Abstract
Anti-P antibodies present in sera from patients with chronic Chagas heart disease (cChHD) recognize peptide R13, EEEDDDMGFGLFD, which encompasses the C-terminal region of the Trypanosoma cruzi ribosomal P1 and P2 proteins. This peptide shares homology with the C-terminal region (peptide H13 EESDDDMGFGLFD) of the human ribosomal P proteins, which is in turn the target of anti-P autoantibodies in systemic lupus erythematosus (SLE), and with the acidic epitope, AESDE, of the second extracellular loop of the β1-adrenergic receptor. Anti-P antibodies from chagasic patients showed a marked preference for recombinant parasite ribosomal P proteins and peptides, whereas anti-P autoantibodies from SLE reacted with human and parasite ribosomal P proteins and peptides to the same extent. A semi-quantitative estimation of the binding of cChHD anti-P antibodies to R13 and H13 using biosensor technology indicated that the average affinity constant was about 5 times higher for R13 than for H13. Competitive enzyme immunoassays demonstrated that cChHD anti-P antibodies bind to the acidic portions of peptide H13, as well as to peptide H26R, encompassing the second extracellular loop of the β1 adrenoreceptor. Anti-P antibodies isolated from cChHD patients exert a positive chronotropic effect in vitro on cardiomyocytes from neonatal rats, which resembles closely that of anti-β1 receptor antibodies isolated from the same patient. In contrast, SLE anti-P autoantibodies have no functional effect. Our results suggest that the adrenergic-stimulating activity of anti-P antibodies may be implicated in the induction of functional myocardial impairments observed in cChHD.
Chronic Chagas heart disease (cChHD) is the most frequent and severe clinical consequence of infection by the haemoflagellate Trypanosoma cruzi. It is essentially a dilated cardiomyopathy with various distinctive features, including a high prevalence of right bundle branch block and/or left anterior hemiblock, sinus node dysfunction, and complex ventricular arrhythmia (1, 2). Sudden cardiac death due to malignant ventricular arrhythmia is exceedingly common (2). Histological examination reveals mononuclear cell infiltration, myocyte damage, and fibrosis (1). Parasite cells are rarely detected in chagasic myocarditis (1). However, PCR amplification (3, 4) and immunohistochemical techniques (5) revealed the presence of parasite DNA and proteins very close to inflammatory foci, implying an active and efficient breakdown of parasite cells at the site of inflammation. These findings suggest that heart parasitation is the primary stimulus for the perpetuation of myocardial inflammation, and is responsible for increased serological response to the parasite (6).
In our search for serological markers of chronically active myocardial damage in human T. cruzi infection, we found that sera from patients with overt Chagas heart disease recognized predominantly, among other antigenic determinants, the C-terminal regions of the T. cruzi ribosomal P proteins (7–9). Two B cell epitopes were found to be implicated. The first one was located in peptide R13, EEEDDDMGFGLFD, the main linear epitope of the low molecular weight ribosomal P1 and P2 proteins (8, 9). The second, defined by the peptide, AESEE, and named P0β, was situated within the C-terminal end of TcP0, the 34-kDa ribosomal P0 protein (10, 11). Both peptides possess stretches of negatively charged residues that may contribute to their antigenicity, as well as to their putative ability to induce antibodies with functional activity against cardiac receptors (12). This is in agreement with the suggestion that the immunological cross-reaction between TcP0 and the β1-adrenergic receptor is caused by the pentapeptide AESEE (11).
Sera from cChHD patients with active myocarditis show significantly higher anti-P antibody levels (measured as anti-R13 antibody) than those cChHD patients without histological evidence of myocarditis (13, 14). However, neither the nature of the stimulus that gives rise to the anti-P antibody response nor its pathogenic relevance have been clearly established. Bestetti (15) suggested that the observed response against R13 was the result of an autoimmune reaction against self-ribosomal P antigens, leaked from injured heart tissue. This interpretation is apparently in line with the following observations: (i) the C-terminal end of the human ribosomal P protein is highly homologous to R13 and is able to elicit autoimmune responses, such as anti-P autoantibodies in systemic lupus erythematosus (SLE) (16); and (ii) anti-P antibodies in cChHD and SLE have the same specificity, defined by the 13 C-terminal residues of the parasite and host ribosomal P proteins (8).
The aim of this study was to determine whether anti-P antibodies induced in cChHD, and recognizing the C-terminal sequence R13, were autoantibodies to human P proteins, similar to those in SLE, or were antibodies directed against the parasite ribosomal P proteins. Moreover, because the C-terminal end of ribosomal P proteins possess a run of acidic residues homologous to the acidic extracellular epitope of the β1-adrenergic receptor, pentapeptide AESDE, and to P0β (11), it was relevant to determine whether antibodies elicited by this portion of the ribosomal proteins had functional properties. We therefore compared the fine specificity and cross-reactivity with the β1-adrenergic receptor of anti-P antibodies from cChHD and SLE. Our results indicate that antibodies elicited against R13 are probably implicated in the induction of functional myocardial impairments occurring in cChHD.
MATERIALS AND METHODS
Human Sera.
Studies were performed using sera from 15 patients with cChHD who came from the same endemic region located in northwest Argentina. They underwent a complete clinical and cardiologic examination, an electrocardiogram at rest, an exercise stress test, a 24-h electrocardiogram Holter monitoring, and a B-mode echocardiogram at the Cardiovascular Division, Ramos Mejía Hospital (Buenos Aires). Anti-P positive sera from 6 SLE patients were provided by S. Müller (Centre National de la Recherche Scientifique, Strasbourg). Sera from 4 patients with leishmaniasis were obtained from the Instituto de Medicina Tropical “Alejandro Von Humboldt” (Lima, Peru). Sera from 4 patients with idiopathic dilated cardiomyopathy and 10 healthy individuals used as negative controls were provided by the Cardiovascular Division, Ramos Mejía Hospital (Buenos Aires).
Recombinant Proteins.
The DNA fragments encoding the T. cruzi ribosomal P proteins, TcP1 (17) and TcP2β (18) and the human ribosomal P proteins, HuP1 and HuP2 (19) were amplified by PCR using the corresponding oligonucleotides and the original λgt11 clones as templates. PCR products were subcloned into the pMal-c2 expression vector (New England Biolabs). The expression and affinity purification of the recombinant proteins were performed as described (20).
Synthetic Peptides.
Peptides were prepared by the solid-phase method of Merrifield as described by Müller et al. (21) with a semiautomatic multisynthesizer NPS 4000 (Neosystem, Strasbourg, France). Cleavage from the resin was done by the high hydrogen fluoride method. Crude peptides were purified by preparative medium-pressure liquid chromatography. The synthetic peptides R13 (EEEDDDMGFGLFD), R11 (EDDDMGFGLFD), and IE (EEEDDDMG) were derived from the T. cruzi ribosomal P1/P2 C-terminal region (9). Peptides H13 (EESDDDMGFGLFD), H11 (SDDDMGFGLFD), and IS (EESDDDMG) were derived from the mammalian ribosomal P proteins (16). Peptides C10 (DDDMGFGLFD) and C7 (MGFGLFD) were derived from a consensus sequence in the ribosomal P protein family (9). Peptide P0β (AESEE) was derived from the C terminus of the P0 protein (11). Peptide H26R (HWWRAESDEARRCYNDPKCCDFVTNR) was derived from the second extracellular loop of the human β1-adrenergic receptor (22). Peptide TMVP (AEAALUKMALMKV) derived from tobacco mosaic virus coat protein was used as a negative control in ELISA.
Peptide Conjugation.
Peptides were coupled at a molar ratio of 1:30 to BSA (Sigma) with 0.05% glutaraldehyde (Serva) as described (23). The products were assessed by analytical HPLC and amino acid analysis was used to calculate the peptide–BSA molar ratio.
ELISA with Fusion Proteins and Synthetic Peptides.
ELISA experiments were performed as described (8). Polystyrene plates were coated with 50 μl of 20 μg/ml of recombinant protein or with 50 μl of 4 μM BSA-conjugated peptides in 0.05 M bicarbonate–carbonate buffer (pH 9.6). Optical densities (OD) were read at 415 nm. In inhibition experiments, sera were first incubated for 2 h at 37°C with increasing amounts of peptides. Results were shown as percent of inhibition.
Antibody Purification.
Antibodies against R13, H13, and H26R were affinity purified using Act-Ultragel AcA 22 resin (Sepracor, Villeneuve La Garenne, France) coupled to the corresponding peptide as described by the manufacturer. Two milliliters of resin with 1 mg of attached peptide was incubated overnight at 4°C with 200 μl of serum. The column was washed with 20 ml of 150 mM sodium chloride/10 mM sodium phosphate, pH 7.4 (PBS) before elution with 0.2 M glycine buffer (pH 2.8). The elution volume (500 μl) was directly neutralized with 100 μl of 1 M Tris buffer (pH 8.0). Specificity of affinity purified anti-P and anti-H26R antibodies was determined as described by Levitus et al. (19) and Tate et al. (24), respectively. Purified antibodies to be used in functional assays were previously dialyzed against PBS.
Immobilization of Immunopurified Antibodies on the Sensor Surface.
The BIAcore system and reagents for interaction analysis were obtained from BIAcore (Uppsala, Sweden) (25). The immobilization of ligands on the carboxylated dextran matrix of CM5 sensor chip was achieved using standard procedures (BIAapplications handbook, BIAcore). The carboxylated matrix was first activated with 30 μl of an N-ethyl-N-[(3-di-methylamino)propyl]carbodiimide hydrochloride (EDC)/N-hydroxy-succinimide (NHS) (1:1) mixture (25). Immobilization of antibodies (100 μl at a 100 μg/ml) was performed at a flow of 5 μl/min in 10 mM acetate buffer (pH 5.0). Remaining NHS-ester groups were blocked by injection of 35 μl of 1 M ethanolamine-HCl (pH 8.5). Noncovalently immobilized molecules were removed by washing the surface with 15 μl of 100 mM NaOH. Finally, the matrix was washed passing 100 μl of HBS buffer (10 mM Hepes/0.15 M NaCl/3.4 mM EDTA/0.05% surfactant P20, pH 7.4).
BIAcore Measurements.
Conjugated peptides (500 μg/ml) diluted in HBS buffer were allowed to interact with the antibodies by passing the peptides over the sensor chip at a flow of 5 μl/min.
Functional Assay.
Spontaneously beating cultured neonatal rat cardiomyocytes were used to assess the functional effects of immunopurified antibodies dialyzed against PBS. Single cells were dissociated from the minced heart of Wistar rats with a trypsin–collagenase solution. Myocytes were cultured for 4 days at 37°C in a 5% CO2 atmosphere as monolayers in DMEM/F-12 (Life Technologies, Gaithersburg, MD) containing 5% calf serum. The baseline beating rate measured in 10 different fields at 37°C on the heated stage of an inverted microscope was 120 ± 24 beats/min. Measurements were repeated 1 h after exposure to immunopurified antibodies at a 1:50 dilution and after subsequent addition of 1 μM bisoprolol or 30 μM of R13, P0β, or H26R peptides.
Statistical Analysis.
Mean and standard deviations (SD) of the OD values obtained by ELISA were calculated by the Statistica for Windows program (release 4.0; StatSoft, Tulsa, OK). Cut-off lines were defined as mean ± 2 SD of the OD415 of the sera from 10 healthy blood donors. Significance among different groups was assessed by the ANOVA module of the same program. Significance of changes in the beating rate of cultured cardiomyocytes was assessed by paired Student’s t test. All reported data were the mean of at least three measurements.
RESULTS
Fine Specificity of Anti-P Antibodies Induced in cChHD and SLE.
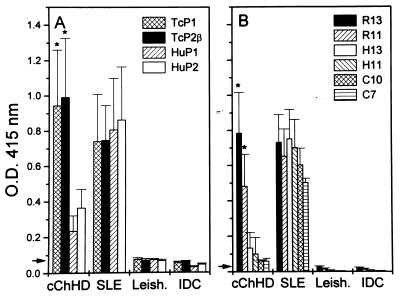
To compare the fine specificities of anti-P antibodies present in chronic T. cruzi infection and SLE, sera obtained from patients with cChHD and SLE were tested by ELISA against a panel of four recombinant ribosomal P proteins and six synthetic peptides. The recombinants were derived from T. cruzi and human ribosomal P proteins (TcP1/TcP2β, and HuP1/HuP2, respectively), whereas the synthetic peptides corresponded to the C termini of the parasite ribosomal P proteins (R13 and R11), the human P ribosomal proteins (H13 and H11), and the region that is common to both (C10 and C7). Fig. 1A shows the reactivity of different serum samples with the recombinant proteins. Patients with cChHD presented mean antibody levels against the T. cruzi recombinants that were significantly higher than those measured against the human proteins (P < 0.005), whereas SLE sera presented similar binding to the four recombinants. As expected, sera from individuals with leishmaniasis or from patients with idiopathic dilated cardiomyopathy did not react with any of the proteins (8, 19). Fig. 1B shows the fine specificity anti-P profile of cChHD and SLE sera. Chagasic sera reacted predominantly with R13 and R11 peptides. The binding to peptides H13 and H11 was higher than the binding to C10 and C7. In contrast, SLE anti-P autoantibodies showed a similar binding to H13 and R13, as well as a strong binding to C7.
Figure 1.
Reactivity of different sera against recombinant ribosomal P proteins and corresponding C-terminal peptides measured by ELISA. Sera from patients with cChHD (n = 15), SLE (n = 6), leishmaniasis (Leish, n = 4), and idiopathic dilated cardiomyopathy (IDC, n = 4) were tested against (A) recombinant proteins derived from T. cruzi (TcP1 and TcP2β) and human (HuP1 and HuP2) ribosomal P proteins, and (B) peptides R13, R11, H13, H11, C10, and C7. Sera were used at a 1:400 dilution. Mean plus standard deviations are shown. ∗, Significant differences (P < 0.01) were observed between antibody levels against TcP1 and TcP2β when compared with anti-HuP2 antibody levels and between anti-R13 and -R11 levels when compared with anti-H13 antibody levels. Arrows indicate the cut-off lines defined as the mean ± 2 SD of the OD415 values obtained from 10 healthy donors.
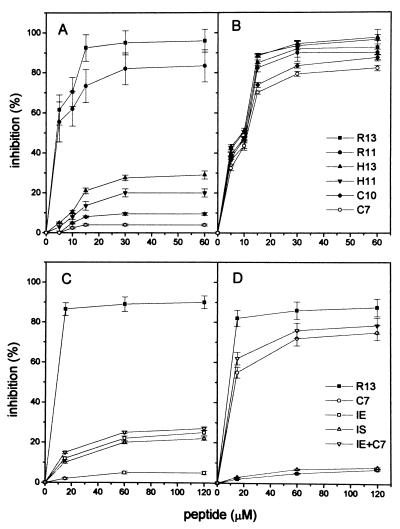
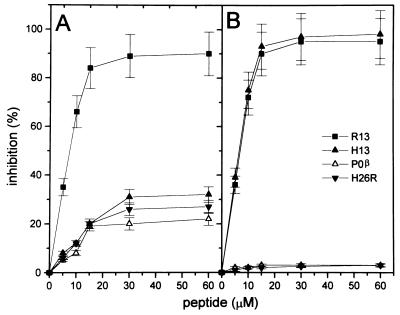
The specificity of the anti-P reactions in both diseases was verified by inhibition experiments. Peptides C7, C10, H11, H13, R11, and R13 were analyzed for their capacity to inhibit binding to R13 of anti-P antibodies from two cChHD sera and two SLE sera (Fig. 2 A and B). The results of the inhibition tests confirmed those of the direct binding assays and indicated that chronic T. cruzi infection does not induce a typical autoimmune anti-P response, but gives rise to an anti-P response typical of infection, characterized by high antibody levels against the C-terminal end of the parasite protein, including R13 and R11, and a weak recognition of the human counterparts.
Figure 2.
Inhibition of binding of anti-P antibodies to R13 by synthetic peptides. Results show the mean ± SD of the percentage of inhibition with increasing amounts of peptides for two sera. (A and C) Inhibition of anti-P activities from cChHD sera 15 and 40. (B and D) Inhibition of anti-P activities from SLE sera 2 and 5. Serum dilution was 1:400.
The data also suggest that the stretch of acidic residues, EEEDDD, and particularly the third Glu residue of peptide R13, may contribute to the specificity of the infection antibody profile. To establish if a discrete epitope was located within the run of acidic residues, sera were preincubated with internal peptides derived from R13 and H13, peptides IE and IS, respectively, and tested by ELISA for reactivity with R13 (Fig. 2 C and D). It was found that both IE and IS partially inhibited the anti-P activities of chagasic sera, inhibition that was not enhanced by addition of peptide C7 (Fig. 2C). The anti-P reactivities of SLE sera were almost completely inhibited by C7, but not by internal peptides (Fig. 2D). In contrast, anti-P antibodies induced by T. cruzi required the complete R13 peptide for maximal binding. Within the R13 sequence the third Glu residue and the terminal FGLFD motif are particularly important for antigenicity, because deletion of the five C-terminal residues or substitution of Glu-3 in R13 reduced binding to the same extent. This is shown by the equivalent inhibition of anti-P reactivities achieved by H13, H11, IE, and IS (Fig. 2 A and C).
Properties of Anti-P Antibodies.
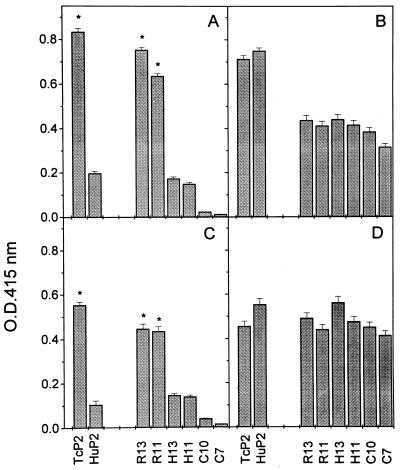
Analysis of the anti-P reactivities in both disorders prompted us to further investigate the binding of anti-P antibodies to homologous and heterologous peptides. Antibodies reacting with R13 and H13 from two cChHD sera were affinity purified, and their binding to different peptides measured and compared with antibodies purified in identical conditions from two SLE sera. Fig. 3 shows binding of affinity purified antibodies from cChHD serum 15 (Fig. 3 A and C) and SLE serum 5 (Fig. 3 B and D). Results indicate that in both diseases the affinity purified antibodies reproduce the reactivity profile of total serum samples.
Figure 3.
Reactivity of affinity-purified antibodies with recombinant proteins and synthetic peptides measured by ELISA. (A and C) Anti-R13 and anti-H13 affinity-purified antibodies from cChHD serum 15. (B and D) Anti-R13 and anti-H13 affinity-purified antibodies from SLE serum 5. Purified antibodies were tested at 1:4 dilution. Mean ± SD of OD415 values are from three independent measurements. ∗, Significant differences (P < 0.01) were observed between antibody levels against TcP2β and HuP2 and between anti-R13 and -R11 antibody levels when compared with anti-H13 antibody levels.
The cChHD anti-P antibody was purified on either R13 or H13 affinity columns, as demonstrated by the fact that the eluted antibodies presented the typical anti-P infection response, with high affinity for R13 and lower affinity for H13 (Fig. 3 A and C). In contrast, anti-P autoantibodies from SLE purified either on R13 or H13 columns reacted to the same degree with homologous and heterologous peptides (Fig. 3 B and D).
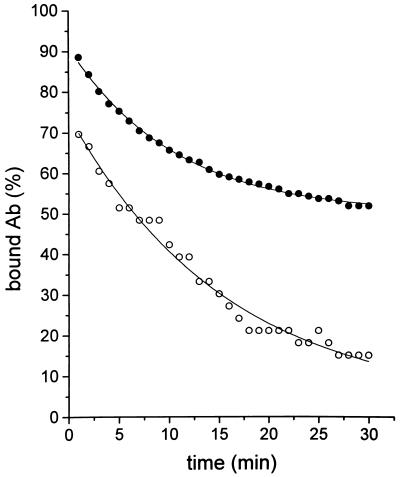
Surface plasmon resonance measurements were used to confirm the nature of the binding of cChHD anti-P antibodies to R13 and H13 peptides. The anti-P antibody was immobilized on the sensor chip and the peptides were used as analytes. The results (Fig. 4) indicated that the antibodies purified on R13 affinity column had an average dissociation rate constant with respect to R13 and H13 of about 2.10−4 s−1 and 1.10−3 s−1, respectively. Because the association rate constants for both peptides are unlikely to differ significantly, the equilibrium affinity constant of the antibodies for R13 is about 5 times higher than that for H13.
Figure 4.
Surface plasmon resonance measurements of dissociation of R13 and H13 peptides from immobilized cChHD anti-P antibodies purified on R13 affinity columns. BIAcore measurements were performed as described. Resonance units (RU) were converted to percentage of bound antibody. •, Interaction between purified antibodies and R13 peptide; ○, interaction between purified antibodies and H13 peptide.
Taken together, these results suggest that the original target of the antibody is the parasite ribosomal peptide. Interestingly, binding of H13 to the cChHD anti-P antibody could be inhibited by peptides containing negatively charged sequences, such as IE, P0β, and H26R, which encompasses the second extracellular loop of the β-adrenergic receptor, including the cross-reactive pentapeptide AESDE (11) (data not shown). In accordance with this result, Fig. 5A shows that peptides H26R, P0β, and H13 inhibit the binding of purified cChHD anti-P antibodies to R13 in a similar manner. However, H26R and P0β did not inhibit the binding of SLE anti-P autoantibodies to R13 (Fig. 5B).
Figure 5.
Inhibition of binding of cChHD anti-P antibodies purified on R13 affinity columns to R13 by synthetic peptides. Results show the mean ± SD of the percentage of inhibition with increasing amounts of peptides for anti-P affinity purified antibodies from cChHD sera 15 and 40 (A) and from SLE sera 2 and 5 (B). Purified antibodies were tested at 1:4 dilution.
Functional Activity of the Anti-P Antibodies Induced by T. cruzi Infection.
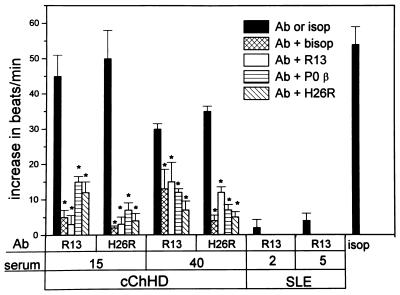
Functional recognition of β1-adrenergic receptor by chagasic anti-P antibodies was assessed on spontaneously beating neonatal rat cardiomyocytes. This system has been used previously for studying anti-receptor antibodies in Chagas disease, as well as in acute myocarditis and idiopathic dilated cardiomyopathy (11, 12, 26). The functional activity of cChHD anti-P antibodies, purified on R13 columns, was compared with that of anti-β1 receptor antibodies, purified on H26R columns from the same patient, and with affinity purified anti-P autoantibodies from SLE patients (Fig. 6).
Figure 6.
Agonist like effect of affinity-purified anti-R13 and anti-H26R antibodies from cChHD and SLE sera. The specificity of the effect was studied by inhibition with the β1-specific antagonist bisoprolol (bisop) and the peptides R13, P0β, and H26R. Cells were subsequently stimulated with the β-adrenergic agonist isoproterenol (isop). Bars represent mean ± SD of the increase in beats/min. ∗, Significant differences (P < 0.01) were observed between results with purified antibodies alone and after treatment with the inhibitors. Ab stands for affinity purified antibodies against peptides R13 or H26R.
The cChHD anti-P antibodies showed a similar positive chronotropic response as the anti-β receptor antibodies obtained from the same patient. After incubation with the cChHD anti-P antibodies, or with the anti-β1-adrenergic antibodies, we observed a significant increase in the rate of beating of the cardiomyocytes (Fig. 6). This was blocked by addition of the β1-specific blocker bisoprolol. In contrast, anti-P autoantibodies from SLE P-positive patients had no effect on cardiomyocytes.
When the cChHD anti-P or anti-β1 receptor antibodies were preincubated with peptides R13, P0β, or H26R, the positive chronotropic effect of the antibodies was almost completely blocked. Peptides alone had no effect. To ensure that the antibody peptide complex had no toxic effects on the cells, the cardiomyocytes were treated with 10 μM isoproterenol, an adrenergic agonist. The cardiomyocytes reacted in a normal way to this stimulant (isop in Fig. 6).
DISCUSSION
Molecular expression cloning techniques showed that patients with overt Chagas heart disease present a strong humoral response against the C-terminal end of the low molecular weight ribosomal P proteins of T. cruzi (7, 8). These sequences are highly conserved throughout evolution and share more than 90% homology with the C-terminal regions of the human ribosomal P proteins, which are in turn the target of anti-P autoantibodies in SLE (16). Analysis of the reactivities of anti-P autoantibodies and antibodies directed against the parasite P proteins in cChHD showed that they share the same specificity (7, 8, 27). This observation led several authors to conclude that mechanisms active in generating the anti-P autoimmune response in SLE are also operative in the generation of the anti-P response in Chagas disease, and could contribute to cChHD pathogenesis (27, 28).
Our results, however, demonstrate that chronic T. cruzi infection does not induce the typical autoimmune anti-P antibody response found in SLE patients. Unlike anti-P autoantibodies which reacted with human and parasite proteins to the same extent (Fig. 1A), cChHD anti-P antibodies showed a marked preference for the parasite proteins, and reacted much less strongly with the human P proteins (Fig. 1A). The binding of these antibodies to peptides R13 and H13, and their shorter fragments R11 and H11, followed the same pattern (Fig. 1B), indicating that the major antigenic determinant and cross-reactive epitope of the low molecular weight ribosomal P proteins in Chagas disease is located in the 13 C-terminal amino acid sequence (8, 19).
Analysis of the binding of cChHD anti-P antibodies to R13 shows that the third Glu residue is crucial for recognition. Replacing Glu-3 by Ser generates the C-terminal end of the human ribosomal P protein (peptide H13), a change that lowered sharply the affinity of the cChHD anti-P antibody for the peptide. However, antibody recognition of Glu-3 was influenced by the five C-terminal residues of R13. Indeed, the octapeptide EEEDDDMG, corresponding to the eight N-terminal residues of R13, inhibited only partially and to the same extent as peptide EESDDDMG, the binding of cChHD anti-P antibodies to R13 (Fig. 2C). Because the addition of peptide C7 to the octapeptide does not enhance this inhibition, it seems that the five C-terminal residues exert their influence in the context of the complete R13 sequence, which suggests that the epitope within R13 is probably discontinuous. This interpretation is further strengthened by the recent observation that together with Glu-3, Asp-13 is indispensable for R13 binding by cChHD anti-P antibodies (I.F., E.M., and M.J.L., unpublished results).
Biosensor experiments provided a semi-quantitative estimation of the binding of cChHD anti-P antibodies to R13 and H13, indicating that the average affinity constant was about 5 times higher for R13 than for H13 (Fig. 4). Thus, in addition to being able to bind to their original target, these antibodies effectively bind H13 albeit with less affinity, a result that agrees with direct ELISA (Figs. 1 and 3) and inhibition experiments (Fig. 2 A and C), as well as with the observed binding of the antibody to H13 affinity columns (Fig. 3).
That cChHD anti-P antibodies react with the acidic portion of the H13 peptide is demonstrated by the following observations: (i) anti-H13 binding is inhibited by peptide IE and other peptides with short runs of acidic residues such as H26R and P0β, and (ii) peptides H26R and P0β, inhibit to the same extent as H13 the binding of the antibodies to R13 (Fig. 5).
The anti-polyanionic nature of the anti-P antibody suggests that this antibody could be responsible, at least in part, for the anti-cardiac receptor activities occurring in patients with cChHD which have been shown to be anti-polyanionic (12). In agreement with this interpretation, the two anti-P and anti-β1 adrenoreceptor affinity-purified antibodies from two different chagasic patients increased the beating rate of heart cells to the same extent. This effect was inhibited by R13, H26R, and P0β, confirming the cross-reactive nature of anti-P and anti-β1 receptor antibodies (Fig. 6). That this functional activity of chagasic antibodies is linked to the recognition of the polyanionic sequences is also demonstrated by the fact that anti-P antibodies of lupus patients isolated on R13 affinity columns, did not have any effect on cardiomyocytes (Figs. 5 and 6) presumably because of the high affinity of these antibodies for the conserved C-terminal heptapeptide.
Interestingly, anti-P antibodies only presented a positive chronotropic effect acting on the β1 receptor. Because chagasic sera possess also anti-muscarinic and anti-β2 receptor activities, these results suggest the existence of other parasite antigens able to induce other types of antireceptor activities (12). Alternatively, anti-muscarinic acetylcholine receptor antibodies could result from a shift of the primary anti-P antibody specificity (12).
The anti-P reaction is a hallmark of cChHD. The R13 peptide is not recognized by sera from patients with malaria or leishmaniasis (Fig. 1) (19, 29). Moreover, anti-P antibodies are not detectable in sera from individuals infected by Trypanosoma brucei (19), a parasite that contains ribosomal P proteins with the same C-terminal end as P proteins in T. cruzi (9), nor in acute Chagas patients, or in sera from patients with digestive forms of Chagas disease (14). These observations imply that in cChHD, parasite ribosomes are directly exposed to the immunological system. Because in cChHD severe inflammatory lesions are only found in heart tissue (30) where they are associated to parasite DNA and antigens (3–5), it is tempting to hypothesize that the recognition of the parasite ribosomal proteins occurs at this location. In accordance with this interpretation, the highest levels of antibodies against the parasite ribosomal epitope R13 were detected in patients with active myocarditis (13, 14).
This report demonstrates the biological relevance of the anti-P reactivity generated during T. cruzi human chronic infection, which is linked to the presence of the parasitic stimulus. Our data suggest that the pathogenicity of antibodies generated against intracellular parasite antigens results from their ability to cross-react with membrane proteins. Because antibodies against the second extracellular loop of the β1-adrenergic receptor are associated with idiopathic ventricular arrhythmia (26), it seems plausible that the adrenergic-stimulating activity of anti-P antibodies may be at the origin of ventricular arrhythmia observed in cChHD. This hypothesis is further reinforced by the fact that immunization with the ribosomal TcP2β protein induces changes in the electrocardiogram of immunized mice (20).
To alleviate these symptoms, functional anti-cardiac receptor reactivities may be immunologically blocked. However, the best long-term solution seems to be an efficient trypanomicidal chemotherapeutic protocol to eliminate the parasite and the resulting induction of anti-parasite antibodies with functional anti-heart reactivity (31).
Acknowledgments
We are indebted to Drs. Mauricio Rosenbaum and Marcelo V. Elizari (Cardiology Division, Ramos Mejía Hospital, Buenos Aires) for comments, discussion, and constant encouragement. This work was supported by the French–Argentinian Cooperation Program (Centre National de la Recherche Scientifique–Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina), Ambassade de France in Argentina-Ministere des Affaires Etrangeres (Paris), and grants from the International Atomic Energy Agency, the World Health Organization/Special Program for Research and Training in Tropical Diseases, the European Commission, Centro Argentino Brasilero de Biotecnologia, Ex203 and Ex283 from University of Buenos Aires, Fundacion Antorchas, and PIA-1997 (Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina).
ABBREVIATIONS
- cChHD
chronic Chagas heart disease
- SLE
systemic lupus erythematosus
- TcP1
Trypanosoma cruzi ribosomal P1 protein
- TcP2β
Trypanosoma cruzi ribosomal P2β protein
References
- 1.Rosenbaum M B. Prog Cardiovasc Dis. 1964;7:199–225. doi: 10.1016/s0033-0620(64)80020-7. [DOI] [PubMed] [Google Scholar]
- 2.Elizari M V, Chiale P A. J Cardiovasc Electrophysiol. 1993;4:598–608. doi: 10.1111/j.1540-8167.1993.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 3.Jones E M, Colley D G, Tostes S, Lopes E R, Vnencak-Jones C L, McCurley T L. Am J Trop Med Hyg. 1993;48:348–57. doi: 10.4269/ajtmh.1993.48.348. [DOI] [PubMed] [Google Scholar]
- 4.Brandariz S, Schijman A, Vigliano C, Arteman P, Viotti R, Beldjord C, Levin M J. Lancet. 1995;346:1370–1371. doi: 10.1016/s0140-6736(95)92388-8. [DOI] [PubMed] [Google Scholar]
- 5.Bellotti G, Bocchi E, Villela de Moraes A, Higuchi M L, Barbero-Marcial M, Sosa E, Esteves-Filho A, Kalil R, Weiss R, Jatene A, Pileggi F. Am Heart J. 1996;131:301–307. doi: 10.1016/s0002-8703(96)90358-0. [DOI] [PubMed] [Google Scholar]
- 6.Levin M J. Parasitol Today. 1996;12:415–416. doi: 10.1016/0169-4758(96)20051-1. [DOI] [PubMed] [Google Scholar]
- 7.Levin M J, Mesri E, Benarous R, Levitus G, Schijman A, Levy-Yeyati P, Chiale P, Ruiz A, Kahn A, Rosenbaum M, Torres H N, Segura E L. Am J Trop Med Hyg. 1989;41:530–539. doi: 10.4269/ajtmh.1989.41.530. [DOI] [PubMed] [Google Scholar]
- 8.Mesri E A, Levitus G, Hontebeyrie-Joskowicz M, Van Regenmortel M H V, Levin M J. J Clin Microbiol. 1990;28:1219–1224. doi: 10.1128/jcm.28.6.1219-1224.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin M J, Vazquez M, Kaplan D, Schijman A G. Parasitol Today. 1993;9:381–384. doi: 10.1016/0169-4758(93)90088-w. [DOI] [PubMed] [Google Scholar]
- 10.Schijman A G, Levitus G, Levin M J. Immunol Lett. 1992;33:15–20. doi: 10.1016/0165-2478(92)90087-5. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari I, Levin M J, Wallukat G, Elies R, Lebesgue D, Chiale P, Elizari M, Rosenbaum M, Hoebeke J. J Exp Med. 1995;182:59–65. doi: 10.1084/jem.182.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elies R, Ferrari I, Wallukat G, Lebesgue D, Chiale P, Elizari M, Rosenbaum M, Hoebeke J, Levin M J. J Immunol. 1996;157:4203–4211. [PubMed] [Google Scholar]
- 13.Levin M J. Res Immunol. 1991;142:157–159. doi: 10.1016/0923-2494(91)90029-i. [DOI] [PubMed] [Google Scholar]
- 14.Aznar C, Lopez Bergami P, Brandariz S, Mariette C, Liegeard P, de Deus Alves M C, Luna Barreiro E, Carrasco R, Lafon S, Kaplan D, Levitus G, Levin M J, Hontebeyrie-Joskowicz M. FEMS Immunol Med Microbiol. 1995;12:231–238. doi: 10.1111/j.1574-695X.1995.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 15.Bestetti R B. Lancet. 1996;347:913–914. doi: 10.1016/s0140-6736(96)91403-8. [DOI] [PubMed] [Google Scholar]
- 16.Elkon K, Bonfa E, Llovet R, Danho W, Weissbach, Brot N. Proc Natl Acad Sci USA. 1988;85:5186–5189. doi: 10.1073/pnas.85.14.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vazquez M P, Shijman A G, Levin M J. Nucleic Acids Res. 1992;20:2599. doi: 10.1093/nar/20.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schijman A G, Dusetti N J, Vazquez M P, Lafon S, Levy-Yeyati P, Levin M J. Nucleic Acids Res. 1990;18:3399. doi: 10.1093/nar/18.11.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levitus G, Hontebeyrie-Joskowicz M, Van Regenmortel M H V, Levin M J. Clin Exp Immunol. 1991;85:413–417. doi: 10.1111/j.1365-2249.1991.tb05741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez Bergami P, Cabeza Meckert P, Kaplan D, Levitus G, Elías F, Quintana F, Van Regenmortel M H V, Laguens R, Levin M J. FEMS Immunol Med Microbiol. 1997;18:75–85. doi: 10.1111/j.1574-695X.1997.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 21.Muller S, Couppez M, Briand J P, Gordon J, Sautiére P, Van Regenmortel M H V. Biochim Biophys Acta. 1985;827:235–246. doi: 10.1016/0167-4838(85)90208-0. [DOI] [PubMed] [Google Scholar]
- 22.Magnusson Y, Höyer S, Lengagne R, Chapot M P, Guillet J G, Hjalmarson A, Strosberg A D, Hoebeke J. Clin Exp Immunol. 1989;78:42–48. [PMC free article] [PubMed] [Google Scholar]
- 23.Briand J P, Muller S, Van Regenmortel M H V. J Immunol Methods. 1985;78:59–69. doi: 10.1016/0022-1759(85)90329-1. [DOI] [PubMed] [Google Scholar]
- 24.Tate K, Magnusson Y, Viguier M, Lengagne R, Hjalmarson A, Guillet J G, Hoebeke J. Biochimie. 1994;76:159–164. doi: 10.1016/0300-9084(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 25.Chatellier J, Rauffer-Bruyere N, Van Regenmortel M H V, Altschuh D. J Mol Recognit. 1996;9:39–51. doi: 10.1002/(sici)1099-1352(199601)9:1<39::aid-jmr239>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 26.Chiale P A, Rosenbaum M B, Elizari M V, Hjalmarson A, Magnusson Y, Wallukat G, Hoebeke J. J Am Coll Cardiol. 1995;26:864–869. doi: 10.1016/0735-1097(95)00262-2. [DOI] [PubMed] [Google Scholar]
- 27.Skeiky Y A W, Benson D, Guderain J A, Sleath P R, Parsons M, Reed S G. J Immunol. 1993;151:5504–5515. [PubMed] [Google Scholar]
- 28.Skeiky Y A W, Benson D, Parsons M, Elkon K, Reed S G. J Exp Med. 1992;176:201–211. doi: 10.1084/jem.176.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levin M J, Franco da Silveira J, Frasch A C C, Camargo M E, Lafon S, Degrave W M, Rangel Aldao R. FEMS Microbiol Immunol. 1991;89:11–20. doi: 10.1111/j.1574-6968.1991.tb04965.x. [DOI] [PubMed] [Google Scholar]
- 30.Andrade Z A. Res Immunol. 1991;142:126–129. doi: 10.1016/0923-2494(91)90021-a. [DOI] [PubMed] [Google Scholar]
- 31.Viotti R, Vigliano C, Armenti H, Segura E. Am Heart J. 1994;127:151–162. doi: 10.1016/0002-8703(94)90521-5. [DOI] [PubMed] [Google Scholar]