Abstract
Study Objectives:
A single subjective question may be an effective screening tool for excessive daytime sleepiness. This study sought to determine whether the following single question about sleepiness can measure subjective sleepiness comparably to the Epworth Sleepiness Scale (ESS): “Please measure your sleepiness on a typical day: (0 = none, 10 is highest).” The relationship between this question and objective sleepiness as measured by the MSLT was also evaluated.
Methods:
303 subjects completed a sleep questionnaire, MSLT, and ESS within 2 months. ROC (receiver-operator characteristic) curves and contingency tables using Fisher's exact test were made using GraphPad Prism software.
Results:
ESS and SS scores showed a significant association at all SS score cut-points. ESS and MSL showed significant associations only at ESS scores 11, 12, and 18. SS scores were significantly related to the MSL only in non–sleep apneics at SS scores 2, 5, 6, and 8, and in sleep apneics at SS score 9. ROC analysis showed the SS could distinguish subjects with an ESS ≥ 11 from those with an ESS < 11 (area = 0.71, p < 0.0001).
Conclusions:
Neither the SS nor the ESS substitutes for the MSLT, which measures objective sleepiness and is not an appropriate screening tool. SS scores ≤ 2 and ≥ 9 reliably predict normal and abnormal ESS scores respectively. Since the ESS is not commonly used in non–sleep specialized practices, the SS may serve as a useful screening tool for patients with disorders of sleepiness.
Citation:
Zallek SN; Redenius R; Fisk H; Murphy C; O'Neill E. A single question as a sleepiness screening tool. J Clin Sleep Med 2008;4(2):143–148.
Keywords: ESS, MSLT, MSL, subjective sleepiness, sleepiness screening
Sleep disorders are common, and most cases remain undiagnosed. Excessive daytime sleepiness (EDS) is an important symptom of many sleep disorders. It can lead to decreased productivity,1 work absenteeism,2 and an increase in motor vehicle accidents.3 Therefore, it is both a risk to the individual and a public health concern. Quantifying EDS is helpful in screening for sleep disorders. It is also useful in measuring changes in sleepiness over time, particularly after an intervention intended to reduce sleepiness. A tool that is simple to administer and reflects objective sleepiness would be most useful.
The multiple sleep latency test (MSLT) is an objective measure of sleepiness. Electrodes are attached to the scalp to record electroencephalography (EEG), to the skin lateral to the eyes to record eye movements, and under the chin to record electromyography (EMG). These electrodes are used to determine sleep stages during 4 or 5 daytime nap opportunities at 2-h intervals. The mean latency to sleep onset (mean sleep latency [MSL]) is then calculated. According to the International Classification of Sleep Disorders (ICSD-2), an MSL of 0-5 min is indicative of severe sleepiness, while an MSL over 10 min is indicative of normal alertness.16 While these values are widely accepted by sleep clinicians, no absolute MSL value defines sleepiness.4,5 The mean MSL in normative populations is 10 min with a 2 SD range of 2-19 min.5 This causes a wide overlap of MSL values and makes the distinction between sleepy and normal groups difficult.4,5 False negatives can occur, as behavioral and environmental factors can prolong the sleep latency. False positives are generally thought not to occur, as a sleep propensity (regardless of reason) must be present to induce sleep.
Although the MSLT may have false negative results, it is currently the “gold standard” objective measure of sleepiness.4–6 It is, however, time consuming and expensive. Therefore, it is neither a good screening tool nor a practical way to follow changes in sleepiness over time.
Subjective verbal reports of EDS are easier and less costly to obtain but difficult to quantify. The Epworth Sleepiness Scale (ESS) is used frequently by sleep specialists. It consists of a questionnaire on a Likert scale of 0-3 that asks a person how likely he or she is to doze in 8 specific daily situations, such as sitting and reading.7 Although some studies have shown the ESS does not correlate well with the MSLT,8,9 it is considered a valid, reliable measure of subjective sleepiness and serves as a good screening tool. In addition to its divergence from the MSLT, the ESS has other inherent limitations. For example, an individual may feel very sleepy, but may also feel that he/she is able to fight off sleep with effort. He/she would therefore score very low on the ESS, which would give the impression that the person is not sleepy. Consequently, the ESS may not be a meaningful measure of EDS in some cases.
The ESS is easy to administer, however, non–sleep specialized clinicians do not commonly use it. This may be due to both a lack of awareness and an unwillingness to add 8 questions to an already lengthy medical inquiry. A simpler, more direct question about sleepiness may be useful as a screening tool for non–sleep specialists.
While many studies have examined the relationship between the ESS and the MSLT,8–11,13,15 these authors could find only 2 studies also examining a single question about sleepiness.8,10 In the first study, 141 subjects were asked, “How often do you have a major problem with sleepiness during the daytime?”8 In the second study, 60 subjects were asked, “How great a problem do you have with sleepiness in the daytime?”10 Each question used a 5-point scale. Although both studies found significant correlations between the ESS and the single subjective question, neither study suggested the application of the single question in non–sleep specialized practices.
The current investigation set out to determine whether a single question about sleepiness could measure sleepiness as well as the ESS and serve as an adequate screening tool in non–sleep specialized practices. We hypothesized that the following single question could measure subjective sleepiness comparably to the ESS: “Please measure your sleepiness on a typical day: (0 = none, 10 is highest).” This question has long been used in our clinical practice; the wording was chosen as a general inquiry about subjective sleepiness.
METHODS
Subjects
A cross-sectional retrospective chart review was conducted using adult subjects (≥ 18 years old at the time of testing) identified from the database of patients who have undergone sleep testing at the Illinois Neurological Institute Sleep Center (SC) at OSF Saint Francis Medical Center in Peoria, Illinois, from April, 2001 to June, 2004. Those who had an MSLT and a clinical evaluation were included as subjects. Subjects were required to have completed the MSLT, ESS, and patient questionnaire (which included the 10-point self-rating of sleepiness) within a 2-month time period. The MSL was determined by calculating the mean latency to sleep onset (MSL) of 4 or 5 naps.
Each patient who undergoes sleep testing in the SC is asked to complete a sleep questionnaire, which includes the statement “Please measure your sleepiness on a typical day: (0 = none, 10 is highest).” This yields a subjective sleepiness (SS) score. Each patient who is seen for a clinical evaluation in the SC (on a day separate from the testing date) is asked to complete the ESS. The office visit notes of each subject were reviewed to determine whether the ESS was completed. Only those subjects completing all three assessments (the MSLT, ESS, and SS) were included as subjects.
Statistical Analysis
Data were analyzed using GraphPad Prism software. ROC (receiver-operator characteristic) curves and contingency tables using Fisher's exact test were made, and sensitivity, specificity, and positive and negative predictive values were calculated. Data were considered significant when p-values were less than 0.05.
RESULTS
Of 559 subjects who completed an MSLT at the SC, 303 met inclusion criteria (n = 189 [62.4%] women). Age range was 18-78 years (mean 43.8 ± 14). Subjects had been diagnosed with a variety of sleep disorders, including obstructive or central sleep apnea (n = 133 [43.9%]), narcolepsy (n = 27 [8.9%]), periodic limb movement disorder (n = 46 [15.2%]), restless legs syndrome (n = 32 [10.6%]), psychophysiological insomnia (n = 60 [19.8%]), inadequate sleep hygiene (n = 70 [23.1%]), and idiopathic hypersomnia (n = 41 [13.5%]). MSL ranged from 0-20 min (mean 7.4 ± 5). ESS scores ranged from 0-24 (mean 12.7 ± 5). SS scores ranged from 0-10 (mean 6.5 ± 2).
In Table 1, subjects with an ESS score ' 11 were placed into the “subjectively sleepy” group while those subjects with an ESS score < 11 were considered “subjectively not sleepy.” Fisher's exact test was performed, and sensitivity, specificity, and predictive values were calculated. All SS scores had a significant association with an ESS score of ≥ 11. Negative predictive values (probability of having an ESS score < 11) were high at SS scores 1 and 2 because no subjects with an SS score ≤ 2 had an abnormal ESS. At SS scores < 3, 82% of subjects had a normal ESS (ESS score < 11). SS scores ≥ 8 yielded positive predictive values greater than 80%, indicating more than 80% of subjects scored ≥ 11 on the ESS. No SS score cut-point resulted in both high sensitivity and specificity. The optimal SS score was 7 with a sensitivity of 0.67 and a specificity of 0.57.
Table 1.
SS Scores Resulting in Sensitivity, Specificity, Fisher's Exact p-Values, Positive Predictive Values (PV+), and Negative Predictive Values (PV−) for an ESS Score ≥ 11
| SS ≥ | n | Sensitivity | Specificity | p | PV+ | PV− |
|---|---|---|---|---|---|---|
| 1 | 300 | 1.00 | 0.03 | 0.0385 | 0.67 | 1.00 |
| 2 | 295 | 1.00 | 0.08 | 0.0001 | 0.68 | 1.00 |
| 3 | 281 | 0.98 | 0.17 | <0.0001 | 0.70 | 0.82 |
| 4 | 260 | 0.93 | 0.27 | <0.0001 | 0.71 | 0.65 |
| 5 | 247 | 0.90 | 0.35 | <0.0001 | 0.73 | 0.64 |
| 6 | 220 | 0.83 | 0.47 | <0.0001 | 0.75 | 0.58 |
| 7 | 174 | 0.67 | 0.57 | 0.0001 | 0.74 | 0.48 |
| 8 | 116 | 0.48 | 0.81 | <0.0001 | 0.83 | 0.44 |
| 9 | 57 | 0.24 | 0.91 | 0.001 | 0.84 | 0.38 |
| 10 | 21 | 0.10 | 0.99 | 0.003 | 0.95 | 0.36 |
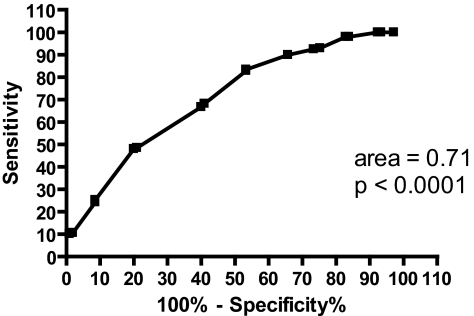
An ROC curve was made to measure the accuracy of the SS in distinguishing between “subjectively sleepy” (ESS ≥ 11) and “subjectively not sleepy” (ESS < 11) groups (Figure 1). The area represents the percentage of subjects in the “subjectively sleepy” group with a higher SS score than subjects in the “subjectively not sleepy” group. An area of 1.0 would indicate that 100% of subjects with an ESS score ≥ 11 would have a higher SS score than subjects with an ESS score < 11. An area of 0.5 would indicate a test no better than chance at distinguishing between “sleepy” and “not sleepy” subjects. The area of 0.71 signifies that 71% of “subjectively sleepy” subjects had a higher SS score than “subjectively not sleepy” subjects. This area was significantly different (p < 0.0001) from the null hypothesis (area = 0.5).
Figure 1.
ROC curve for the SS when groups were defined by ESS scores
In Table 2, subjects were placed into “objectively sleepy” (MSL ≤ 10) and “objectively not sleepy” (MSL > 10) groups based on their MSL values. None of the SS scores had a significant association with the MSL. Negative predictive values were low at all SS scores, with the highest being 50% at SS scores less than 2. Positive predictive values were high at all SS scores, with no value less than 72%. SS scores ≥ 8 yielded positive predictive values of 0.76, signifying 76% of subjects who scored ≥ 8 on the SS had an abnormal MSLT. No SS score resulted in both high sensitivity and specificity.
Table 2.
SS Scores Resulting in Sensitivity, Specificity, Fisher's Exact p-Values, Positive Predictive Values (PV+), and Negative Predictive Values (PV−) for an MSL ≤ 10 Min
| SS ≥ | n | Sensitivity | Specificity | p | PV+ | PV− |
|---|---|---|---|---|---|---|
| 1 | 300 | 0.99 | 0.01 | 0.5753 | 0.75 | 0.33 |
| 2 | 295 | 0.98 | 0.05 | 0.1075 | 0.76 | 0.50 |
| 3 | 281 | 0.93 | 0.08 | 0.7988 | 0.75 | 0.27 |
| 4 | 260 | 0.86 | 0.13 | 0.9091 | 0.75 | 0.23 |
| 5 | 247 | 0.83 | 0.23 | 0.3048 | 0.77 | 0.30 |
| 6 | 220 | 0.74 | 0.31 | 0.4596 | 0.76 | 0.28 |
| 7 | 174 | 0.57 | 0.41 | 0.8930 | 0.75 | 0.24 |
| 8 | 116 | 0.39 | 0.63 | 0.8916 | 0.76 | 0.25 |
| 9 | 57 | 0.18 | 0.79 | 0.5012 | 0.72 | 0.24 |
| 10 | 21 | 0.07 | 0.95 | 0.7932 | 0.81 | 0.25 |
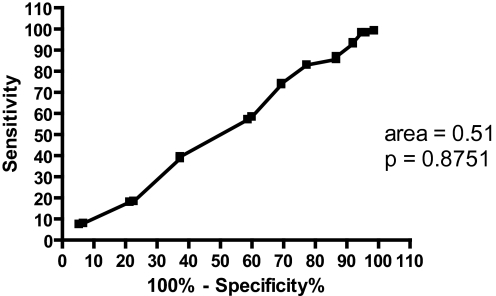
The ROC curve in Figure 2 was made to determine the accuracy of the SS in distinguishing between “objectively sleepy” (MSL ≤ 10) and “objectively not sleepy” (MSL > 10) groups with respect to the MSLT. The obtained area of 0.51 shows that 51% of subjects with an MSL ≤ 10 had higher SS scores than those subjects with an MSL > 10. This area was not significantly different (p = 0.8751) from the null hypothesis.
Figure 2.
ROC curve for the SS when groups were defined by MSL
In Table 3, the ESS and MSL were compared. Subjects were placed into “objectively sleepy” (MSL ≤ 10) and “objectively not sleepy” (MSL > 10) groups based on MSL values. Significance was seen at ESS scores 11, 12, 16, and 18. No ESS score resulted in both high sensitivity and specificity. The optimal ESS score was 12, with a sensitivity of 0.64 and a specificity of 0.51. Positive predictive values at this score were 0.80, indicating 80% of subjects with an ESS score ≥ 12 had an abnormal MSLT.
Table 3.
ESS Scores Resulting in Sensitivity, Specificity, Fisher's Exact p-Values, Positive Predictive Values (PV+), and Negative Predictive Values (PV−) for an MSL ≤ 10 Min
| ESS ≥ | n | Sensitivity | Specificity | p | PV+ | PV− |
|---|---|---|---|---|---|---|
| 11 | 198 | 0.69 | 0.45 | 0.0354 | 0.79 | 0.32 |
| 12 | 183 | 0.64 | 0.51 | 0.0293 | 0.80 | 0.32 |
| 13 | 163 | 0.56 | 0.53 | 0.1820 | 0.79 | 0.29 |
| 14 | 145 | 0.50 | 0.59 | 0.2306 | 0.79 | 0.28 |
| 15 | 129 | 0.45 | 0.65 | 0.1385 | 0.80 | 0.28 |
| 16 | 104 | 0.38 | 0.76 | 0.0353 | 0.83 | 0.29 |
| 17 | 89 | 0.32 | 0.79 | 0.0817 | 0.82 | 0.28 |
| 18 | 70 | 0.26 | 0.87 | 0.0263 | 0.86 | 0.28 |
| 19 | 49 | 0.18 | 0.89 | 0.1516 | 0.84 | 0.26 |
| 20 | 29 | 0.11 | 0.96 | 0.0700 | 0.90 | 0.26 |
| 21 | 19 | 0.07 | 0.97 | 0.1754 | 0.89 | 0.26 |
| 22 | 8 | 0.03 | 0.99 | 0.6844 | 0.88 | 0.25 |
| 23 | 6 | 0.02 | 0.99 | 1.0000 | 0.83 | 0.25 |
| 24 | 3 | 0.01 | 0.99 | 0.5753 | 0.67 | 0.25 |
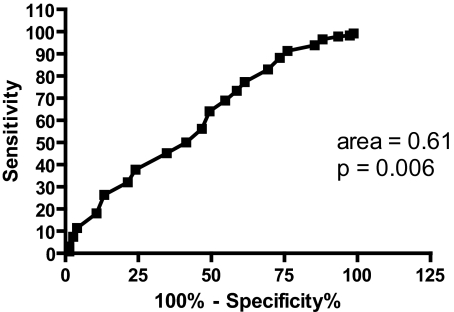
The ability of the ESS to distinguish between “objectively sleepy” (MSL ≤ 10) and “objectively not sleepy” (MSL > 10) groups was determined (Figure 3). An area of 0.61 indicates that 61% of subjects with an MSL ≤ 10 had a higher ESS score than those subjects with an MSL > 10. This area is significant (p = 0.006) from the null hypothesis.
Figure 3.
ROC curve for the ESS when groups were defined by MSL
ROC curves for sleep apneics, non–sleep apneics, and narcoleptics were analyzed separately (Table 4). ROC analysis for sleep apneics when groups were defined by the ESS yielded an area of 0.67, which was significantly different from the null hypothesis (p = 0.002). When an MSL ≤ 10 min defined “objectively sleepy” and an MSL > 10 min defined “not objectively sleepy,” the ROC curve for sleep apneics yielded an area of 0.61 and was significantly different from the null hypothesis (p = 0.0423). An ROC analysis of the ability of the ESS to distinguish between “objectively sleepy” and “not objectively sleepy” in sleep apneics generated an area of 0.51 and was not significant from chance (p = 0.8892). When an MSL ≤ 5 min was used to define “objectively sleepy” and an MSL > 5 min was used to define “not objectively sleepy,” an ROC analysis of the SS yielded an area of 0.52 and was not significant from chance (p = 0.7043). ROC analysis of the ESS when groups were defined in the same manner yielded an area of 0.51, which was also not significant from chance (p = 0.8112).
Table 4.
Areas of ROC Curves Separated by Diagnostic Groups
| Diagnostic Group | Area of ROC curve for SS defined by ESS | Area of ROC curve for SS defined by MSL (10 min) | Area of ROC curve for ESS defined by MSL (10 min) | Area of ROC curve for SS defined by MSL (5 min) | Area of ROC curve for ESS defined by MSL (5 min) |
|---|---|---|---|---|---|
| Sleep Apneics | 0.67 | 0.61 | 0.51 | 0.52 | 0.51 |
| (n = 133) | (p = 0.002) | (p = 0.0423) | (p = 0.8892) | (p = 0.7043) | (p = 0.8112) |
| Non-Sleep Apneics* | 0.74 | 0.64 | 0.74 | 0.57 | 0.62 |
| (n = 160) | (p < 0.0001) | (p = 0.009) | (p < 0.0001) | (p = 0.1289) | (p = 0.007) |
| Narcoleptics | 0.86 | Data | Data | 0.51 | 0.64 |
| (n = 27) | (p = 0.0052) | inconclusive** | inconclusive** | (p = 0.9606) | (p = 0.2267) |
All other subjects, including those diagnosed with other sleep disorders
Only 2 subjects had an MSL > 10 min
ROC analysis for non–sleep apneics when groups were defined by the ESS yielded an area of 0.74 and was significantly different from the null hypothesis (p < 0.0001). SS scores of non–sleep apneics were compared to the MSL at both 10-min and 5-min cut-points. When an MSL ≤ 10 defined “objectively sleepy”, the area of the ROC curve for non–sleep apneics was 0.64, which was significantly different from the null hypothesis (p = 0.009). When the ESS was compared to the MSL (MSL ≤ 10 used for “objectively sleepy”) for non–sleep apneics, the area of the ROC curve was 0.74 and was significant from chance (p < 0.0001). ROC curves for non–sleep apneics when groups were defined by an MSL ≤ 5 had an area of 0.57, which was not significant from the null hypothesis (p = 0.1289). An ROC analysis of the ability of the ESS to distinguish between “objectively sleepy” (MSL ≤ 5) and “not objectively sleepy” (MSL > 5) yielded an area of 0.62 and was significantly different from the null hypothesis (p = 0.007).
The ROC curve for narcoleptics when groups were defined by the ESS yielded an area of 0.86, which was significantly different from chance (p = 0.0052). SS scores of narcoleptics were analyzed separately at both MSL cut-points of 10 min and 5 min. Analysis at an MSL ≤ 10 min was inconclusive, since only 2 subjects had an MSL > 10 min. The ROC curve of the SS when groups were defined by MSL ≤ 5 yielded an area of 0.51 and was not significant from chance (p = 0.9606). ROC analysis of the ESS when groups were defined in the same manner generated an area of 0.64, which was also not significant from chance (p = 0.2267).
Analysis of each diagnostic group with Fisher's exact test showed no SS score resulting in both high sensitivity and specificity. When subjects with sleep apnea were analyzed separately, the ESS and SS were significantly associated at all SS score cut-points except 1 (p = 0.1179), 2 (p = 0.1179), 8 (p = 0.0558), and 9 (p = 0.2277). The highest positive predictive values were seen at an SS score of 10, with 100% of subjects (n = 9) having an ESS ≥ 11. The highest negative predictive values were at SS scores 1 and 2, with 100% of subjects (n = 131) having an ESS < 11. When an MSL cut-point of 10 min was used to define “objectively sleepy” and “not objectively sleepy,” only SS scores ≥ 9 had a significant relationship with the MSL (p = 0.0380).
SS scores of non–sleep apneics were significantly associated with the ESS at all cut-points except SS ≥ 1 (p = 0.3688). Negative predictive values were high (100%) at SS scores 1 and 2 because no subjects (n = 131) with an SS score ≤ 2 had an abnormal ESS. The highest positive predictive values were at an SS score of 10, with 91% of subjects (n = 11) having an ESS ≥ 11. When an MSL ≤ 10 defined “objectively sleepy”, significance was seen at SS scores 2 (p = 0.0235), 5 (p = 0.0028), 6 (p = 0.0398), and 8 (p = 0.0063). When an MSL ≤ 5 defined “objectively sleepy,” significant associations occurred at SS scores 2 (p = 0.0356), 5 (p = 0.0467), and 10 (p = 0.0110). At an SS score of 10, 82% of subjects (n = 11) had an MSL ≤ 5 min.
When subjects with narcolepsy were analyzed separately, ESS and SS scores were significantly associated only at SS scores 6 (p = 0.0120), 7 (p = 0.0047), and 8 (p = 0.0237). Only SS scores within the range 4-10 were considered since only one subject had an SS score less than 4. No subjects with an ESS ≥ 11 scored lower than 6 on the SS. When an MSL ≤ 5 defined “objectively sleepy,” no SS scores were significantly associated with the MSL.
DISCUSSION
This study presents an important new measure of sleepiness that may change the detection of sleep disorders in non–sleep specialized medical practice. Although the ESS is a valid screening tool for sleepiness that is easy to administer, non–sleep specialists do not commonly use it. The finding that the single question used in this study had significant associations with the ESS in all subject groups and was able to distinguish between “subjectively sleepy” (ESS ≥ 11) and “subjectively not sleepy” (ESS < 11) groups suggests the SS is a good measure of subjective sleepiness. Its simplicity means non–sleep specialists may be more likely to use it and therefore recognize sleep disorders in more patients.
The relationship between the ESS and MSL in this study is comparable to findings in previous studies for subjects of similar ages and diagnoses.8,10 These investigators found significant associations between the ESS and MSL at only 4 ESS score cut-points (11, 12, 16, and 18), with no cut-point resulting in both high sensitivity and specificity. The results of our ROC analysis, however, did indicate the ESS had some ability to distinguish subjects with an MSL ≤ 10 from those with an MSL > 10 min. Chervin et al. found no significant association between the ESS and MSL when an MSL of 10 min was used as the cut-point.10 When an MSL of 8 min was used as the cut-point, significant relationships between the ESS and MSL were found at ESS scores 14-21, and the optimal range was determined to be ESS scores 16-18. Benbadis et al. found no ESS score resulting in both high sensitivity and specificity when an MSL of 10 min was used as the cut-point.9 In addition, their ROC analysis yielded an area of 0.61, identical to our area, but their result was not significantly different from chance. The average MSL found in the present study (7.4 min) is similar to that found by Olson et al.11 ESS scores greater than 10 are commonly thought to indicate sleepiness, while the average ESS score in normal subjects is approximately 6.7 The average ESS score of 12.7 in the present study is as expected, considering most subjects (90%) had at least one sleep disorder.
Sangal et al. found a significant, but low correlation between the ESS and MSL in 522 narcoleptics.15 Despite a significant correlation, Sangal believes, and these authors agree, that physicians should not rely on one specific measure alone. Subjective measures are both helpful as screening tools to detect previously undiagnosed sleep disorders and as tools to track sleepiness within individual patients over time. For example, a primary care physician might become aware of a patient's likely sleep apnea only after seeing an abnormal ESS. A sleep specialist might then use the ESS over time to help determine effectiveness of treatment. A different group of patients would benefit from the MSLT.
Chervin et al. compared the following subjective question to the ESS and MSLT: “How great a problem do you have with sleepiness in the daytime?”10 Despite the differences between this question and the single question used in the present investigation, both inquire about general sleepiness. Chervin's results showed a significant correlation between the single subjective question and the ESS; we found similar results using our single question. In addition, the MSL did not correlate with the single question used in Chervin's study. Because of distinct variables between subjective and objective measurements, the ESS and MSLT may actually evaluate separate components of sleepiness.14 For example, the ESS measures perception of sleep tendency at any time, while the MSLT measures physiologic sleep tendency at a specific time in one standardized setting. This may account at least in part for the lack of correlation between subjective measures of sleepiness and the MSLT. This does not necessarily diminish the importance of these subjective measures; rather, it suggests that they assess different facets of sleepiness, as proposed by Johns.12 Thus, the ESS and SS may be as important in patient evaluations of sleepiness as the MSLT, despite their inconsistent correlations with it in different patient groups.
Although previous analyses of the ESS demonstrated its validity, reliability, and internal consistency with the MSLT,7,12 the present study sought to determine whether a single question could measure subjective sleepiness comparably to the ESS. The relationship between this single question and objective sleepiness as measured by the MSLT was also evaluated. The ESS and SS scores had significant associations in all subjects and when subjects were separated by sleep apnea and narcolepsy diagnoses. The strongest relationship was observed when all subjects were analyzed together. In addition, ROC analysis showed the SS was able to distinguish between 71% of “subjectively sleepy” and “subjectively not sleepy” subjects. This number was higher when non–sleep apneics (74%) and narcoleptics (86%) were analyzed separately, perhaps suggesting these groups are more accurate in their self-assessment of sleepiness than apneics. An ESS score of 11 corresponds most closely to an SS score of 7 (sensitivity 0.67 and specificity 0.57). In all groups, negative predictive values were 100% at SS scores 1 and 2. If non–sleep specialists see their patient scored a 1 or 2 on the SS, they may determine this patient is not sleepy. Alternatively, SS scores of 9 and 10 yielded high positive predictive values of an abnormal ESS.
Although the relationship between the ESS and SS was significant in most cases, the relationship between the MSLT and the SS was not. Non–sleep apneics showed a significant association between the SS and MSL at SS scores 2, 5, and 10 when an MSL ≤ 5 min was used as a cut-point and at SS scores 2, 5, 6, and 8 when an MSL ≤ 10 min was used as a cut-point. This finding again indicates that non–sleep apneics may be more accurate in their self-assessments of sleepiness. Sleep apneics showed a significant association between the SS and MSL only at SS score of ≥ 9. The ROC analysis for the SS when groups were defined by the MSL was only significantly different from chance in sleep apneics and non–sleep apneics analyzed separately when “objectively sleepy” was defined by an MSL ≤ 10 min. In both cases this significance was weak, which suggests that while the SS can distinguish between subjective sleepiness as measured by the ESS, it has limited ability to distinguish between objective sleepiness. The ESS, however, distinguished between “objectively sleepy” and “not objectively sleepy” in non–sleep apneics at both MSL cut-points of 5 and 10 min.
Neither the SS nor the ESS substitutes for the MSLT, which measures objective sleepiness and is not an appropriate screening tool. SS scores of ≤ 2 and ≥ 9 reliably reflect normal and abnormal ESS scores respectively. As SS scores 3-8 are not clearly predictive of a normal or abnormal ESS, these authors recommend following the SS in those cases with the ESS or other further evaluations for sleepiness. This would allow the introduction of a useful and simplified single question into non–sleep specialized practices, which may help identify more patients with disorders of sleepiness and trigger further evaluation when indicated.
Limitations of the Study
Our sample of subjects was drawn from a sleep center database and thus were probably sleepier than normal subjects. No data existed on a group with no sleep complaints. Assigning patients into “objectively sleepy” and “objectively not sleepy” groups based on MSL values may be confounded since the MSLT is known to provide false-negatives and no single MSL value can separate sleepy and normal populations. The single question used in this study differs from other single questions previously studied by other investigators and cannot be compared directly.
ACKNOWLEDGMENTS
Institution where work was performed: Illinois Neurological Institute Sleep Center at OSF Saint Francis Medical Center.
Financial Support: OSF Saint Francis Foundation
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Ulfberg J, Carter N, Talback M, Edling D. Excessive daytime sleepiness at work and subjective work performance in the general population and among heavy snorers and patients with obstructive sleep apnea. Chest. 1996;110:659–63. doi: 10.1378/chest.110.3.659. [DOI] [PubMed] [Google Scholar]
- 2.Melamed S, Oksenberg A. Excessive daytime sleepiness and risk of occupational injuries in non-shift daytime workers. Sleep. 2002;25:315–22. doi: 10.1093/sleep/25.3.315. [DOI] [PubMed] [Google Scholar]
- 3.Lyznicki JM, Doege TC, Davis RM, Williams MA. Sleepiness, driving, and motor vehicle crashes. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279:1908–13. doi: 10.1001/jama.279.23.1908. [DOI] [PubMed] [Google Scholar]
- 4.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 5.Arand D, Bonnet M, Hurwitz T, Mitler M, Rosa R, Sangal RB. The clinical use of the MLST and MWT. Sleep. 2005;28:123–44. doi: 10.1093/sleep/28.1.123. [DOI] [PubMed] [Google Scholar]
- 6.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 7.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 8.Chervin RD, Aldrich MS. The Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apnea. Neurology. 1999;52:125–31. doi: 10.1212/wnl.52.1.125. [DOI] [PubMed] [Google Scholar]
- 9.Benbadis SR, Mascha E, Perry MC, et al. Association between the Epworth sleepiness scale and the multiple sleep latency test in a clinical population. Ann Intern Med. 1999;130:289–92. doi: 10.7326/0003-4819-130-4-199902160-00014. [DOI] [PubMed] [Google Scholar]
- 10.Chervin RD, Aldrich MS, Pickett R, Guilleminault C. Comparison of the results of the Epworth Sleepiness Scale and the Multiple Sleep Latency Test. J Psychosom Res. 1997;42:145–55. doi: 10.1016/s0022-3999(96)00239-5. [DOI] [PubMed] [Google Scholar]
- 11.Olson LG, Cole MF, Ambrogetti A. Correlations among Epworth Sleepiness Scale scores, multiple sleep latency tests and psychological symptoms. J Sleep Res. 1998;7:248–53. doi: 10.1046/j.1365-2869.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- 12.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 13.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the Epworth Sleepiness Scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 14.Leng PH, Low SY, Hsu A, Chong SF. The clinical predictors of sleepiness correlated with the multiple sleep latency test in an Asian Singapore population. Sleep. 2003;26:878–81. doi: 10.1093/sleep/26.7.878. [DOI] [PubMed] [Google Scholar]
- 15.Sangal RB, Mitler MM, Sangal JM. Subjective sleepiness ratings (Epworth sleepiness scale) do not reflect the same parameter of sleepiness as objective sleepiness (maintenance of wakefulness test) in patients with narcolepsy. Clin Neurophysiol. 1999;110:2131–35. doi: 10.1016/s1388-2457(99)00167-4. [DOI] [PubMed] [Google Scholar]
- 16.American Academy of Sleep Medicine. The international classification of sleep disorders. second edition. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]