Abstract
The specific mechanisms underlying the varied susceptibility of HIV-infected (HIV+) individuals to opportunistic infections (OI) are still incompletely understood. One hypothesis is that quantitative differences in specific T cell responses to a colonizing organism determine the development of an AIDS-defining OI. We evaluated this hypothesis for herpes simplex virus (HSV) infection, a common OI in HIV+ patients. Using limiting dilution analyses, the frequency of HSV-specific CD8+ cytotoxic T lymphocyte precursors (pCTL) and proliferative precursors were quantitated in peripheral blood mononuclear cells from 20 patients coinfected with HIV and HSV-2. The frequency of HSV-specific CD8+ pCTL in HSV+HIV+ individuals was significantly lower than in HSV+HIV− individuals (1 in 77,000 vs. 1 in 6,000, P = .0005) and was not different than in HSV-HIV− individuals (1 in 100,000, P = .24). HIV+ patients who suffered more severe genital herpes recurrences had significantly lower HSV-specific CD8+ pCTL frequencies than those patients with mild recurrences (1 in 170,000 vs. 1 in 26,000, P = .03). In contrast, no significant difference was seen in proliferative precursor frequencies between those patients with mild vs. severe genital herpes (1 in 3,800 vs. 1 in 6,600, P > .5). Quantitative differences in pCTL frequency to HSV appear to be the most important host factor influencing the frequency and severity of HSV reactivation in HIV+ patients. Studies to reconstitute such immunity, especially in people with acyclovir-resistant HSV, appear warranted.
Herpes simplex virus 2 (HSV-2) infection is a significant opportunistic infection (OI) in HIV-infected (HIV+) individuals, often resulting in frequent reactivation of latent HSV-2 and severe, long-lasting genital lesions. It is estimated that 50–90% of HIV+ individuals are coinfected with HSV-2 compared with approximately 20% of HIV-negative (HIV−) individuals (1–3). The prevalence of HSV-2 shedding is 4–5 times greater in HIV+ individuals than in HIV− individuals (4), likely increasing HSV transmission. Genital HSV reactivations have been implicated in increasing the efficiency of HIV transmission (5, 6) and in up-regulating HIV replication by transactivation of the HIV-long terminal repeat by HSV proteins (7). Moreover, an increase in acyclovir-resistant strains of HSV-2 are complicating the treatment of HSV infection in HIV+ individuals (8–10). Thus, the high seroprevalence of HSV-2, the high rate of HSV-2 shedding and frequent HSV disease, the presence of HIV in herpetic lesions, and the increase in acyclovir-resistant HSV strains make HSV infection a major health problem for HIV+ individuals.
The mechanism(s) involved in the worsening of HSV disease in HIV+ individuals is not known. Because individuals with depressed T cell function suffer more serious HSV-related illnesses than immunocompetent individuals or patients with Ig defects, the cellular arm of the adaptive immune system has long been implicated in preventing and resolving HSV reactivations. Although CD4+ and CD8+ T cells specific for HSV are present in blood (11–16) and in genital herpes lesions (17, 18), the relative importance of HSV-specific cellular immune responses compared with other components of the immune response in controlling HSV disease expression is unclear. HIV infection and subsequent immunosuppression in individuals provide a unique model system to study critical immune functions necessary for control and resolution of human HSV infections. In addition, the study of HSV-specific immune responses in HIV+ individuals provides an opportunity to study the mechanisms involved in the emergence of a clinically relevant OI. Many OIs among HIV+ patients are related to ubiquitous colonizing agents. Yet even with severe immunosuppression, clinical disease is seen in only 15–30% of HIV+ individuals. The mechanisms underlying what determines the development of an OI in HIV+ individuals are unclear.
Several studies have demonstrated that HIV+ individuals have significantly lower HSV-specific proliferative responses compared to HIV−, HSV-seropositive patients (19, 20). This observation has been correlated with absolute CD4+ lymphocyte counts (19, 21). Cytotoxic T lymphocyte (CTL) responses have been shown to be critical in the control of HIV-1 replication. However, no studies evaluating the relationship between CTL responses and the development of an OI have been reported.
Therefore, the goal of this study was to determine if there were quantitative differences in cellular immune responses to HSV in HIV+ individuals compared with HIV− individuals. More importantly, were HSV-specific immune responses quantitatively different between HIV+ patients with severe and frequent genital herpes infections vs. HIV+ individuals with mild HSV disease? Using limiting dilution analysis, we measured the frequency of HSV-specific CD8+ CTL memory precursors (pCTL) and proliferative precursors (pProlif) in a cross-sectional study of 20 HIV+ patients with varying severity of HSV disease.
MATERIALS AND METHODS
Patients.
We studied 20 HIV+ individuals coinfected with HSV-2 (16 of whom also were infected with HSV-1) and 14 immunocompetent people with genital HSV-2. Patients were enrolled into a University of Washington Institutional Review Board-approved protocol of the natural history of recurrent genital herpes. Patients were given diary cards to record the frequency and duration of their genital lesions. Patients were seen during their genital recurrences and at 6- to 8-week intervals at which time standardized interviews eliciting the severity and duration of their genital recurrences were recorded. At the end of the follow-up (6–12 months), one of the authors (M.S.) classified the severity of patients’ genital herpes into five categories: 0, no recurrences; 1+, mild (<6 recurrences/yr, minimum symptoms lasting 3–4 days); 2+, moderate (<6 recurrences/yr, mean duration of recurrences 3–10 days); 3+, frequent (>6 recurrences/yr, mean duration of recurrences of 3–10 days); and 4+, long and frequent (duration of recurrence lasting >10 days and/or >6 recurrences/yr). Five additional patients who were seronegative for HSV-1, HSV-2, and HIV− were included as controls. All the laboratory work was performed without knowledge of the clinical data.
Cell Lines.
Peripheral blood mononuclear cells (PBMC) were isolated from peripheral blood by Ficoll/Hypaque density gradient centrifugation and cryopreserved for use as stimulator cells, responder cells, or feeder cells. Epstein–Barr virus (EBV)-transformed lymphoblastoid cell lines were prepared from PBMC as previously described (22). Lymphoblastoid cell line and K562 cells were maintained in RPMI-F [RPMI 1640 medium containing 10% fetal bovine serum, 50 μg of streptomycin per ml, 50 units of penicillin per ml, 2 × 10−5 M 2-mercaptoethanol, 1 mM pyruvate, 2 mM l-glutamine, 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Hepes)].
HSV-Specific CD8+ pCTL.
The frequency of HSV-specific CD8+ CTL precursors (pCTL frequency) was determined by limiting dilution analysis, using methods and calculations previously described (16). Briefly, HSV-infected phytohemagglutinin (PHA) blasts (HSV blasts) were used as stimulator cells and were prepared by infecting autologous PHA blasts for 18 hr with HSV-2 strain 333, propagated in Vero cells (10 pfu/cell), in RPMI-H (RPMI-F but replacing 10% fetal bovine serum with 10% heat-inactivated human serum) followed by fixation in 1% paraformaldehyde. CD8+ responder cells were positively selected from freshly isolated or cryopreserved donor PBMC using CD8 microbeads (Miltenyi Biotec, Auburn, CA). Flow cytometric analysis revealed that positively selected cells were 94–100% CD8+ and 0–6% CD4+. Positively selected CD8+ T cells were aliquoted in 100 μl in seven serial 2-fold dilutions starting at 3 × 104 to 1 × 105 as the highest initial cell number per well. Twenty-four replicates were plated for each dilution in round-bottomed 96-well culture plates. Then, 1 × 104 HSV blasts and 5 × 104 irradiated autologous PBMC (3,300 rad) (feeder cells) were added to each well in 100 μl total. Twenty-four control wells also were set up with HSV blasts and feeders only. After 2, 5, and 8 days, 100 μl of supernatant was removed and replaced with 100 μl RPMI-H containing 10 units/ml interleukin 2 (5 units/ml final) (Schiaperelli Biosystems, Columbia, MD).
Cell-Mediated Cytotoxicity Assays.
Limiting dilution analysis wells were split into 2–3 aliquots between 10 and 12 days after initial setup and assayed for cytotoxic activity against the following target cells: autologous lymphoblastoid cell line that were mock-infected or infected for 18 hr with HSV-2 (multiplicity of infection 10) or K562 cells. Target cells were incubated overnight with 100 μCi Na251CrO4 (New England Nuclear) and washed three times with RPMI-F before use in 51Cr-release assay. Target cells (2 × 103/well) were incubated with limiting dilution analysis test cells for 4 hr, 30 μl supernatant removed, and radioactivity measured by a gamma counter. Target cells also were incubated with 1% Nonidet P-40 (maximum release) or in RPMI-F alone (spontaneous release). The percent specific 51Cr release was calculated as follows: [(cpm experimental release-cpm spontaneous release)/(cpm maximum release - cpm spontaneous release)] × 100%. The spontaneous release was always less than 30% of the maximum release. A well was scored as positive if the radioactivity in the experimental well was greater than 6% specific 51Cr release. Precursor frequencies were calculated by the χ2 minimization method (23) with a computer program (12) written by L. Sirinek (provided by C. Orosz, both Ohio State University, Columbus, OH).
Lymphoproliferation Assays.
PBMC (1 × 105) were incubated in triplicate in 200 μl RPMI-H in 96-well round-bottomed plates with 1:500 dilution of UV-inactivated HSV-2 (HSV-Ag), mock Ag, or PHA-P (0.4 μg/ml). After 5 days, 1 μCi [3H]thymidine (New England Nuclear) was added to each well for 18 hr, and its incorporation into DNA was measured. Stimulation indices (SI) were calculated as follows: cpm HSV-Ag/cpm (mock Ag). Δcpm were calculated by subtracting cpm from mock Ag wells from cpm from HSV-Ag wells.
The PBMC precursor frequency of HSV-specific proliferative responses (pProlif frequency) was performed as previously described (17). Briefly, limiting dilution analysis was performed by plating PBMC in seven serial 2-fold dilutions starting at 5 × 104 PBMC/well (HIV−, HSV-seropositive people) or 1 × 105 PBMC/well (HSV-seronegative and HIV+ people) in 24 replicates per dilution in 96-well round-bottomed culture plates. Autologous irradiated PBMC (2.5 × 104/well) and a 1:500 dilution of HSV-Ag were added to each well. Twelve control wells were set up as above but replacing HSV-Ag with mock Ag. After 5 days, 1 μCi [3H]thymidine (New England Nuclear) was added to each well for 18 hr, and its incorporation into DNA was measured. Wells were scored as positive if the cpm from experimental wells were greater than 3 SD above the mean cpm from the appropriate 12 control wells. Precursor frequencies were calculated by the χ2 minimization method as described above.
Statistics.
Relationships between pCTL and pProlif frequencies and CD4+ and CD8+ lymphocyte counts were evaluated by Spearman rank order correlation using log transformed values. The Mann–Whitney test was used to evaluate the significance between groups.
RESULTS
HSV-Specific pCTL Responses in HIV+ Individuals.
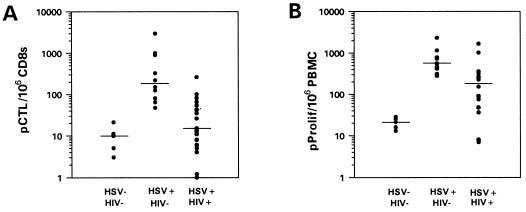
The frequency of HSV-specific CD8+ pCTL was measured in individuals who were HSV-seronegative, HSV-seropositive, or HSV-seropositive and HIV+ (HIV+/HSV+). Because several studies have noted reduced HSV-specific proliferative function during the onset of HSV lesions (24–26), PBMC used in proliferative and CTL assays were obtained from blood drawn at times when no genital lesions were noted. Table 1 lists the CD4 counts and HSV disease history in the HIV+ patients. Fig. 1A demonstrates that HSV-specific CD8+ pCTL frequencies were significantly different between HSV-seronegative subjects (median: 1 in 100,000) and immunocompetent HSV+ subjects (median: 1 in 6,000) (P = .003) (16). Whereas 7 of the 15 HIV+/HSV+ individuals had higher levels of CD8+ pCTL than HSV-seronegative individuals, overall the median response was not significantly different from seronegative individuals (1 in 77,000 vs. 1 in 100,000, P = .24). Immunocompetent individuals infected with HSV had significantly higher CD8+ pCTL than HIV+/HSV+ patients (P = .0005).
Table 1.
Clinical characteristics of HSV infections and CD4 and CD8 T cell counts in HIV-infected patients
| Patient | Gender | CD4 count | CD8 count | HSV serology | Severity of HSV disease* | Duration of HSV, yr | Acyclovir use† |
|---|---|---|---|---|---|---|---|
| A | M | 73 | 1,059 | 1 and 2 | 4+ | 13 | E |
| B | F | 126 | 620 | 1 and 2 | 3+ | 2 | N |
| C | M | 172 | 1,395 | 1 and 2 | 1+ | 11 | N |
| D | M | 159 | 382 | 1 and 2 | 2+ | 15 | E |
| E | M | 468 | 1,319 | 1 and 2 | 3+ | 17 | E + C |
| F | M | 303 | 1,264 | 1 and 2 | 4+ | 30 | E |
| G | M | 13 | 525 | 1 and 2 | 3+ | 5 | N |
| H | M | 380 | 503 | 2 | 2+ | 3 | E |
| I | M | 407 | 785 | 2 | 2+ | 15 | E |
| J | M | 222 | 556 | 1 and 2 | 4+ | 12 | E |
| K | M | 570 | 528 | 1 and 2 | 2+ | 3 | E + C |
| L | F | 310 | 849 | 1 and 2 | 2+ | 10 | E |
| M | F | 573 | 1,023 | 1 and 2 | 2+ | 4 | C |
| N | M | 405 | 1,450 | 1 and 2 | 3+ | 1 | Z |
| O | M | 267 | 499 | 2 | 0 | Unknown | N |
| P | M | 474 | 1,842 | 1 and 2 | 1+ | 12 | E |
| Q | M | 50 | 599 | 1 and 2 | 3+ | Unknown | E |
| R | M | 180 | 1,083 | 2 | 4+ | Unknown | E |
| S | M | 378 | 1,435 | 1 and 2 | 1+ | 6 | E |
| T | M | 111 | 953 | 1 and 2 | 0 | Unknown | N |
Severity rated as follows: 4+, long and frequent recurrences (mean duration of lesions >10 days and >6/yr; moderate to severe pain reported with each lesion); 3+, frequent recurrences (6/yr; mean duration of lesions 4–10 days; mild to moderate symptoms); 2+, moderate recurrences (<6/yr; mean duration of lesions 3–10 days; minimal symptoms with recurrences); 1+, mild (<6/yr, minimal symptoms, average duration of lesions 3–4 days); 0, not symptomatic.
E, episodic; C, chronic; Z, used for zoster; N, not used.
Figure 1.
Impaired HSV-specific pCTL and pProlif responses in HIV+ individuals. The HSV-specific pCTL frequency per million CD8s from PBMC (A) and the HSV-specific pProlif frequency per million PBMC (B) were measured in individuals who were HSV-seronegative and HIV− (HSV-HIV−), HSV-seropositive and HIV− (HSV+HIV−), or HSV-seropositive and HIV-positive (HSV+HIV+). The median precursor frequency in each group of individuals is displayed. pCTL data for HSV-HIV− and HSV+HIV− are from ref. 16.
HSV-Specific pProlif Responses in HIV+ Individuals.
HSV-specific proliferation of PBMC was measured in two ways: bulk PBMC proliferation in response to HSV-Ag vs. mock Ag, reported as SI and Δcpm, and HSV-specific PBMC pProlif frequency determined by limiting dilution analysis. Numerous studies have shown that HSV-Ag stimulation of PBMC results in the activation and proliferation of CD4+ T cells (11, 27). To ensure that the proliferation we measured in PBMC was due to the expansion of CD4+ HSV-specific T cells, CD4+ cells were depleted from PBMC from several HIV+ and HIV− individuals and stimulated with HSV-Ag. Depletion of CD4+ T cells from PBMC reduced the [3H]thymidine incorporation by greater than 80% (data not shown). Thus, for proliferation studies, proliferation was measured in bulk PBMC cultures.
The median HSV-specific pProlif frequency in HSV-seronegative individuals was 1 in 48,000 vs. 1 in 1,800 in immunocompetent individuals with culture-proven HSV-2 infection (P = .003) (Fig. 1B). HIV+ individuals coinfected with HSV-2 had an intermediate pProlif frequency of 1 in 5,300, which was significantly different from immunocompetent seropositive individuals (P = .006). Of interest, bulk proliferative responses to HSV antigens, as measured by stimulation index and Δcpm, were similar between the immunocompetent vs. HIV+ patients (Table 2), suggesting that the pProlif assay was more indicative of impairment in immune responses than the bulk stimulation index. HSV-specific lymphoproliferative responses from HSV-seropositive individuals, with or without HIV infection, were significantly greater than responses in the HSV-seronegative population. The pProlif frequencies of the seven HIV+ patients with pCTL greater than those in HSV-seronegatives was 326/106 vs. 90/106 in the eight HIV+ patients with pCTL similar to HSV-seronegatives (P = .3, not significant). Proliferative responses to PHA were similar in HIV− individuals regardless of HSV status and were significantly higher than in HIV+ individuals (P = .03). All the HIV+ patients we studied had PHA responses >3,000 cpm.
Table 2.
Bulk proliferation specific for HSV-Ag and PHA in HIV+ and HIV− patients
| Patients | n | HSV-AG
|
PHA Median cpm, range§ | |
|---|---|---|---|---|
| Median SI, range | Median Δcpm, range | |||
| HSV−*† | 5 | 11 (4–16) | 2,963 (339–6,053) | 24,584 (10,667–44,931) |
| HSV+*‡ | 7 | 119 (20–165) | 23,436 (7,836–45,469) | 27,391 (15,386–50,603) |
| HSV+HIV+†‡ | 17 | 42 (3–194) | 20,075 (334–43,783) | 14,289 (3,304–49,796) |
PBMC from HSV-seronegative (HSV−), HSV-seropositive (HSV+), and HIV+-HSV-seropositive (HSV+HSV+) subjects were tested for proliferation in response to HSV-AG and PHA and the median values for SI, Δcpm (HSV-AG), and total cpm (PHA) with ranges are displayed.
*HSV− vs. HSV+: SI, P = .003, Δcpm, P = .003.
HSV− vs. HSV+HIV+: SI, P = .01; Δcpm, P = .02
HSV+ vs. HSV+HIV+: SI, P = .24; Δcpm, P = .31.
HIV− vs. HIV+: cpm (PHA), P = .03.
HSV Disease Severity Correlates with HSV-Specific pCTL and Not pProlif Frequencies in HIV+ Individuals.
HIV+ patients with more severe genital herpes defined by both more frequent and more prolonged episodes of disease had a significantly lower pCTL frequency (median: 1 in 167,000) than those individuals with mild disease (0–2+) (median: 1 in 26,000) (P = .03) (Table 3). In contrast, no significant difference was seen in pProlif frequencies between those patients with mild (median: 1 in 3,800) and severe (median: 1 in 6,600) genital herpes (P = 1) (Table 3). No difference was seen in CD4+ or CD8+ lymphocyte counts between patients with mild vs. severe genital herpes disease (Table 3). Similarly, no significant difference was observed in SI or Δcpm in response to HSV-Ag or in PHA responses between individuals with mild or severe genital herpes (data not shown). Therefore, lower HSV-specific pCTL frequencies, and not HSV-specific pProlif frequencies, SIs or Δcpm, were observed in HIV+ patients who experienced longer and more frequent genital HSV recurrences than those individuals who had mild disease.
Table 3.
Frequency of HSV-specific CD8+ pCTL and pProlif in HIV+ patients with severe vs. mild HSV disease
| Severity of HSV disease | n | Median lymphocyte count/μl, range
|
HSV-specific CD8+ pCTL
|
HSV-specific pProlif
|
|||
|---|---|---|---|---|---|---|---|
| CD4+ | CD8+ | Frequency* | Range | Frequency | Range | ||
| HSV-specific CD8+ pCTL | |||||||
| 0–2+ | 8 | 323 (111–570) | 656 (382–1,842) | 1/26,000 | 1/4,000–1/83,000 | ||
| 3+–4+ | 9 | 180 (13–468) | 1,059 (525–1,450) | 1/167,000 | 1/12,000–<1/106 | ||
| HSV-specific pProlif | |||||||
| 0–2+ | 7 | 267 (111–474) | 953 (382–1,842) | 1/3,800 | 1/1,000–1/123,000 | ||
| 3+–4+ | 7 | 246 (50–405) | 1,083 (556–1,450) | 1/6,600 | 1/600–1/142,000 | ||
Frequency of CD8+ pCTL in 0–2 vs. 3+–4+, P = .03; all other comparisons not significant (P > .1).
pCTL and pProlif Frequencies Correlate with CD4+ and Not CD8+ Lymphocyte Counts in HIV+ Individuals.
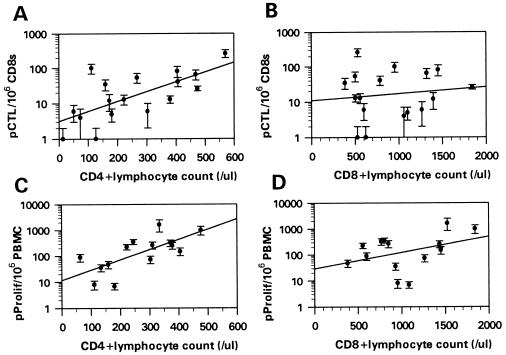
HSV-specific pCTL and pProlif frequencies from HIV+ individuals were plotted against their absolute CD4+ and CD8+ lymphocyte counts. Fig. 2 demonstrates that HSV-specific pCTL frequencies were positively correlated with CD4+ lymphocyte counts (r = .68, P < .01) but not correlated with CD8+ lymphocyte counts (r = .09, P > .2). Likewise, pProlif frequencies specific for HSV antigens also correlated with CD4+ lymphocyte counts (r = .70, P < .01) but not with CD8+ lymphocyte counts (r = .37, P > .1) (Fig. 2). No significant correlations were seen between CD4+ or CD8+ lymphocyte counts and SI or Δcpm or between PHA responses and CD4+ or CD8+ lymphocyte counts (data not shown). Therefore, in HIV+ individuals, the CD4+ lymphocyte count, and not the CD8+ lymphocyte count, was predictive of HSV-specific pCTL and pProlif frequencies.
Figure 2.
HSV-specific pCTL and pProlif frequencies positively correlate with CD4+ and not CD8+ lymphocyte counts. pCTL and pProlif frequencies were plotted against absolute CD4+ (A and C) and CD8+ (B and D) lymphocyte counts. pCTL and pProlif frequencies are displayed as number of precursors per million CD8s (pCTL) or million PBMC (pProlif). Error bars represent the 95% confidence interval of pCTL and pProlif frequencies.
Role of Irradiated PBMC (Feeders) and HSV Blasts in Generating HSV-Specific pCTL and pProlif Responses.
One potential explanation for the low pCTL frequencies measured in vitro among patients with low CD4+ lymphocyte counts is defective antigen presentation from the accessory cells used in the in vitro culture system. In an attempt to address this issue, pCTL and pProlif frequencies were measured in patient T, an HIV+ (CD4 count = 111), HSV-2 seropositive subject. These pCTL and pProlif frequencies were compared with frequencies measured when the patient’s feeders/antigen-presenting cells (APC) (pCTL and pProlif) and HSV blasts (pCTL only) were replaced with feeders/APC and HSV blasts from patient U, the HSV-1 seropositive HIV− identical twin brother of patient T (Fig. 3). The frequency of HSV-specific pCTL (1 in 10,000) was the same regardless of which HSV blasts and feeders were used. The frequency of HSV-specific pProlif was also similar when feeders/APC from patient U were used compared with autologous feeders/APC (1 in 63,000 vs. 1 in 123,000). The frequency of pProlif to HSV was still below the median pProlif frequency of HSV-seronegative patients (1 in 48,000) and HIV− patients (1 in 5,300). Thus, the pProlif response to HSV in an HIV+ patient was modestly increased with irradiated PBMC from a syngeneic HIV− patient but not restored to the level of HSV+HIV− patients. Of interest, patient T is an example of a HIV+ individual with HSV-2 infection with a very low pProlif response but a normal pCTL response to HSV. He reactivated HSV-2 only infrequently.
Figure 3.
The role of feeders and stimulators in generating HSV-specific pCTL and pProlif responses in an HIV+ individual. The frequency of HSV-specific pCTL (f) in patient T was measured (A) using autologous HSV blasts and irradiated PBMC (T blasts/feeders) or HSV blasts and irradiated PBMC from patient U (U blasts/feeders). Similarly, the frequency of HSV-specific pProlif in patient T was measured (B) using autologous irradiated PBMC as APC (T feeders/APC) or cells from patient U (U feeders/APC).
DISCUSSION
HIV+ patients with genital herpes had significantly lower numbers of HSV-specific CTL and proliferative precursors in PBMC compared with immunocompetent individuals with genital herpes. On an individual basis, the severity of genital herpes lesions in HIV+ individuals was directly related to the frequency of HSV-specific CD8+ pCTL and not related to the frequency of proliferative precursors or to the level of bulk proliferation to HSV. HIV+ individuals with higher pCTL to HSV had much fewer and milder recurrences of genital herpes than those with lower pCTL irrespective of their pProlif responses or their CD4 count.
We did find an association between the absolute CD4 T cell count and frequency of pCTL to HSV. The observation that the frequency of HSV-specific CD8+ pCTL correlates with CD4 count is similar to that observed for HIV-specific CD8+ pCTL but in contrast to the lack of correlation between CD4 count and EBV-specific CD8+ pCTL frequencies. The apparent maintenance of EBV-specific pCTL during CD4+ lymphocyte loss and the decline of HSV- and HIV-specific pCTL may reflect differential requirements for the generation of CTL responses to each virus.
Whereas we were able to show a correlation between absolute CD4 count and memory proliferative responses to HSV, there was no correlation with disease severity. Recently, Schrier et al. (28) suggested that HIV+ patients were at a higher risk of HIV disease progression when higher proliferative responses to HSV and cytomegalovirus were present in bulk PBMC cultures. We found no correlation between CD4+ lymphocyte counts and bulk proliferation to HSV in HIV+ people. However, the frequency of HSV-specific pProlif as determined by limiting dilution analysis declined with declining CD4+ lymphocyte counts. This decline was less marked than that seen with memory CTL responses. Technical differences likely explain the difference seen between the two measures of HSV-specific proliferation. Bulk proliferation represents the mean of triplicate wells with 100,000 responder PBMC, whereas limiting dilution analysis is more sensitive and quantitative in that the proliferative capacity of 24 replicates diluted seven times is measured. In several patients, we found reasonably high memory proliferative responses to HSV but no HSV-specific CD8+ CTL precursors. Whether these HSV-specific proliferative T cells can provide help to HSV-specific CD8+ CTL is unclear. That a significant proliferative response can be measured in the absence of a measurable CD8+ pCTL response would suggest that either these cells cannot provide appropriate help to activate HSV-specific CD8+ pCTL or that there is a selective impairment or loss of HSV-specific CD8+ pCTL in HIV+ patients. Although we did not measure HSV-specific CD4+ CTL responses in this cohort of patients, it is likely that CD4+ CTL make up part of the proliferating pool of cells responding to HSV antigens in our proliferation assays. Future studies will determine the role of HSV-specific CD4+ CTL in controlling HSV disease in HIV+ patients.
The association between absolute CD4 count and the severity of an opportunistic infection is often not linear. In larger studies, we have shown that the frequency of HSV reactivation varies greatly among individuals with similar CD4 counts (L.C., unpublished data), similar to the findings presented here. Our data suggest that antigen-specific immune responses are likely to be a closer determinant of clinical disease than absolute CD4 count.
Whether replenishment of HSV-specific pCTL through adoptive immunotherapy will alter HSV disease among HIV+ individuals with low pCTL remains to be determined. Successful reconstitution of cellular immunity to cytomegalovirus and EBV by adoptive transfer of donor-derived T cells has been proven safe and effective in bone marrow transplant patients (29) and in patients at risk for the development of EBV lymphoproliferative disease (30).
Our data also have some implications toward defining what are the key components of the cellular immune response to HSV-2. Previous work has suggested that CD4+ T cell responses to HSV were the predominate HSV-specific T cell response. Recently, we developed methods to accurately measure HSV-specific CD8+ pCTL from patients with HSV disease and demonstrated high frequencies of class I-restricted CTL to HSV in all seropositive individuals (16). The data in this report extend those findings to show a clinical association between pCTL frequency and severity of HSV disease in HIV+ individuals. We evaluated HIV+ patients because we felt that the differences in severity of HSV lesions between patients would be great enough to potentially note differences in pCTL frequencies. In our study, six of eight patients with severe genital HSV had HSV-specific CD8+ pCTL were in the lowest quartile of all responses. The fact that two patients still had severe HSV reactivations despite what appeared to be adequate pCTL suggests that other mechanisms of host resistance are also likely operative in HSV disease. Of interest, those two patients had high CD4 T cell counts (468/μl and 407/μl).
In summary, our study demonstrates a quantitative threshold between memory CD8+ T cell responses and severe reactivation of an endemic opportunistic pathogen in HIV+ patients. Moreover, our data suggest that resolution of mucosal T cell infection by HSV appears related to host CD8+ T cell responses.
Acknowledgments
We thank Drs. Luwy Musey and Jeff Vieira for many helpful discussions, Dr. Mark Gilbert for critical reading of the manuscript, Gail Barnum and Theresa Shea for specimen collection, and Matthew Johnson and Excel Guerrero for excellent technical assistance. This work was supported by National Institutes of Health Grants AI34616, CA70017 (D.M.K.) and AI30731 (L.C.), the Medical Research Council of Canada, and the National Research Development Program of Canada (postdoctoral fellowship to C.M.P.).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: HIV-infected, HIV+; CTL, cytotoxic T lymphocyte; pCTL, cytotoxic T lymphocyte precursor; pProlif, proliferative precursor; HSV-2, herpes simplex virus 2; OI, opportunistic infection; PBMC, peripheral blood mononuclear cells; PHA, phytohemagglutinin; SI, stimulation indices; EBV, Epstein–Barr virus; APC, antigen-presenting cells.
References
- 1.Halbert S P, Kiefer D J, Friedman-Kien A E, Poiesz B. J Clin Microbiol. 1986;23:318–321. doi: 10.1128/jcm.23.2.318-321.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enzensberger R, Braun W, July C, Helm E B, Doerr H W. Infection. 1991;19:140–145. doi: 10.1007/BF01643233. [DOI] [PubMed] [Google Scholar]
- 3.Stamm W E, Handsfield H H, Rompalo A M, Ashley R L, Roberts P L, Corey L. J Amer Med Assoc. 1988;260:1429–1433. [PubMed] [Google Scholar]
- 4.Augenbraun M, Feldman J, Chirgwin K, Zenilman J, Clarke L, DeHovitz J, Landesman S, Minkoff H. Ann Int Med. 1995;123:845–847. doi: 10.7326/0003-4819-123-11-199512010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Kinghorn G R. J Int Med Res. 1994;22:14A–23A. [PubMed] [Google Scholar]
- 6.Augenbraun M H, McCormack W M. Infect Dis Clin N Amer. 1994;8:439–448. [PubMed] [Google Scholar]
- 7.Margolis D M, Rabson A B, Straus S E, Ostrove J M. Virology. 1992;186:788–791. doi: 10.1016/0042-6822(92)90048-t. [DOI] [PubMed] [Google Scholar]
- 8.Englund J A, Zimmerman M E, Swierkosz E M, Balfour H H. Ann Int Med. 1990;112:416–422. doi: 10.7326/0003-4819-76-3-112-6-416. [DOI] [PubMed] [Google Scholar]
- 9.Safrin S, Kemmerly S, Plotkin B, Smith T, Weissbach N, De Veranez D, Phan L D, Cohn D. J Infect Dis. 1994;169:193–196. doi: 10.1093/infdis/169.1.193. [DOI] [PubMed] [Google Scholar]
- 10.Boivin G, Edelman C K, Pedneault L, Talarico C L, Biron K K. J Infect Dis. 1994;170:68–75. doi: 10.1093/infdis/170.1.68. [DOI] [PubMed] [Google Scholar]
- 11.Yasukawa M, Zarling J M. J Immunol. 1984;133:2736–2742. [PubMed] [Google Scholar]
- 12.Clouse K A, Adams P W, Orosz C G. J Clin Microbiol. 1989;27:2316–2323. doi: 10.1128/jcm.27.10.2316-2323.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikloska Z, Kesson A M, Penfold M E T, Cunningham A L. J Infect Dis. 1996;173:7–17. doi: 10.1093/infdis/173.1.7. [DOI] [PubMed] [Google Scholar]
- 14.Carmack M A, Yasukawa L L, Chang S Y, Tran C, Saldana F, Arvin A M, Prober C G. J Infect Dis. 1996;174:899–906. doi: 10.1093/infdis/174.5.899. [DOI] [PubMed] [Google Scholar]
- 15.Tigges M A, Koelle D M, Hartog K, Sekulovich R E, Corey L, Burke R L. J Virol. 1992;66:1622–1634. doi: 10.1128/jvi.66.3.1622-1634.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Posavad C M, Koelle D M, Corey L. J Virol. 1996;70:8165–8168. doi: 10.1128/jvi.70.11.8165-8168.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koelle D M, Corey L, Burke R L, Eisenberg R J, Cohen G H, Pichyangkura R, Triezenberg S J. J Virol. 1994;68:2803–2810. doi: 10.1128/jvi.68.5.2803-2810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koelle D M, Abbo H, Peck A, Ziegweid K, Corey L. J Infect Dis. 1994;169:956–961. doi: 10.1093/infdis/169.5.956. [DOI] [PubMed] [Google Scholar]
- 19.Hersh E M, Gutterman J U, Spector S, Friedman H, Greenberg S B, Reuben J M, LaPushin R, Matza M, Mansell P W A. Cancer Res. 1985;45:406–410. [PubMed] [Google Scholar]
- 20.Wainberg M A, Portnoy J, Tsoukas C, Gilmore N. Immunology. 1987;60:275–280. [PMC free article] [PubMed] [Google Scholar]
- 21.Carrega G, Canessa A, Argenta P, Cruciani M, Bassetti D. AIDS Res Hum Retroviruses. 1995;11:741–746. doi: 10.1089/aid.1995.11.741. [DOI] [PubMed] [Google Scholar]
- 22.Posavad C M, Rosenthal K L. J Virol. 1992;66:6264–6272. doi: 10.1128/jvi.66.11.6264-6272.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taswell C. J Immunol. 1981;126:1614–1619. [PubMed] [Google Scholar]
- 24.Kuo Y C, Lin C Y. J Med Virol. 1990;31:183–189. doi: 10.1002/jmv.1890310303. [DOI] [PubMed] [Google Scholar]
- 25.Vestey J P, Norval M, Howie S, Maingay J, Neill W A. Clin Exper Immunol. 1989;77:384–390. [PMC free article] [PubMed] [Google Scholar]
- 26.Vestey J P, Norval M, Howie S E M. J Roy Soc Med. 1990;83:308–311. doi: 10.1177/014107689008300510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasukawa M, Inatsuke A, Kobayashi Y. J Immunol. 1988;140:3419–3425. [PubMed] [Google Scholar]
- 28.Schrier R D, Wiley C A, Spina C, McCutchan J A, Grant I. J Clin Invest. 1996;98:731–740. doi: 10.1172/JCI118845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walter E A, Greenberg P D, Gilbert M J, Finch R J, Watanabe K S, Thomas E D, Riddell S R. New Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 30.Heslop H E, Ng C Y C, Li C, Smith C A, Loftin S K, Krance R A, Brenner M K, Rooney C M. Nat Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]