Abstract
During differentiation in vitro, embryonic stem (ES) cells generate progenitors for most hemato-lymphoid lineages. We studied the developmental potential of two ES cell subpopulations that share the fetal stem cell antigen AA4.1 but differ in expression of the lymphoid marker B220 (CD45R). Upon transfer into lymphoid deficient mice, the B220+ population generated a single transient wave of IgM+ IgD+ B cells but failed to generate T cells. In contrast, transfer of the B220− fraction achieved long-term repopulation of both T and B lymphoid compartments and restored humoral and cell-mediated immune reactions in the recipients. To assess the hemato-lymphopoietic potential of ES cell subsets in comparison to their physiological counterparts, cotransplantation experiments with phenotypically homologous subsets of fetal liver cells were performed, revealing a more potent developmental capacity of the latter. The results suggest that multipotential and lineage-committed lymphoid precursors are generated during in vitro differentiation of ES cells and that both subsets can undergo complete final maturation in vivo.
Murine embryonic stem (ES) cells can spontaneously differentiate in vitro to structures resembling yolk sac blood islets (1) and, therefore, have become a model system for the study of cellular and molecular aspects of hematopoiesis (for review, see ref. 2). Initial reports on differentiating ES cells described the presence of hematopoietic cytokines and their respective receptors (3) and of myeloid (4, 5) and erythroid (1) progenitors. In the latter, the sequential expression of hemoglobin genes resembled the transition of “primitive” to “definitive” erythroid cells in vivo (6–8). Furthermore, the observation of angiogenesis in embryoid bodies (9) further underlines the similarities between ES differentiation in vitro and yolk sac development in vivo (extensively reviewed in ref. 10). More recently, relatively mature lymphoid progenitors have been demonstrated, either within in vitro-derived embryoid bodies (11) or by cocultivation on stroma cell lines (12). In addition to these in vitro approaches, unseparated differentiated ES cells have been shown to restore the lymphoid compartment of SCID (13, 14) or Rag-deficient mice (15). The capacity of ES cells to develop in vitro into virtually all hematopoietic lineages suggests the presence of a hematopoietic stem cell (HSC) or a primitive multilineage progenitor in these culture systems. Indeed, during a limited time span of ES cell differentiation, primitive multilineage progenitors have recently been isolated (16). In addition, by cocultivating ES cells with stroma cell lines in medium supplemented with various cytokines, a stem cell-like activity has been described (17).
In our previous studies of ES cell-derived lymphopoiesis, uncommitted hematopoietic precursor cells appeared to coexist with relatively advanced progenitors for both T and B cell lineages. To further characterize these stages in hemato-lymphopoiesis, it was our aim to identify ES cell subpopulations that harbor the different types of precursor activities. Moreover, we asked the question which of these progenitor/precursor populations gave rise to the lymphoid reconstitution reported by other groups upon in vivo transfer of unseparated ES cells (13–15). To this end, phenotypically defined subpopulations of in vitro-differentiated ES cells were tested for their ability to reconstitute the lymphoid system of Rag-deficient mice. The results identify two distinct subsets of in vitro-differentiated ES cells, harboring lymphoid potential with distinct developmental capacities. The direct comparison of these subsets to progenitors developing in vivo will allow the further definition of the developmental capacity of ES cell-derived hematopoiesis.
MATERIALS AND METHODS
Animals and Cell Lines.
Rag-1-deficient mice, backcrossed to C57/BL6 (Ly-5.2+, Ly-9.1−), were maintained under specific-pathogen-free conditions in the animal facility of our institute. Breeding stocks of C57/BL6 Ly-5.1 (Ly-5.1+, Ly-9.1−) were provided by H.-R. Rodewald (Basel Institute for Immunology, Basel, Switzerland). Fetal liver (FL) was isolated from timed matings of C57/BL6 Ly-5.1 mice. The day of the vaginal plug was counted as day 0.5 of pregnancy. The C57/BL6-derived ES cell lines Bl6-III or 129/Sv-derived D3/M were maintained in an undifferentiated state by culture on a monolayer of mitomycin C-inactivated embryonic fibroblasts.
Differentiation of ES Cells and Preparation of Cells.
For differentiation cultures, ES cells were dissociated by trypsinization and cultured in Iscove’s modified Dulbecco’s medium (Biochrom, Berlin) supplemented with 15% fetal calf serum and 4.5 × 10−4 M monothioglycerol. For the induction of differentiation, ES cells were plated in gas-permeable 60-mm hydrophobic culture dishes (Heraeus) at a concentration of 0.8–1.4 × 104 cells per ml in a total volume of 5 ml. In some experiments embryoid bodies were induced by culturing 15–20 ES cells in 20 μl in inverted hanging drops. Embryoid bodies were transferred after 2–3 days and seeded on gas-permeable culture dishes. During the whole differentiation period, cultures were maintained at 37°C in an atmosphere of 7.5% CO2/5% O2 by using an incubator with adjustable oxygen content (Heraeus). After 15 days of differentiation, embryoid bodies were harvested and dissociated by gentle digestion with collagenase (0.1 unit/mg) and dispase (0.8 unit/mg; Boehringer Mannheim) in PBS. Cells were labeled with purified anti-CD45 monoclonal antibody (mAb) M1/9.3.4.HL.2, followed by goat anti-rat immunoglobin (Ig) conjugated to paramagnetic microbeads (Miltenyi, Bergisch Gladbach, Germany), and enriched by single passage over a MACS (Miltenyi) system. Enrichment was found to increase the number of CD45+ cells from 21% (range: 16.7–32.5%) to 48% (range: 41.2–57.3%), as analyzed by flow cytometry. FL cells were prepared similarly, except that the initial digestion step was omitted. Bone marrow, spleen, thymus, and lymph node cells of reconstituted animals were prepared by standard procedures. Bone marrow and lymph node cells were depleted for surface IgM+ cells by incubation with a biotinylated goat anti-mouse IgG/IgM serum (Jackson-Dianova, Hamburg, Germany) detected by streptavidin-paramagnetic microbeads (Miltenyi), followed by separation on MACS. Both preparations were found to contain less than 1% IgM+ cells.
Staining, Flow Cytometry, and Cell Sorting.
For further purification of MACS-enriched ES or FL populations, cells were purified by flow cytometry. After blocking nonspecific antibody binding with normal rat Ig, cells were stained with fluorescein isothiocyanate-labeled anti-B220 mAb (RA3–6B2, PharMingen) and biotinylated mAb AA4.1 (18), followed by streptavidin-phycoerythrin (Southern Biotechnology Associates) in PBS/5% fetal calf serum on ice. Cells were separated into AA4.1+ B220− and AA4.1+ B220+ fractions by using a FACStar Plus cell sorter (Becton Dickinson). Sorted cells were found to be ≥98% pure upon reanalysis. For phenotypic analysis, single cell suspensions were stained with mAbs as indicated. Dead cells were excluded from analysis on a FACScan (Becton Dickinson) by propidium iodide (1 μg/ml) counterstaining. For each diagram, at least 2 × 104 cells were analyzed on a logarithmic scale.
Cell Transfer into Rag-1-Deficient Animals.
Sorter-purified cell populations were injected intravenously in the lateral tail vein of 4- to 6-week-old Rag-1−/− mice in 100 μl of PBS. To facilitate hematopoietic development in vivo, animals were sublethally irradiated with 400 rads (1 rad = 0.01 Gy) from an x-ray source prior to transfer. Mice were housed in a positive-pressure cabinet and received neomycin (0.1 g/100 ml) and Borgal (10% final concentration; Hoechst) for the first 2 weeks after irradiation. Injected cell numbers are indicated in the text.
ELISAs.
Ig production in the serum of reconstituted mice was tested by standard ELISA. Briefly, Nunc-Immuno plates (Nunc) were coated with goat anti-mouse Ig F(ab)2 (Jackson-Dianova) or purified mAbs from allotype-specific hybridomas RS3.1 (anti-mouse IgMa) or AF6 (anti-mouse IgMb). Serum samples were serially diluted and detected with alkaline phosphatase-conjugated goat anti-mouse IgM or IgG (Southern Biotechnology Associates). Results were quantified by using external standards (all from Southern Biotechnology Associates). Relative serum titers of different antibody isotypes (IgM, IgG1, IgG2a, IgG2b, and IgG3) were determined by incubating serum samples on trinitrophenyl-ovalbumin (50 μg/ml)-coated plates, followed by detection with alkaline-phosphatase-conjugated isotype-specific secondary antibodies (all from Southern Biotechnology Associates). Absorption was read at 405/490 nm by using p-nitrophenyl phosphate (Boehringer Mannheim) as a substrate.
Immunization and Proliferation Experiments.
Reconstituted Rag-1−/− mice were injected s.c. (base of the tail) with 100 μl of an emulsion of 100 μg of trinitrophenyl-ovalbumin in PBS and incomplete Freund’s adjuvant. Two weeks later, immunization was repeated s.c. as well as i.p. Serum was taken at the indicated times before and after booster immunization. Mesenteric and inguinal lymph nodes were prepared and depleted of IgM+ cells as described above. For proliferation assays, 105 T cells were stimulated with Con A (5 μg/ml) and ovalbumin (100 μg/ml or 250 μg/ml) for 48 h and pulsed for 8 h with 0.5 μCi of [3H]thymidine (1 Ci = 37 GBq). All results represent the means of triplicates, with standard deviations shown by error bars.
RESULTS
Restoration of the Lymphoid Compartment of Rag-1-Deficient Mice by Transfer of in Vitro-Differentiated ES Cell Subsets.
Upon in vitro differentiation, ES cells express genes and surface antigens indicative for hemato-lymphoid commitment (4, 11). We first addressed the question whether a combination of surface molecules could be used to enrich, and/or to distinguish between, different stages of hemato-lymphopoietic development. To concentrate on hematopoietic cells, day 15 differentiated ES cells were first enriched for CD45 (T200) by MACS. The enriched population was then analyzed by flow cytometry for expression of B220 and AA4.1. We have reported the expression of B220 (CD45R) on a small proportion (≈ 3%) of ES cells differentiated in vitro for 15–20 days (11). The AA4.1 antibody has been described to recognize fetal HSCs (19) and primitive multilineage progenitors (20). Double staining for these markers reveals four distinct populations (21): The majority is negative for both markers (≈57%), a small population (≈3%) expresses B220 only, and the remaining AA4.1+ cells can be divided into a B220− (≈32%) fraction and a B220+ (≈8%) fraction. In preliminary transfer experiments, the B220 single-positive population did not give rise to any lymphoid cell type in vivo and was, therefore, not further investigated. The AA4.1+ B220− and AA4.1+ B220+ fractions were isolated by preparative flow cytometry and transferred into Rag-1-deficient mice. These mice are convenient hosts because, in contrast to lethally irradiated mice, their long-term survival does not require HSC transfer. In addition, because of the complete block of lymphocyte maturation, all mature lymphocytes in these mice are donor-derived. To facilitate hematopoietic reconstitution, Rag-1−/− recipients were sublethally irradiated with 400 rad and subsequently i.v.-injected with 104 AA4.1+ B220− or AA4.1+ B220+ ES cells.
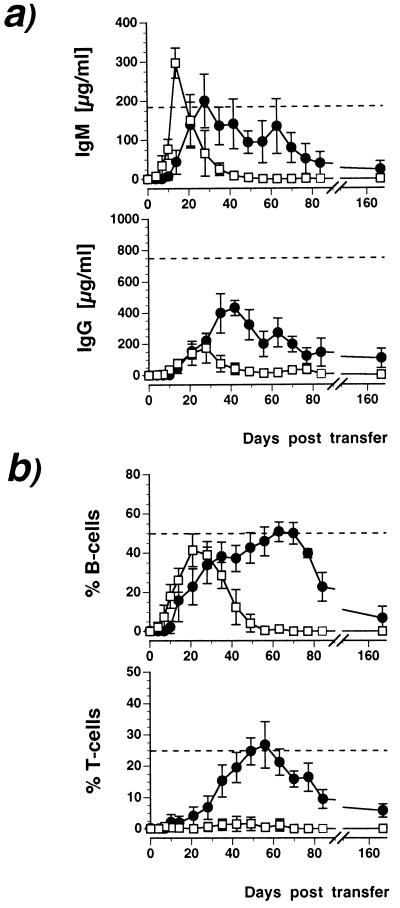
Transfer of the AA4.1+ B220+ population rapidly reconstituted serum IgM, reaching C57BL/6 wild-type levels within 2 weeks (Fig. 1a). These IgM levels quickly returned to background and became undetectable at 8 weeks. IgG was restored by the AA4.1+ B220+ population only to a small extent and with delayed kinetics compared with IgM (Fig. 1a). Thus, AA4.1+ B220+ cells gave rise to a single and transient wave of serum Ig. In contrast, transfer of the AA4.1+ B220− fraction reconstituted serum Ig with slower kinetics, reaching maximal levels within 4 weeks (IgM) or 5 weeks (IgG) (Fig. 1a). Serum levels declined more slowly and both Ig classes were still detectable 24 weeks after transfer, i.e., for the entire period of observation. The analysis of peripheral blood lymphocytes in Rag-1−/− recipients reconstituted with the AA4.1+ B220+ fraction revealed the complete absence of T cells and the emergence of a single wave of B cells (Fig. 1b). In contrast, the AA4.1+ B220− subset developed into both T and B lymphoid lineages, which remained detectable until week 24 (Fig. 1b). Progeny of either ES cell subset were able to repopulate secondary lymphoid organs, including spleen (Table 1) and lymph node (data not shown). As in peripheral blood, AA4.1+ B220+ derived cells were detectable in the spleen for up to week 12 but not thereafter. In bone marrow, they gave rise to IgM+ IgD+ cells only (Fig. 2b), suggesting reentry from the circulation rather than local development. AA4.1+ B220−-derived cells were detectable in the spleen until week 24 (Table 1), and most were IgM single-positive in the bone marrow (Fig. 2b), suggesting local development. These experiments were performed with the congenic C57/BL6-derived ES cell line Bl6-III and allogenic 129/Sv-derived D3/M cells, without apparent strain-dependent variations in lymphoid reconstitution. Thus, these results are consistent with the notion that the AA4.1+ B220+ subset contains a relatively mature precursor committed to the B cell lineage that is unable to self renew, whereas the AA4.1+ B220− population harbors multilineage progenitor activity giving rise to long-term reconstitution of the entire lymphoid compartment.
Figure 1.
Kinetics of peripheral lymphoid reconstitution in Rag-1-deficient mice with ES-derived lymphoid progenitors. At indicated times after transfer, blood was analyzed for serum IgM and IgG (a) and for peripheral blood B and T cells (b). (a) Appearance of IgM (Upper) and IgG (Lower) after the injection of 104 AA4.1+ B220+ (□) or 104 AA4.1+ B220− (•) ES-derived progenitors. Cellular reconstitution was assessed by two-color flow cytometry for IgM/CD45 (b Upper) or CD3/CD45 (b Lower) by using the same samples as in a. Results are given either as total amount of Ig (a) or as numbers in percent relative to total (CD45+) leukocytes in blood. Dashed lines indicate wild-type levels of Ig and B or T cells in C57/BL6 mice for comparison. The data represent results (mean ± SD) from three experiments with four to six animals per group.
Table 1.
Development of AA4.1+ B220+ and AA4.1+ B220− in vitro-differentiated ES cells into mature lymphocytes upon transfer into Rag-1−/− mice
| Flow cytometry analysis, % positive cells
|
|||||||
|---|---|---|---|---|---|---|---|
| 8 weeks after transfer
|
12 weeks after transfer
|
24 weeks after transfer
|
|||||
| AA4.1+ B220+ | AA4.1+ B220− | AA4.1+ B220+ | AA4.1+ B220− | AA4.1+ B220+ | AA4.1+ B220− | ||
| (experiments 1, 2, and 4; n = 8) | (experiments 1, 2, 3, and 5; n = 11) | (experiments 3 and 5; n = 5) | |||||
| Thymus | TCR αβ | 0.0 | 38.2 | 0.0 | 33.8 | 0.0 | 24.2 |
| TCR γδ | 0.0 | 6.9 | 0.0 | 1.8 | 0.0 | 2.5 | |
| Spleen | CD3 | 0.4 | 17.8 | 0.2 | 22.4 | 0.1 | 12.6 |
| IgM | 19.4 | 20.4 | 9.6 | 22.6 | 0.8 | 12.2 | |
| IgD | 16.8 | 18.3 | 6.8 | 20.9 | 0.6 | 13.6 | |
| Bone marrow | CD3 | 0.1 | 0.2 | 0.0 | 0.1 | 0.2 | 0.0 |
| IgM | 7.8 | 8.9 | 1.6 | 9.4 | 0.9 | 3.8 | |
| IgD | 5.9 | 0.8 | 0.9 | 1.2 | 0.4 | 0.7 | |
Sublethally irradiated Rag-1−/− mice were reconstituted by intravenous injection of 104 cells of the indicated subsets of day 15 ES cells. Data from five experiments with ES cell line Bl6-III (C57/BL6 derived, experiments 1 and 2) or D3/M (129/Sv, experiments 3, 4, and 5) are shown. In every experiment, each group contained initially five or six animals. Results are given as the mean of antigen expression as determined by flow cytometry. The origin of the ES cells and the number of animals analyzed are given for every time separately. TCR, T cell receptor.
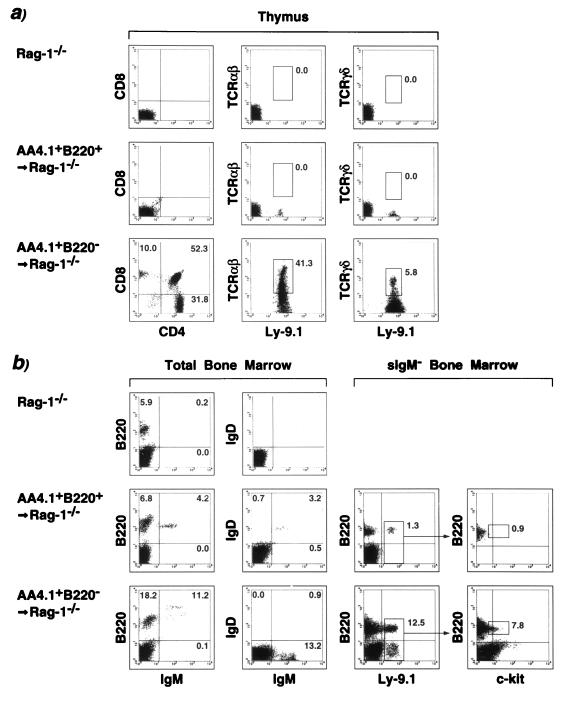
Figure 2.
Lymphoid reconstitution of Rag-1−/− mice by subsets of in vitro-differentiated ES cells. ES cells were separated in AA4.+ B220+ and AA4.1+ B220− populations as detailed. Eight weeks after intravenous injection of 104 cells of the respective populations, mice were analyzed for lymphoid reconstitution in the thymus (a) or in the bone marrow (b). Thymocytes were stained with CD4/CD8 or T cell receptor (TCR) αβ, TCR γδ, and the donor-specific marker Ly-9.1. (b) Total bone marrow was investigated for the presence of IgM- or IgD-expressing cells. To detect donor-derived pro-B cells, bone marrow was depleted for surface IgM+ cells and stained simultaneously for B220, c-kit, and Ly-9.1. Numbers given in the individual quadrants and gates indicate percentages.
To examine the contribution of ES-derived cells to earlier stages of lymphoid development, it was necessary to use genetic markers that distinguish donor (129/Sv, marker Ly-9.1)-derived cells from host (Rag-1−/− mice backcrossed to C57/BL6, Ly-9.1−). Bone marrow was examined after depletion of surface IgM+ cells (Fig. 2b). Donor-derived B220+ c-kit+ pro-B cells were completely absent in animals reconstituted with AA4.1+ B220+ ES cells. In contrast, the AA4.1+ B220− fraction contributed to the most immature B220+ c-kit+ pro-B subset and the B220− c-kithigh fraction, known to contain multilineage progenitors (Fig. 2b). In some animals (5 of 21 mice analyzed after 8 weeks), we detected various amounts of donor-derived bone marrow myeloid cells (data not shown). Consistent with the appearance of peripheral blood T cells, AA4.1+ B220− ES cells developed into αβ as well as γδ T cells in the thymus of reconstituted mice (Fig. 2a). No intrathymic T cell development was observed with the AA4.1+ B220+ fraction (Fig. 2a and Table 1). Interestingly, intrathymic T cell development was initially biased toward the γδ lineage, although the αβ lineage predominated at all times (Table 1). Collectively, the data in Figs. 1 and 2 and Table 1 demonstrate that both AA4.1+ B220+ and AA4.1+ B220− ES-derived populations contain precursor activity for B cells, whereas only AA4.1+ B220− ES-derived cells give rise to T and B cells. The data suggest that the two subsets of differentiated ES cells represent distinct stages of lymphoid differentiation, the AA4.1+ B220+ subset being more advanced than the AA4.1+ B220− cells. The long-term T cell development in the thymus, in combination with the contribution to the B220− c-kithigh population in the bone marrow, argues for the presence of an early multilineage progenitor within the AA4.1+ B220− ES cells.
ES-Derived Lymphocytes Acquire Full Functional Competence in Vivo.
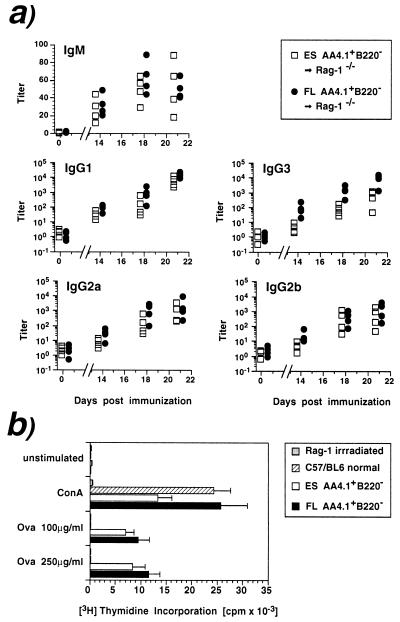
We reconstituted Rag-1−/− hosts with either 5 × 104 ES-derived AA4.1+ B220− cells or the same number of embryonic day 14.5 FL cells, which were isolated ex vivo by using an identical protocol. Reconstitution was verified by serum Ig levels and recipient mice were immunized with trinitrophenyl-ovalbumin by a priming injection 8 weeks after transfer and a booster injection 2 weeks later. Both ES- and FL-derived populations mounted similar humoral immune responses (Fig. 3a) in IgM and all IgG classes except for IgG3, which remained lower in the case of ES cells (mean titer 21 days after immunization: 607) compared with FL (mean: 9,400). Moreover, the proliferative responses of IgM-depleted lymph node T cells to ovalbumin were of similar magnitude in both groups of reconstituted mice (Fig. 3b), demonstrating the presence of antigen-reactive T cells. These results indicate that ES-derived progenitors can undergo final lymphoid differentiation in vivo, thereby acquiring full functional capacity.
Figure 3.
ES-derived lymphoid progenitors acquire full-functional competence after in vivo transfer. AA4.1+ B220− cells (5 × 104) from ES cells differentiated for 15 days or isolated from FL were transferred into Rag-1−/−. Recipients were immunized with trinitrophenyl-ovalbumin by a priming injection 8 weeks after transfer and a booster injection 2 weeks later. (a) Antigen-specific titer for each individual mouse (n = 4, two experiments) reconstituted with ES (□) or FL-derived (•) cells. (b) Three weeks after immunization, surface-IgM-depleted lymph node cells were stimulated with Con A (5 μg/ml) or indicated concentrations of ovalbumin and tested for proliferation. Results are the mean ± SD of triplicate samples.
Competitive Lymphoid Reconstitution of Rag-Deficient Mice with ES- and FL-Derived Populations.
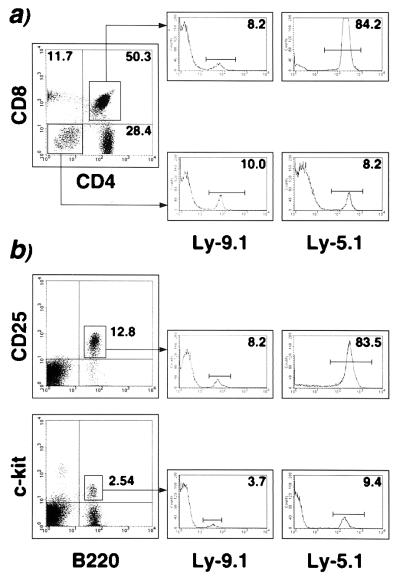
Next we tested the repopulation capacity of ES-derived AA4.1+ B220− cells in competition with the homologous population isolated from embryonic day 14.5 FL. AA4.1+ B220− cells were isolated from C57/BL6 FL (Ly-5.1+, Ly-9.1−, IgMb) and from D3/M ES cells derived from 129/Sv mice (Ly-5.2+, Ly-9.1+, IgMa) and were transferred into Rag-1−/− mice backcrossed to C57/BL6 (Ly-5.2+, Ly-9.1−) separately or in mixtures at various ratios (Table 2). The allotype titers in the serum of reconstituted animals showed a considerable bias toward the FL-derived Ig allotype for all three cell mixtures transferred (Table 2). A similar predominance of the progeny of FL is observed among CD4+ CD8+ thymocytes and among B220+ CD25+ bone marrow cells (Table 2). Both populations are absent in Rag-deficient mice (22–24) and originate exclusively from the inoculum (Fig. 4). When the ratio of ES- to FL-derived cells in the inoculum was 9:1, the ratios of the progenies in either thymus (Fig. 4a) or bone marrow (Fig. 4b) was shown to be the inverse (1 ES-derived to 8.5 FL-derived progeny). The predominance of FL is less striking in lymphoid populations prior to the genetic block in Rag-1−/− recipients. CD4− CD8− thymocytes and B220+ c-kit+ bone marrow cells of ES or FL origin occurred in comparable proportions in animals that received a 9:1 mixture of ES/FL cells (Fig. 4). In all experiments, the predominance of FL was more pronounced in bone marrow than in thymus (Table 2). Thus, these results clearly demonstrate a superior reconstitution potency for FL compared with ES cells but nevertheless define ES-derived progenitors as capable of developing into mature lymphocytes even in a competitive protocol.
Table 2.
Competitive lymphoid repopulation of Rag-1−/− recipients with ES- or FL-derived AA4.1+ B220− subpopulations
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Origin | |||
|---|---|---|---|---|---|---|---|---|
| Allotype titers (% of respective allotype) | ||||||||
| Serum | IgMa | 100.0 | 31.9 | 16.4 | 1.2 | 0.1 | ES | |
| IgMb | 0.1 | 65.0 | 83.9 | 88.2 | 100.0 | FL | ||
| Flow cytometry analysis (% positive cells) | ||||||||
| Thymus | CD4+ CD8+ | Ly-9.1+ | 90.1 | 11.3 | 8.1 | 5.4 | 1.4 | ES |
| Ly-5.1+ | 3.1 | 85.6 | 90.7 | 92.3 | 94.1 | FL | ||
| CD4− CD8− | Ly-9.1+ | 15.1 | 8.5 | 5.8 | 2.4 | 1.3 | ES | |
| Ly-5.1+ | 1.6 | 8.4 | 19.1 | 21.6 | 25.2 | FL | ||
| Ly-5.2+ | 83.6 | 83.9 | 76.1 | 76.6 | 73.9 | Rag-1−/− | ||
| Bone marrow | B220+ CD25+ | Ly-9.1+ | 86.8 | 7.5 | 6.9 | 1.6 | 0.9 | ES |
| Ly-5.1+ | 1.7 | 84.8 | 86.4 | 92.3 | 93.5 | FL | ||
| B220+ c-kit+ | Ly-9.1+ | 6.7 | 5.1 | 0.8 | 1.1 | 0.5 | ES | |
| Ly-5.1+ | 1.4 | 9.1 | 16.4 | 19.5 | 17.6 | FL | ||
| Ly-5.2+ | 92.0 | 86.4 | 82.9 | 79.6 | 82.0 | Rag-1−/− | ||
Mixtures of AA4.1+ B220− cells were transferred into Rag-1−/− mice at various ratios. Ratios of ES AA4.1+ B220− cells to FL AA4.1+ B220− cells are as follows: Group 1, 50,000/0; 2, 45,000/5,000; 3, 25,000/25,000; 4, 5,000/45,000; 5, 0/50,000. Two experiments were performed with two animals in each experimental group. Eight weeks after transfer, animals were sacrificed and analyzed. Allotype titers were determined by using an allotype-specific ELISA. Results are given in percent, setting serum levels in animals receiving exclusively ES or FL populations at 100%. Lymphoid populations in the thymus and the bone marrow were assessed by three-color flow cytometry, staining for the indicated subpopulations with either Ly-9.1 (indicative for ES origin) or Ly-5.1 (FL).
Figure 4.
Competitive reconstitution of Rag-deficient mice with ES- and FL-derived progenitors. Purified AA4.1+ B2220− cells from either in vitro-differentiated ES cells (ES cell line D3/M, Ly-9.1) or from embryonic day 14.5 FL (C57/BL6 Ly-5.1) were coinjected in sublethally irradiated Rag-1−/− (C57/BL6 Ly-5.2). A representative three-color cytofluormetric analysis 8 weeks after transfer of a reconstituted recipient initially injected with 4.5 × 104 ES-derived and 0.5 × 104 FL-derived cells is shown. (a) Thymocytes were stained with CD4/CD8 and Ly-9.1, indicative of ES-derived cells, or Ly-5.1, as a marker for FL-derived cells. B cell development was analyzed by staining surface-IgM-depleted bone marrow cells for B220/CD25 (b Upper) or B220/c-kit (b Lower). Double positive cells were analyzed by using Ly-9.1 or Ly-5.1 as a third parameter (single parameter histograms), allowing the distinction among endogenous or ES- or FL-derived lymphoid precursors. Numbers shown indicate percentages in quadrants (a) or individual regions (b).
DISCUSSION
We have examined the in vivo reconstitution potential of phenotypically defined subsets of differentiated ES cells by transfer into sublethally irradiated Rag-1-deficient mice. The two transplanted populations were found to represent two distinct stages of lymphoid development on the basis of (i) the lymphoid lineages restored, (ii) the kinetics of reconstitution, and (iii) the persistence, tissue distribution, and subset relationships of the progeny of ES-derived cells.
The population defined by the combined expression of AA4.1 and B220 gave rise exclusively to B cell development and showed no evidence for self-renewal in vivo. The restriction of this differentiated ES cell subpopulation to the B lymphoid lineage correlates with the presence of immunoglobin rearrangements (11, 15) and Rag gene expression (11, 15, 16). Commitment to the B cell lineage upon in vitro differentiation is demonstrated by the rapid appearance of surface IgM+ cells in the peripheral blood of reconstituted animals. The IgM+ cells appeared in a single wave and no long-lived B cell reconstitution was observed. Thus, these observations suggest that stem cells or primitive multilineage progenitors are absent from the AA4.1+ B220+ ES cell population. Alternatively, the in vitro differentiation might induce distortions in the putative progenitor/stem cells in this subset, for example, in the expression of adhesion molecules resulting in impaired migration or survival. We think that this is unlikely, however, because multilineage potential could be readily demonstrated in our in vitro differentiated cell cultures.
In contrast to the exclusive and transient restoration of the B cell lineage after the transfer of AA4.1+ B220+ cells, the corresponding AA4.1+ B220− fraction fully reconstitutes the lymphoid defect in Rag-deficient hosts. Several observations argue in favor of a primitive multilineage precursor and/or a stem cell in this population. In all experiments using several ES cell lines with different genetic backgrounds, AA4.1+ B220− cells contributed to the pro-B cell pool, i.e., a developmental stage prior to the endogenous block of lymphocyte maturation in Rag-deficient mice. Intrathymic T cell maturation was restored for an extended period of time, suggesting a continuous replenishment of, presumably, bone marrow-derived pro-T cells. This developmental potential of in vitro differentiated ES cells could either be due to the transfer of an early lymphoid precursor, a primitive multilineage progenitor, or HSCs. Although the lymphoid reconstitution persisted for more than 24 weeks, a decline over time was obvious, suggesting a limitation in self-renewal capacity. This is in line with the properties of HSCs early in embryogenesis or with a progenitor activity downstream of the stem cell stage. In either case, the development of some ES-derived macrophages and c-kit+ lineage-marker-negative bone marrow cells suggests multipotent precursor activity in the AA4.1+ B220− population. Previous studies have described lymphoid reconstitution of SCID (13, 25) or Rag-deficient mice (15) with unseparated ES cells and reported the generation of mature T and B cells. In the case of Nisitani et al. (15), successful restoration of B cell development in the bone marrow was also observed. Although these studies provided evidence for lymphopoietic precursor activity in differentiated ES cell, the present work defines two distinct stages of lymphopoiesis during in vitro differentiation, both of which are able to undergo final maturation upon in vivo transfer.
To assess the functional competence of a lymphoid system derived entirely from ES cells, we employed a T cell-dependent immunization protocol to analyze antibody production and T cell responsiveness. Immune responses were similar, both quantitatively and qualitatively, in mice reconstituted with the homologous precursor populations isolated from ES cells or from FL. These results suggest that the ES cell-derived lymphoid system can mature to full functional competence. In addition, a direct comparison of the reconstitution potencies of both precursor populations was carried out by cotransplantation of ES- and FL-derived AA4.1+ B220− preparations. By using allotypes and a combination of surface markers to identify the source of antibodies or cells, respectively, we consistently found a marked predominance of FL-derived cells. The decreased efficiency of ES cell progenitors might reflect a partially deregulated gene expression pattern during in vitro differentiation resulting in impaired adaptation to the in vivo environment. Interestingly, ES cell-derived populations also contributed significantly less than expected to the CD4+ CD8+ cells in the thymus, which are generated by rapid proliferation. This could indicate a reduced proliferative potential of ES-derived cells in vivo. Our data are in line with the limited lympho-hematopoietic potential reported for unseparated ES cells (16). It is not clear from these results whether the reconstitution deficiencies of ES cells arise at the stem cell level or further downstream during in vitro differentiation.
To what extent do ES cells recapitulate fetal hematopoiesis in vitro? In birds, the definitive HSCs originate intraembryonically from the para-aortic splanchnopleural mesoderm (26) rather than from the extraembryonic yolk sac, which has been proposed to be the main source of fetal HSCs in mammals (27). The emergence of erythroid and myeloid precursors during in vitro differentiation of ES cells has been taken as evidence for a yolk sac equivalent of hematopoietic development (1). During embryogenesis, the yolk sac contains progenitors that can undergo in vitro (20, 28, 29) or upon transfer in animals (29, 30) differentiation to lymphocytes. Most recently, Cumano et al. (31) provided evidence for a restriction of the lymphoid potential to the intraembryonic para-aortic splanchnopleura when analyzed before the onset of circulation. An interpretation that integrates the different experimental results would, therefore, place the hematopoietic potential of differentiated ES cells at a similar developmental stage as that of the para-aortic splanchnopleura. The splanchnopleura develops at later times into the aorta–gonad–mesonephros (AGM) region. Interestingly, hematopoietic long-term repopulating activity is not present before embryonic day 10 in the AGM (25, 32), just prior to the colonization of the FL by HSCs (33). One possible explanation for the impairment of ES cells in long-term hematopoiesis could be a lack of the functional transition within the HSC pool, generating the long-term repopulating activity. In vivo, this transition has been mapped to the anterior AGM region (32). Experiments involving cocultures of differentiated ES cells with AGM region-derived cells might provide a suitable system to distinguish between an alteration at the stem cell level and the absence of factors provided by a specialized microenvironment. Thus, with the present study, this should be helpful in understanding the mechanism of in vitro hematopoiesis and might provide additional insights into the physiological process of lymphopoiesis during ontogenesis.
Acknowledgments
We thank Dr. H.-R. Rodewald (Basel Institute for Immunology, Switzerland) for generously providing breeding pairs of C57/BL6 Ly-5.1 mice, Dr. H. Mossmann for his care in the maintenance of mouse colonies in our institute, and Ms. G. Nerz for excellent technical assistance. We are very grateful to Drs. I. Haidl and P. J. Nielsen for helpful discussion and critical reading of the manuscript.
ABBREVIATIONS
- ES cell
embryonic stem cell
- FL
fetal liver
- HSC
hematopoietic stem cell
- AGM region
aorta–gonad–mesonephros region
References
- 1.Doetschman T C, Eistetter H, Katz M, Schmidt W, Kemler R. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 2.Keller G M. Curr Opin Cell Biol. 1995;7:862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 3.Schmitt R M, Bruyns E, Snodgrass H R. Genes Dev. 1991;5:728–740. doi: 10.1101/gad.5.5.728. [DOI] [PubMed] [Google Scholar]
- 4.Keller G, Kennedy M, Papayannopoulou T, Wiles M V. Mol Cell Biol. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkert U, von Rüden T, Wagner E F. New Biol. 1991;3:698–708. [PubMed] [Google Scholar]
- 6.Lindenbaum M H, Grosveld F. Genes Dev. 1990;4:2075–2085. doi: 10.1101/gad.4.12a.2075. [DOI] [PubMed] [Google Scholar]
- 7.Nakano T, Kodama H, Honjo T. Science. 1996;272:722–724. doi: 10.1126/science.272.5262.722. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy M, Firpo M, Chol K, Wall C, Robertson S, Kabrun N, Keller G. Nature (London) 1997;386:488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- 9.Wang R, Clark R, Bautch V L. Development (Cambridge, UK) 1992;114:303–316. doi: 10.1242/dev.114.2.303. [DOI] [PubMed] [Google Scholar]
- 10.Auerbach R, Huang H, Lu L. Stem Cells (Dayton) 1996;14:269–280. doi: 10.1002/stem.140269. [DOI] [PubMed] [Google Scholar]
- 11.Potocnik A J, Nielsen P J, Eichmann K. EMBO J. 1994;13:5274–5283. doi: 10.1002/j.1460-2075.1994.tb06861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakano T, Kodama H, Honjo T. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez-Ramos J C, Palacios R. Proc Natl Acad Sci USA. 1992;89:9171–9175. doi: 10.1073/pnas.89.19.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller A M, Dzierzak E A. Development (Cambridge, UK) 1993;118:1343–1351. doi: 10.1242/dev.118.4.1343. [DOI] [PubMed] [Google Scholar]
- 15.Nisitani S, Tsubata T, Honjo T. Int Immunol. 1994;6:909–916. doi: 10.1093/intimm/6.6.909. [DOI] [PubMed] [Google Scholar]
- 16.Hole N, Graham G J, Menzel U, Ansell J D. Blood. 1996;88:1266–1276. [PubMed] [Google Scholar]
- 17.Palacios R, Golunski E, Samaridis J. Proc Natl Acad Sci USA. 1995;92:7530–7534. doi: 10.1073/pnas.92.16.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKearn J P, Baum C, Davie J M. J Immunol. 1984;132:332–339. [PubMed] [Google Scholar]
- 19.Jordan C T, McKearn J P, Lemischka I R. Cell. 1990;61:953–963. doi: 10.1016/0092-8674(90)90061-i. [DOI] [PubMed] [Google Scholar]
- 20.Godin I, Dieterlen-Lièvre F, Cumano A. Proc Natl Acad Sci USA. 1995;92:773–777. doi: 10.1073/pnas.92.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potocnik A J, Nerz G, Kohler H, Eichmann K. Immunol Lett. 1997;57:131–137. doi: 10.1016/s0165-2478(97)00089-8. [DOI] [PubMed] [Google Scholar]
- 22.Mombaerts P, Iacomini J, Johnson R S, Herrup K, Tonegawa S, Papaioannou V E. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 23.Shinkai Y, Rathbun G, Lam K P, Oltz E M, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall A M, Alt F W. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 24.Rolink A, Grawunder U, Winkler T H, Karasuyama H, Melchers F. Int Immunol. 1994;6:1257–1264. doi: 10.1093/intimm/6.8.1257. [DOI] [PubMed] [Google Scholar]
- 25.Müller A M, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 26.Dieterlen-Lièvre F. J Embryol Exp Morphol. 1975;33:607–619. [PubMed] [Google Scholar]
- 27.Moore M A, Metcalf D. Br J Haematol. 1970;18:279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu C P, Auerbach R. Development (Cambridge, UK) 1991;113:1315–1323. doi: 10.1242/dev.113.4.1315. [DOI] [PubMed] [Google Scholar]
- 29.Huang H, Zettergren L D, Auerbach R. Exp Hematol. 1994;22:19–25. [PubMed] [Google Scholar]
- 30.Palacios R, Imhof B A. Proc Natl Acad Sci USA. 1993;90:6581–6585. doi: 10.1073/pnas.90.14.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cumano A, Dieterlen-Lièvre F, Godin I. Cell. 1996;86:907–916. doi: 10.1016/s0092-8674(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 32.Medvinsky A, Dzierzak E. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 33.Dzierzak E, Medvinsky A. Trends Genet. 1995;11:359–366. doi: 10.1016/s0168-9525(00)89107-6. [DOI] [PubMed] [Google Scholar]