.5) | IDET (42) | ||
| Placebo (40.2) | Placebo (40) | |||
| Disc height | Up to 50% loss | Up to 20% loss | ||
| Disc morphology | Discrete annular tear or global degeneration | Posterior annular tear only | ||
| Worker compensation in each group (%) | IDET (55.3) | Excluded from study | ||
| Placebo (57.90) | ||||
| Duration of symptoms (months) | IDET (41) | IDET 78% over 24 | ||
| Placebo (66) | Placebo 74% over 24 | |||
| ODI baseline | IDET | 41.4 | IDET | 32 |
| Placebo | 40.7 | Placebo | 33 | |
| SF-36 PF baseline | IDET | 41.8 | IDET | 54 |
| Placebo | 35.0 | Placebo | 48 | |
| SF-36 BP baseline | IDET | 33.1 | IDET | 35 |
| Placebo | 24.4 | Placebo | 35 | |
| Definition of success | No neurological deficit | Comparison of mean | ||
| LBOS > 7.0 | Categorical outcomes | |||
| SF-36 PF > 1 SD | ||||
| SF-36 BP > 1 SD | ||||
PF physical function subset of SF-36, BP bodily pain subset of SF-36
Safety issues
Eckel and Ortiz reported on the complications following IDET in a retrospective multi-centre registry of 1,675 treated patients [10]. These included 19 catheter breakages, five transient nerve root injuries, one partially resolved nerve root injury and six cases of post-IDET disc herniation.
In 2002, Djurasovic et al. reported a case of vertebral osteonecrosis associated with the use of IDET [9]. The patient continued to have severe unrelenting symptoms and subsequently underwent an L5-S1 anterior interbody fusion. Scholl et al. reported a second case of vertebral osteonecrosis in a 26-year-old male undergoing at L2-L3 [27]. The procedure was performed by sequential placement of intradiscal catheters bilaterally. The bilateral approach was judged necessary at the time of the IDET because the catheter tip could not be manipulated through a unilateral approach to heat the entire posterior annulus. This technique is described in the instructional course manual [23]. The patient continues to complain of severe disabling low back pain.
There have been three reports of cauda equina syndrome following IDET [1, 14, 31] and one report of a giant herniated disc following IDET [6]. It has been suggested that IDET weakens the posterior annular wall predisposing to disc prolapse.
Orr and Thomas present a case whereby a broken IDET catheter tip migrated from the disc space into the thecal sac leading to radiculopathy [19]. The symptoms improved after surgical removal of the catheter.
Davis et al. reported a case of discitis at L4/5 commencing within 4 weeks of her L4-L5 IDET procedure [7]. Conservative treatment failed to resolve the infection and the patient underwent an L4/5 lumbar interbody fusion 10 months later.
The incidence of complications following IDET in one series was reported at 15% [5]. Complications included radicular pain, paraesthesia and numbness in the thighs, foot drop, cerebral spinal fluid leaks and severe headaches.
Conclusions
Initial reports from the originators of IDET were impressive with respect to improvements in subjective outcome measures in highly selected cases. Further level II and III studies carried out at beta sites appear much less impressive. The two randomised controlled trials (level I evidence) addressing the effectiveness of IDET provide inconsistent evidence [12, 21]. Pauza et al. [21] showed modest overall benefit, although many patients did not seem to improve at all. Freeman et al. [12] showed no substantial benefit from the procedure. It is clear one must exercise caution in recommending this treatment for patients [4]. The current published evidence does not provide clear evidence of benefit.
References
- 1.Ackerman III WE. Cauda equina syndrome after intradiscal electrothermal therapy. Reg Anaesth Pain Med. 2002;27:622. doi: 10.1053/rapm.2002.37120. [DOI] [PubMed] [Google Scholar]
- 2.Barendse GAM, berg SGM, Kessels AHF, et al. Randomised controlled trial of percutaneous intradiscal radio-frequency thermo-coagulation for chronic discogenic back pain. Lack of effect from a 90-second 70°C lesion. Spine. 2001;26:287–292. doi: 10.1097/00007632-200102010-00014. [DOI] [PubMed] [Google Scholar]
- 3.Bogduk N, Karasek M. Two-year follow-up of a controlled trial of intradiscal electrothermal anuloplasty for chronic low back pain resulting from internal disc disruption. Spine J. 2002;2:343–350. doi: 10.1016/S1529-9430(02)00409-6. [DOI] [PubMed] [Google Scholar]
- 4.Carey TS. Point of view. Spine. 2005;30:2378. doi: 10.1097/01.brs.0000186471.59654.73. [DOI] [Google Scholar]
- 5.Cohen SP, Larkin T, Abdi S. Risk factors for failure and complications of intradiscal electrothermal therapy: a pilot study. Spine. 2003;28:1142–1147. doi: 10.1097/00007632-200306010-00011. [DOI] [PubMed] [Google Scholar]
- 6.Cohen SP, Larkin T, Polly DW., Jr A giant herniated disc following intradiscal electrothermal therapy. J Spinal Disord Tech. 2002;15:537–541. doi: 10.1097/00024720-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Davis TT, Delamarter RB, Sra P, et al. The IDET procedure for chronic discogenic low back pain. Spine. 2004;29:752–756. doi: 10.1097/01.BRS.0000119403.11472.40. [DOI] [PubMed] [Google Scholar]
- 8.Derby R, Eek B, Chen Y, et al. Intradiscal electrothermal annuloplasty (IDET): a novel approach for treating chronic discogenic back pain. Neuromodulation. 2000;3:82–88. doi: 10.1046/j.1525-1403.2000.00082.x. [DOI] [PubMed] [Google Scholar]
- 9.Djurasovic M, Glassman SD, Dimar JR, et al. Vertebral osteonecrosis associated with the use of intradiscal electrothermal therapy. A case report. Spine. 2002;27:E325–E328. doi: 10.1097/00007632-200207010-00023. [DOI] [PubMed] [Google Scholar]
- 10.Eckel TS, Ortiz AO. Intradiscal electrothermal therapy in the treatment of discogenic low back pain. Tech Vasc Interv Radiol. 2002;5:217–222. doi: 10.1053/tvir.2002.36430. [DOI] [PubMed] [Google Scholar]
- 11.Freedman BA, Cohen SP, Kuklo TR, et al. Intradiscal electrothermal therapy (IDET) for chronic low back pain in active-duty soldiers: 2-year follow-up. Spine J. 2003;3:502–509. [PubMed] [Google Scholar]
- 12.Freeman BJC, Fraser RD, Cain CMJ, et al. A randomised double-blind controlled trial: intra-discal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine. 2005;30:2369–2377. doi: 10.1097/01.brs.0000186587.43373.f2. [DOI] [PubMed] [Google Scholar]
- 13.Gerszten PC, Welch WC, McGrath PM, et al. A prospective outcomes study of patients undergoing intradiscal electrothermy (IDET) for chronic low back pain. Pain Physician. 2002;5:360–364. [PubMed] [Google Scholar]
- 14.Hsia AW, Isaac K, Katz JS. Cauda equina syndrome from intradiscal electrothermal therapy. Neurology. 2000;55:320. doi: 10.1212/wnl.55.2.320. [DOI] [PubMed] [Google Scholar]
- 15.Kapural L, Hayek S, Malak O, et al. Intradiscal thermal annuloplasty versus intradiscal radiofrequency ablation for the treatment of discogenic pain: a prospective matched control trial. Pain Med. 2005;6:425–431. doi: 10.1111/j.1526-4637.2005.00073.x. [DOI] [PubMed] [Google Scholar]
- 16.Karasek M, Bogduk N. Twelve-month follow-up of a controlled trial of intradiscal thermal anuloplasty for back pain due to internal disruption. Spine. 2000;25:2601–2607. doi: 10.1097/00007632-200010150-00010. [DOI] [PubMed] [Google Scholar]
- 17.Lee MS, Cooper G, Lutz GE, et al. Intradiscal electrothermal therapy (IDET) for treatment of chronic lumbar discogenic pain: a minimum 2-year clinical outcome study. Pain Physician. 2003;6:443–448. [PubMed] [Google Scholar]
- 18.Lutz C, Lutz GE, Cooke PM. Treatment of chronic lumbar diskogenic pain with intradiskal electrothermal therapy: a prospective outcome study. Arch Phys Med Rehabil. 2003;84:23–28. doi: 10.1053/apmr.2003.50059. [DOI] [PubMed] [Google Scholar]
- 19.Orr RD, Thomas S. Intradural migration of broken IDET catheter causing a radiculopathy. J Spinal Disord Tech. 2005;18:185–187. doi: 10.1097/01.bsd.0000164732.24906.e2. [DOI] [PubMed] [Google Scholar]
- 20.Park SY, Mood SH, Park MS, et al. Intradiscal electrothermal treatment for chronic lower back pain patients with internal disc disruption. Yonsei Med J. 2005;46:539–545. doi: 10.3349/ymj.2005.46.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pauza KJ, Howell S, Dreyfuss P, et al. A randomised, placebo-controlled trial of intradiscal electrothermal therapy for the treatment of discogenic low back pain. Spine J. 2004;4:27–35. doi: 10.1016/j.spinee.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Philips B, Ball C, Sackett D et al (2001) The oxford centre for evidence-based levels of evidence. www.cebm.net
- 23.Saal JA, Saal JS. Intradiscal electrothermal therapy (IDET) training course syllabus. Menlo Park: Oratec Interventions Inc; 1999. [Google Scholar]
- 24.Saal JS, Saal JA. Management of chronic discogenic low back pain with a thermal intradiscal catheter: a preliminary report. Spine. 2000;25:382–388. doi: 10.1097/00007632-200002010-00021. [DOI] [PubMed] [Google Scholar]
- 25.Saal JA, Saal JS. Intradiscal electrothermal treatment for chronic discogenic low back pain. A prospective outcome study with a minimum 1-year follow-up. Spine. 2000;25:2622–2627. doi: 10.1097/00007632-200010150-00013. [DOI] [PubMed] [Google Scholar]
- 26.Saal JA, Saal JS. Intradiscal electrothermal treatment for chronic discogenic low back pain. A prospective outcome study with a minimum 2-year follow-up. Spine. 2002;27:966–973. doi: 10.1097/00007632-200205010-00017. [DOI] [PubMed] [Google Scholar]
- 27.Scholl BM, Theiss SM, Lopez-Ben R, et al. Vertebral osteonecrosis related to intradiscal electrothermal therapy: a case report. Spine. 2003;28:E161–E164. doi: 10.1097/00007632-200305010-00022. [DOI] [PubMed] [Google Scholar]
- 28.Smith and Nephew Sustainability report published 28th April 2003 (www.smith-nephew.com)
- 29.Spruit M, Jacobs WCH. Pain and function after intradiscal electrothermal treatment (IDET) for symptomatic lumbar disc degeneration. Eur Spine J. 2002;11:589–593. doi: 10.1007/s00586-002-0450-6. [DOI] [PubMed] [Google Scholar]
- 30.Webster BS, Verma S, Pransky GS. Outcomes of workers’ compensation claimants with low back pain undergoing intradiscal electrothermal therapy. Spine. 2004;29:435–441. doi: 10.1097/01.BRS.0000092376.41688.1A. [DOI] [PubMed] [Google Scholar]
- 31.Wetzel FT. Cauda equina syndrome from intradiscal electrothermal therapy. Neurology. 2001;56:1607. doi: 10.1212/wnl.56.11.1607. [DOI] [PubMed] [Google Scholar]