Abstract
CD38 ligation on mouse B cells by CS/2, an anti-mouse CD38 mAb, induced proliferation, interleukin 5 (IL-5) receptor α chain expression, and tyrosine phosphorylation of Bruton tyrosine kinase (Btk) from wild-type, but not from X chromosome-linked, immunodeficient mice. B cells from fyn-deficient (Fyn−/−) and lyn-deficient (Lyn−/−) mice showed an impaired response to mAb CS/2 for proliferation and IL-5 receptor α chain expression, and B cells from fyn/lyn double-deficient (Fyn/Lyn−/−) mice did not respond at all to mAb CS/2. The Btk activation by CD38 ligation was observed in B cells from Fyn−/− mice, and it was severely impaired in B cells from Lyn−/− and Fyn/Lyn−/− mice. CD38 expression on B cells from three mutant strains was comparable to that on control B cells. We infer from these results that both Fyn and Lyn are required and that their signals are synergistic for B cell triggering after CD38 ligation. Lyn is upstream of Btk activation in the CD38 signaling. Stimulation of B cells with IL-5 together with CD38 ligation induces not only IgM but also IgG1 secretion. Analysis of the synergistic effects of IL-5 and CD38 ligation on IgG1 secretion revealed the impaired IgG1 secretion of B cells from Lyn−/− and Fyn/Lyn−/− mice. These data imply that Lyn is involved in B cell triggering by CD38 ligation plus IL-5 for isotype switching.
Keywords: src kinase, Btk, class-switching, cytokine
CD38 is an ectoenzyme that possesses both ADP ribosyl cyclase and cADP ribosyl hydrolase activities, which generate cADP ribose and ADP ribose from NAD+ (1–4). A role for CD38 in regulation of B cell activation has been suggested by analysis using agonistic mAb to mouse CD38 (5–9). We have reported that CD38 ligation on B cells by anti-mouse CD38 mAb induced proliferation, IgM secretion, and tyrosine phosphorylation of Bruton tyrosine kinase (Btk) in B cells from wild-type mice. B cells from X chromosome-linked immunodeficient (Xid) mice did not respond at all to anti-CD38 mAb although CD38 expression on these B cells was comparable to that on wild-type B cells (8, 9). We infer from these results that Btk activity is involved in B cell triggering after cross-linkage of CD38.
Interleukin 5 (IL-5) induces proliferation and differentiation of B cells, eosinophils, and basophils (10, 11). IL-5 signals through the IL-5 receptor (IL-5R) complex, which is comprised of an α chain (IL-5Rα) and a β chain (12–18). Analysis of the synergistic effects of various cytokines with CD38 ligation of B cell activation revealed that IL-5 showed the most potent effect on B cell proliferation, Blimp-1 gene expression, and IgM production (9). Although neither CD38 nor IL-5R carries the catalytic domain for protein tyrosine kinases, CD38 ligation induces activation of Btk (9), and IL-5 activates Btk and JAK2 kinases (18–20). The B cells from Xid mice that carry the xid gene with a point mutation in the pleckstrin homology domain of Btk (21, 22) have a global defect represented by low responses to CD38 ligation, IL-5, and anti-IgM stimulation (23–26), further supporting the requirement of Btk in CD38-dependent B cell signaling.
Lyn and Fyn are expressed in a broad range of cell types and tissues (27). Lyn has been shown to be physically associated with a number of hematopoietic cell surface receptors, including the B cell antigen receptor (28–30), CD40 (31), the lipopolysaccharide (LPS) receptor (32), the high affinity FcɛRI complex (33), the granulocyte–macrophage colony-stimulating factor receptor (34), and IL-5R (11, 35). Furthermore, Lyn and Fyn were shown to be associated with Btk (36). Lyn is most likely the predominant src family kinase involved in activation of Btk in B cells (37). It is not clear, however, whether Fyn and Lyn are involved in the signaling cascade of CD38 ligation. In this study, using fyn-deficient (Fyn−/−), lyn-deficient (Lyn−/−), and fyn/lyn double-deficient (Fyn/Lyn−/−) mice, we have examined the role of Fyn and Lyn in signaling through CD38 ligation and in the synergistic effect of CD38 ligation and IL-5 on B cell triggering. We describe that CD38 ligation and IL-5 induce IgG1 secretion, which is impaired in B cells from Lyn−/− and Fyn/Lyn−/− mice.
MATERIALS AND METHODS
Reagents.
Mouse recombinant IL-5 was prepared and purified using anti-IL-5 mAb coupled beads as described (12). Rat anti-mouse CD38 mAb, CS/2, and anti-mouse IL-5Rα mAb, H7, were prepared as described (9, 38). Rabbit anti-mouse Btk polyclonal antibodies were prepared by immunizing rabbits with glutathion S-transferase–Btk fusion protein (9). Anti-phosphotyrosine mAb, 4G10, was obtained from Upstate Biotechnology (Lake Placid, NY).
Flow Cytometry.
One million cells were stained with 50 μl of biotinylated CS/2, H7, or bet-2 mAb (anti-mouse IgM, 2 μg/ml) and 50 μl fluorescein isothiocyanate-labeled RA3–6B2 mAb (anti-mouse B220, 10 μg/ml) for 30 min at 4°C, with the addition of 2.4G2 mAb (anti-mouse FcγR, 10 μg/ml) to avoid nonspecific binding of the labeled mAb. After being washed, cells were incubated with 50 μl of phycoerythrin-conjugated streptavidin at 4°C for 20 min. After being stained, cells were suspended in PBS buffer containing 0.1% 7-amino-actinomycin D (Sigma) to exclude dead cells from the analysis. Fluorescence intensity was measured with a FACScan instrument (Becton Dickinson).
Assays for B Cell Proliferation and Ig Secretion.
To generate Fyn/Lyn−/− mice, we crossed Fyn−/− mice (39) with Lyn−/− mice (40). All single mutant animals used to generate Fyn/Lyn−/− mice had been back-crossed at least two generations to C57BL/6 mice. Mice were anesthetized with ether and then killed. Splenic B cells were isolated from 7- to 9-week-old mice after T cell depletion by treatment with anti-mouse Thy1.2 mAb for 30 min on ice and then with rabbit complement for 30 min at 37°C as described (23). Small dense splenic B cells were further purified by fractionation on discontinuous gradient of Percoll (Pharmacia LKB) as described (40). Approximately 90–93%, 88–91%, 83–86%, and 85–88% of isolated cells from wild-type, Fyn−/−, Lyn−/−, and Fyn/Lyn−/− mice, respectively, were found to express the B cell marker B220. Small dense B cells were cultured at a concentration of 1 × 105 cells/200 μl/well with or without stimulants in RPMI 1640 medium, 8% fetal calf serum, 2 mM l-glutamine, and 50 μM 2-mercaptoethanol/penicillin at 50 units/ml/streptomycin at 50 μg/ml in 96-well flat-bottom microtiter plates. Cultures were performed in triplicate. The cells were pulse-labeled with [3H]thymidine (0.2 μCi per well; 1 Ci = 37 GBq) during the last 6 h of a 72-h culture period, and incorporation of radioactivity was measured as described (9). Results were expressed as the mean cpm ± SEMs of triplicate cultures.
For determining Ig secretion, cells were cultured for 7 days, and IgM and IgG1 in cultured supernatants were determined by ELISA (9).
Immunoprecipitation and Immunoblot Analysis.
Cells (4 × 107/2 ml) were stimulated with CS/2 (final 20 μg/ml) or anti-IgM mAb (20 μg/ml) for 5 min at 37°C. Cell lysates were prepared and precleared with protein G-Sepharose 4B. The lysates (400 μl) were incubated at 4°C for 60 min with 5 μg of anti-Btk antibody. Immune complexes were collected on protein G-Sepharose during a 60-min incubation at 4°C, washed with lysis buffer, and boiled for 5 min with 2X Laemmli’s sample buffer. Samples were electrophoresed on SDS/8% polyacrylamide gels and transferred to an Immobilon-P membrane (Nihon Millipore, Tokyo). After blocking with Tris-buffered saline (TBS) containing 5% BSA, membranes were incubated with the appropriate primary antibody and washed in TBS/0.05% Tween 20 (TBS-T). After incubation with rabbit anti-mouse or donkey anti-rabbit secondary antibodies coupled to horse radish peroxidase, membranes were washed with TBS-T and subjected to an enhanced chemiluminescence detection system (Amersham).
RESULTS
Impaired Proliferative Response of B Cells from Lyn Knock-Out Mice to IL-5 and CD38 Ligation.
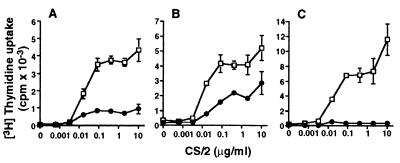
Small dense splenic B cells were stimulated with mAb CS/2 for 3 days, and uptake of [3H]thymidine was determined. mAb CS/2 alone (>10 ng/ml) induced proliferation of B cells from wild-type mice (Fig. 1). This response reached a plateau at a mAb CS/2 concentration of 0.1 μg/ml. The proliferative response was significantly reduced by 80% and 50% of control response in B cells from Fyn−/− and Lyn−/− mice (Fig. 1 A and B), respectively, and was abolished in B cells from Fyn/Lyn−/− mice (Fig. 1C).
Figure 1.
Effect of CD38 ligation on B cell proliferation. T cell-depleted splenic B cells (1 × 105/well) from (A) Fyn−/−, (B) Lyn−/−, or (C) Fyn/Lyn−/− mice were cultured for 3 days with various concentrations of CS/2. Cells were pulse-labeled with [3H]thymidine, and incorporation of radioactivity was measured. Results are expressed as mean cpm ± SEMs of triplicate cultures. (□) and (•) denote wild-type littermates and gene-deficient mice, respectively.
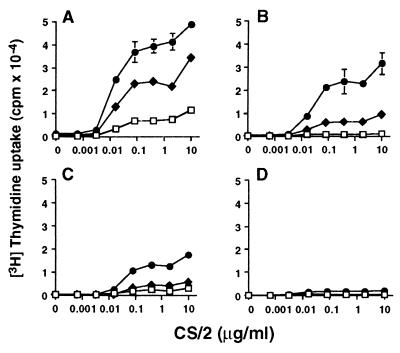
A remarkable proliferative response was observed when B cells from wild-type mice were stimulated with mAb CS/2 plus IL-5. A significant proliferative response was demonstrated down to a mAb CS/2 concentration of 10 ng/ml (Fig. 2A) when B cells from wild-type mice were cultured with a fixed concentration of IL-5 (0.5 units/ml or 5 units/ml). The B cells from Fyn−/− mice significantly responded to mAb CS/2 plus IL-5, but their responses were severely reduced (Fig. 2B). The B cells from Lyn−/− mice showed more profoundly decreased proliferative response (Fig. 2C). Splenic B cells from Fyn/Lyn−/− mice did not show a significant proliferative response (Fig. 2D). These results indicate that Lyn and Fyn are involved in proliferative response to mAb CS/2 and IL-5.
Figure 2.
Synergistic effect of mAb CS/2 and IL-5 on B cell proliferation. T cell-depleted splenic B cells (1 × 105/well) from (A) wild-type, (B) Fyn−/−, (C) Lyn−/−, or (D) Fyn/Lyn−/− mice were cultured for 3 days with various concentrations of mAb CS/2 (□), mAb CS/2 plus 0.5 units/ml of IL-5 (♦), or mAb CS/2 plus 5 units/ml of IL-5 (•). Cells were pulse-labeled with [3H]thymidine, and incorporation of radioactivity was measured. Results are expressed as mean cpm ± SEMs of triplicate cultures.
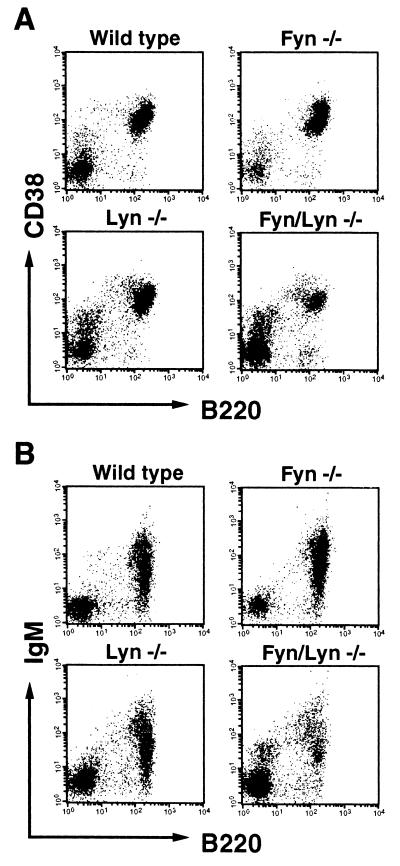
The decreased proliferative response of B cells from Fyn−/−, Lyn−/−, and Fyn/Lyn−/− mice to CD38 ligation may be due to the decreased expression of CD38 on their cell surface. We compared the expression of CD38 vs. B220 (Fig. 3A) and surface IgM vs. B220 (Fig. 3B) on spleen cells from each strain of mice with that from wild-type mice by flow cytometry. The results revealed that intensity of the CD38 and surface IgM expression on B220+ B cells from Fyn−/− and Lyn−/− mice were comparable to B cells from wild-type mice although numbers of B220+ cells in spleen from Fyn/Lyn−/− slightly decreased. We infer from these results that the impaired responsiveness of B cells from Fyn−/−, Lyn−/−, and Fyn/Lyn−/− mice may not simply be due to a reduced level of CD38 expression but rather due to an impaired signaling pathway through CD38.
Figure 3.
Flow cytometric analysis. Spleen cells from wild-type, Fyn−/−, Lyn−/−, or Fyn/Lyn−/− mice were stained with (A) biotinylated mAb CS/2 or (B) anti-IgM mAb followed by avidin-phycoerythrin and fluorescein isothiocyanate-labeled RA3–6B2.
Impaired Tyrosine Phosphorylation of Btk in B Cells from Lyn−/− and Fyn/Lyn−/− Mice.
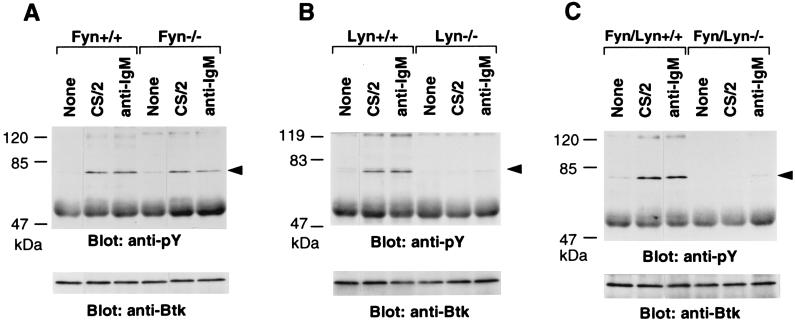
Activation of B cells through B cell antigen receptor, IL-5R, or CD38 ligation is accompanied with tyrosine phosphorylation of Btk (9, 18, 42). To gain further insight into the impaired responsiveness of B cells from Lyn−/− and Fyn/Lyn−/− mice to CD38 ligation, we focused on tyrosine phosphorylation of Btk. Splenic B cells were unstimulated or stimulated with mAb CS/2 or anti-IgM mAb for 5 min. Cell lysates from each group were analyzed by immunoblot using anti-phosphotyrosine mAb and anti-Btk antibody. Results revealed that significant tyrosine phosphorylation of Btk was induced in splenic B cells of wild-type mice stimulated with mAb CS/2 or anti-IgM mAb but was not induced in unstimulated cells (Fig. 4). Splenic B cells did not show from either Lyn−/− or Fyn/Lyn−/− mice a significant tyrosine phosphorylation of Btk with either mAb CS/2 or anti-IgM stimulation (Fig. 4 B and C) even though equal amounts of Btk were immunoprecipitated with anti-Btk antibody. B cells from Fyn−/− mice showed a significant tyrosine phosphorylation of Btk (Fig. 4A).
Figure 4.
Induction of tyrosine phosphorylation of Btk by cross-linking of CD38 or surface IgM. Small dense splenic B cells (4 × 107/2 ml) from (A) Fyn−/−, (B) Lyn−/−, or (C) Fyn/Lyn−/− mice were treated with mAb CS/2 at 40 μg or anti-IgM mAb at 40 μg for 5 min at 37°C. Cells were lysed, and lysates were immunoprecipitated with anti-Btk antibodies. Immunoprecipitates were subjected to SDS/8% PAGE analysis and immunoblotted with anti-phosphotyrosine mAb. The membrane was reprobed with anti-Btk antibody (Lower). Positions to which Btk kinase migrated are indicated by arrowheads, and marker proteins (in kilodaltons) are shown at left.
Impaired Production of IgG1 Antibody by B Cells from Lyn−/− and Fyn/Lyn−/− Mice in Response to mAb CS/2 and IL-5.
To examine the effect of mAb CS/2 on IgM and IgG1 production, splenic B cells from wild-type, Fyn−/−, Lyn−/−, and Fyn/Lyn−/− mice were cultured for 7 days with either mAb CS/2, IL-5, or mAb CS/2 plus IL-5, and the amounts of secreted IgM and IgG1 were measured. As a control, B cells also were stimulated with LPS. The B cells from Lyn−/− mice showed spontaneous production of relatively high amounts of IgM, as described (40, 41). Stimulation of B cells with mAb CS/2 alone induced a basal level of IgM by B cells from each strain of mice (Table 1). When the B cells from wild-type mice were stimulated with mAb CS/2 plus IL-5, IgM production was dramatically enhanced (Table 1). The B cells from either Fyn−/− or Lyn−/− mice responded to mAb CS/2 plus IL-5 for IgM production; however, the amounts of secreted IgM were lower than that secreted by control B cells. In contrast, B cells from Fyn/Lyn−/− mice showed markedly decreased IgM production upon stimulation with either mAb CS/2 plus IL-5 or LPS.
Table 1.
Secretion of IgM by splenic B cells from Fyn/Lyn-deficient mice
| Stimulation | Donor of spleen cells
|
|||||
|---|---|---|---|---|---|---|
| Fyn
|
Lyn
|
Fyn/Lyn
|
||||
| +/+ | −/− | +/+ | −/− | +/+ | −/− | |
| None | 24 ± 8 | 25 ± 8 | 131 ± 7 | 1181 ± 47 | 21 ± 10 | 101 ± 9 |
| CS/2 | 49 ± 7 | 103 ± 24 | 529 ± 26 | 1039 ± 41 | 81 ± 9 | 136 ± 29 |
| IL-5 | 143 ± 22 | 270 ± 23 | 1167 ± 45 | 4120 ± 200 | 519 ± 43 | 443 ± 39 |
| CS/2 + IL-5 | 13,469 ± 816 | 7024 ± 580 | 22,375 ± 818 | 6574 ± 262 | 6407 ± 749 | 777 ± 65 |
| LPS | 85,719 ± 6870 | 82,839 ± 4690 | 97,568 ± 2221 | 81,514 ± 1882 | 66,195 ± 4665 | 5512 ± 454 |
T cell-depleted splenic B cells (1 × 105 well) from wild-type (+/+) or gene-deficient (−/−) mice were cultured for 7 days. Cells were unstimulated and stimulated with mAb CS/2 (1 μg/ml), IL-5 (100 units/ml), mAb CS/2 plus IL-5, or LPS (10 μg/ml). After culturing, the amount of IgM (ng/ml) in the culture supernatant was determined by ELISA. Data are expressed as mean ± SE of triplicate cultures.
Table 2 shows that stimulation of B cells from wild-type and Fyn−/− mice with mAb CS/2 and IL-5 dramatically enhanced IgG1 production and was to a extent similar to that induced by LPS plus IL-4 (data not shown). Stimulation with mAb CS/2 plus IL-2, IL-4, or granulocyte–macrophage colony-stimulating factor showed only marginal enhancement of IgG1 production (data not shown). Conversely, stimulation of splenic B cells from Lyn−/− and Fyn/Lyn−/− mice induced marginal IgG1 production when stimulated with IL-5 plus mAb CS/2. IgG1 production by B cells in response to LPS rather was enhanced in B cells from Fyn−/−, Lyn−/−, and Fyn/Lyn−/− mice.
Table 2.
Effect of mAb CS/2 and IL-5 on IgG1 secretion by splenic B cells
| Stimulation | Donor of spleen cells
|
|||||
|---|---|---|---|---|---|---|
| Fyn
|
Lyn
|
Fyn/Lyn
|
||||
| +/+ | −/− | +/+ | −/− | +/+ | −/− | |
| None | 5 ± 2 | 8 ± 4 | 57 ± 5 | 35 ± 5 | 9 ± 1 | 8 ± 1 |
| CS/2 | 10 ± 4 | 21 ± 3 | 36 ± 26 | 41 ± 5 | 12 ± 1 | 14 ± 3 |
| IL-5 | 5 ± 2 | 18 ± 4 | 70 ± 45 | 61 ± 13 | 14 ± 1 | 7 ± 1 |
| CS/2 + IL-5 | 3659 ± 266 | 5678 ± 335 | 2629 ± 350 | 113 ± 15 | 1200 ± 31 | 136 ± 7 |
| LPS | 193 ± 13 | 1962 ± 98 | 5611 ± 728 | 18,276 ± 1929 | 178 ± 7 | 2749 ± 52 |
T cell-depleted splenic B cells (1 × 105/well) from wild-type (+/+) or gene-deficient (−/−) mice were cultured for 7 days. Cells were unstimulated and stimulated with mAb CS/2 (1 μg/ml), IL-5 (100 units/ml), mAb CS/2 plus IL-5, or LPS (10 μg/ml). After culturing, the amount of IgG1 (ng/ml) in the culture supernatant was determined by ELISA. Data are expressed as mean ± SE of triplicate cultures.
Role of Fyn or Lyn in the Enhancement of IL-5 Receptor α Chain Expression by CD38 Ligation.
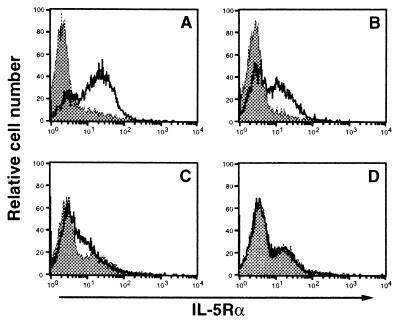
As we described (9), CD38 ligation of B cells from wild type mice enhances the expression of IL-5Rα. The impaired secretion of IgM and IgG1 by B cells from Fyn−/−, Lyn−/−, and Fyn/Lyn−/− mice in response to mAb CS/2 plus IL-5 raised the possibility that stimulation of B cells from Fyn−/−, Lyn−/−, and Fyn/Lyn−/− mice by mAb CS/2 may not enhance the expression of IL-5Rα on B cells. To evaluate this possibility, we stimulated splenic B cells with mAb CS/2 for 72 h. The IL-5Rα expression was then determined by flow cytometry using an anti-IL-5Rα mAb. An increased expression of IL-5Rα was observed in B cells from wild-type mice in response to mAb CS/2 but was not observed in B cells from Fyn/Lyn−/− mice, and significantly reduced expression was observed in B cells from Fyn−/− and Lyn−/− mice (Fig. 5).
Figure 5.
Flow cytometric analysis. Splenic B cells from (A) wild-type, (B) Fyn−/−, (C) Lyn−/−, or (D) Fyn/Lyn−/− mice were cultured with medium (shaded) or with mAb CS/2 at 1 μg/ml (unshaded) for 72 h and stained with biotinylated H7 (anti-IL-5Rα mAb), followed by avidin-phycoerythrin. Cells in the lymphoid gate were analyzed by flow cytometry.
DISCUSSION
We demonstrated four major observations in this study. (i) We showed an agonistic effect of mAb CS/2 on proliferation, tyrosine phosphorylation of Btk, and IL-5Rα expression of small dense splenic B cells. (ii) However, the agonistic effect of mAb CS/2 on B cell proliferation was impaired in B cells from Fyn−/−, Lyn−/−, and Fyn/Lyn−/− mice. Btk activation by CD38 ligation was observed in B cells from Fyn−/− mice whereas it was not observed in B cells from Lyn−/− and Fyn/Lyn−/− mice. (iii) IL-5 and mAb CS/2 synergistically acted on B cells to enhance proliferation and secretion of IgM and IgG1 by B cells from wild-type and to a lesser extent Fyn−/− mice. These synergistic effects were severely impaired in B cells from Lyn−/− and Fyn/Lyn−/− mice. And (iv) enhanced IgG1 production in response to LPS in B cells from Fyn−/−, Lyn−/−, and Fyn/Lyn−/− mice was observed. We have demonstrated that Xid B cells do not respond to CD38 for proliferation and Btk activation (9). Furthermore, stimulation of Xid B cells with mAb CS/2 plus IL-5 did not induce at all the IgG1 production (data not shown). Based on these observation, we conclude that Lyn, Fyn and Btk are required for B cell triggering by CD38 ligation.
The number of B cells in the spleen was not normal in Lyn−/− mice and was quite a bit lower in the Fyn/Lyn−/− mice (Fig. 3B). One trivial possibility is that the decreased responses may be due to decreased numbers of B cells in the populations being studied. As described in Materials and Methods, B cell populations obtained from wild-type, Fyn−/−, Lyn−/−, and Fyn/Lyn−/− mice were not different so much from each other. In the experiment shown in Fig. 1, percentages of CD38-positive cells in B cell populations from wild-type, Fyn−/−, Lyn−/−, and Fyn/Lyn−/− mice were 85%, 84%, 80%, and 82%, respectively. Furthermore, we obtained essentially the same results shown in this study by culturing twice as many B cells from Lyn−/− and Fyn/Lyn−/− mice as from wild-type mice. So we conclude that the decreased responses are not due to decreased numbers of B cells in the populations being studied.
As described (40, 41), the Lyn−/− mice showed splenomegaly with age and reduced numbers of peripheral B cells (35–70% of wild-type mice). They showed elevated levels of serum IgM, the number of IgM-secreting cells, and the existence of circulating autoantibodies (40, 41). Intriguingly, the Lyn−/− mice exhibited hyperresponsiveness to anti-IgM-induced proliferation (43). Thus, Lyn has an indispensable role in B cell antigen receptor-mediated signal transduction that is required for T cell-independent B cell proliferation and in triggering the elimination of autoreactive B cells. The proportion of IL-5Rα-positive B cells in spleen was increased in Lyn−/− mice and was not enhanced upon mAb CS/2 stimulation. Although thymocytes from Fyn−/− mice showed hyporesponsiveness to T cell antigen receptor ligation, their splenic B cells did not show abnormality to B cell antigen receptor ligation and LPS stimulation for proliferation (44). The B cells from Fyn−/− and Lyn−/− mice showed a significantly decreased proliferative response to IL-5 plus dextran sulfate (data not shown), suggesting the involvement of Fyn and Lyn in the IL-5 signaling pathway for proliferation. Phenotypes of Fyn/Lyn−/− mice used in this study were very similar to those of Lyn−/− mice.
CD38 ligation by mAb CS/2 induced B cell proliferation, IgM production, and tyrosine phosphorylation of Btk in B cells from wild-type mice. B cells from Xid mice showed no significant responsiveness to mAb CS/2 plus IL-5 (9). So, Btk must be required, directly or indirectly, for CD38-mediated signal transduction. Knowledge of how CD38 control Btk activity is necessary for understanding CD38-dependent signaling. The CD38 ligation enhanced tyrosine phosphorylation of Lyn and Fyn (data not shown), suggesting the involvement of Lyn and Fyn in CD38 signal transduction cascade. Thus, an important question is whether activations of Lyn and Fyn are indispensable, and if so, are they upstream or downstream of Btk in CD38-dependent signaling? As shown in Fig. 4, a significant Btk activation was not observed in B cells from Lyn−/− and Fyn/Lyn−/− mice when stimulated with mAb CS/2 or anti-IgM mAb, although it was observed in B cells from Fyn−/− mice. This was not due to a reduced level of the CD38 and surface IgM expression (Fig. 3).
CD38-induced proliferative response does not correlate with Btk activation; the Fyn−/− mice have Btk phosphorylation but a substantial decrease in proliferation (80% reduction) (Figs. 1 and 4). Thus, there is a Btk-independent pathway controlled by Fyn that is important for CD38-induced B cell proliferation, in addition to the Fyn/Lyn signaling pathway. These results imply that Fyn and Lyn are required for the CD38 signaling pathway and play a different role in CD38-dependent B cell signaling. Lyn is probably involved upstream of Btk in the system. Recent studies in transient expression assays have provided evidence that Btk can be activated by src-kinases and have suggested that Btk is downstream of src-family kinases in B cell signal transduction pathway (45, 46). Our results are in agreement with theirs.
Stimulation of B cells from wild-type mice with mAb CS/2 plus IL-5 dramatically enhanced B cell proliferation and IgM production (Fig. 2; Table 1). By contrast, the same stimulation of B cells from Fyn/Lyn−/− mice did not induce at all any of these effects. Splenic B cells from either Fyn−/− or Lyn−/− mice responded to mAb CS/2 plus IL-5 for significant proliferation and IgM production but to a lesser extent to control B cells (Table 1). These results indicate that Fyn and Lyn kinases are synergistic in B cell triggering by CD38 ligation and IL-5.
An intriguing observation in this study was the remarkable IgG1 production by B cells in response to mAb CS/2 and IL-5 (Table 2). Stimulation of B cells with mAb CS/2 and IL-4 did not induce the significant IgG1 production (data not shown). Neither IgA nor IgG2a production was enhanced upon stimulation with mAb CS/2 and IL-5. The CD38-dependent IgG1 production was not observed in B cells from Lyn−/− and Fyn/Lyn−/− mice, indicating the critical role of Lyn in the system. These results suggest that there are at least two different pathways for polyclonal IgG1 production; the one pathway is dependent on IL-4, and the other pathway is inducible by CD38 ligation and IL-5 in an IL-4-independent manner. Lyn and Btk are required for the latter pathway. This may be the first demonstration of the involvement of Lyn kinase in preferential IgG1 production. Although the role of IL-5 in IgG1 production in conjunction with mAb CS/2 remains unclear, the results suggest that CD38 ligation may induce isotype-switching from μ to γ1. LPS has been shown to induce IgM and IgG3 production mainly. The B cells from Lyn−/− and Fyn−/− mice produced more IgG1 upon LPS stimulation than B cells from wild-type mice. Fyn and Lyn may regulate LPS-induced isotype-switching negatively.
In conclusion, B cell triggering by CD38 ligation that is synergistically enhanced by IL-5 requires activation of Fyn, Lyn, and Btk. The signals from Fyn and Lyn kinases are synergistic in B cell triggering by CD38 ligation and IL-5. Further study of Btk-associated molecules, substrates for Btk, the natural ligand for CD38, and molecular mechanisms of enhanced IL-5Rα expression and src-kinase activation by CD38 ligation should provide an important opportunity for understanding CD38-dependent B cell signaling, in particular for IgG1 production.
Acknowledgments
We are grateful to Drs. Taku Kouro and Masashi Baba for their valuable suggestions. We thank Drs. Kimishige Ishizaka and Tatsuo Kinashi for critical review of the manuscript. This study was supported in part by a special grant for Advanced Research on Immunology and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
ABBREVIATIONS
- IL-5
interleukin 5
- LPS
lipopolysaccharide
- Lyn−/−
lyn-deficient
- Fyn−/−
fyn-deficient
- Fyn/Lyn−/−
fyn and lyn double- deficient
- Btk
Bruton tyrosine kinase
References
- 1.Malavasi F, Funaro A, Roggero S, Hornstein A, Calosso L, Mehta K. Immunol Today. 1994;15:95–97. doi: 10.1016/0167-5699(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 2.Howard M, Grimaldi J C, Bazan J F, Lund F E, Santos-Argumedo L, Parkhouse R M E, Walseth T F, Lee H C. Science. 1993;262:1056–1059. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- 3.Zocchi E, Franco L, Guida L, Benatti U, Bargellesi A, Malavasi F, Lee H C, Flora A D. Biochem Biophys Res Commun. 1993;196:1459–1465. doi: 10.1006/bbrc.1993.2416. [DOI] [PubMed] [Google Scholar]
- 4.Gelman L, Deterre P, Gouy H, Boumsell L, Debre P, Bismuth G. Eur J Immunol. 1993;23:3361–3364. doi: 10.1002/eji.1830231245. [DOI] [PubMed] [Google Scholar]
- 5.Harada N, Santos-Argumedo L, Chang R, Grimaldi J C, Lund F E, Brannan C I, Copeland N G, Jenkins N A, Heath A W, Parkhouse R M E, Howard M. J Immunol. 1993;151:3111–3118. [PubMed] [Google Scholar]
- 6.Santos-Argumedo L, Teixeira C, Preece G, Kirkham P A, Parkhouse R M E. J Immunol. 1993;151:3119–3130. [PubMed] [Google Scholar]
- 7.Kirkham P A, Santos-Arugumedo L, Harnett M M, Parkhouse R E M. Immunology. 1994;83:513–516. [PMC free article] [PubMed] [Google Scholar]
- 8.Yamashita Y, Miyake K, Kikuchi Y, Takatsu K, Noda T, Kosugi A, Kimoto M. Immunology. 1995;85:248–255. [PMC free article] [PubMed] [Google Scholar]
- 9.Kikuchi Y, Yasue T, Miyake K, Kimoto M, Takatsu K. Proc Natl Acad Sci USA. 1995;92:11814–11818. doi: 10.1073/pnas.92.25.11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takatsu K. Curr Opin Immunol. 1992;4:299–306. doi: 10.1016/0952-7915(92)90080-x. [DOI] [PubMed] [Google Scholar]
- 11.Takatsu K, Takaki S, Hitoshi Y. Adv Immunol. 1994;57:145–190. doi: 10.1016/s0065-2776(08)60673-2. [DOI] [PubMed] [Google Scholar]
- 12.Takaki S, Tominaga A, Hitoshi Y, Mita S, Sonoda E, Yamaguchi N, Takatsu K. EMBO J. 1990;9:4367–4374. doi: 10.1002/j.1460-2075.1990.tb07886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murata Y, Takaki S, Migita M, Kikuchi Y, Tominaga A, Takatsu K. J Exp Med. 1992;175:341–351. doi: 10.1084/jem.175.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takaki S, Mita S, Kitamura T, Yonehara S, Yamaguchi N, Tominaga A, Miyajima A, Takatsu K. EMBO J. 1991;10:2833–2838. doi: 10.1002/j.1460-2075.1991.tb07832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devos R, Plaetinck G, Heyden J V D, Cornelis S, Vandekerckhove J, Fiers W, Tavernier J. EMBO J. 1991;10:2133–2137. doi: 10.1002/j.1460-2075.1991.tb07747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takaki S, Murata Y, Kitamura T, Miyajima A, Tominaga A, Takatsu K. J Exp Med. 1993;177:1523–1529. doi: 10.1084/jem.177.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavernier J, Devos R, Cornelis S, Tuypens T, Heyden J V D, Fiers W, Plaetinck G. Cell. 1991;66:1175–1184. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- 18.Takaki S, Kanazawa H, Shiiba M, Takatsu K. Mol Cell Biol. 1994;14:7404–7413. doi: 10.1128/mcb.14.11.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satoh S, Katagiri T, Takaki S, Kikuchi Y, Hitoshi Y, Yonehara S, Tsukada S, Kitamura D, Watanabe T, Witte O N, Takatsu K. J Exp Med. 1994;180:2101–2111. doi: 10.1084/jem.180.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kouro T, Kikuchi Y, Kanazawa H, Hirokawa K, Harada N, Shiiba M, Wakao H, Takaki S, Takatsu K. Int Immunol. 1996;8:237–245. doi: 10.1093/intimm/8.2.237. [DOI] [PubMed] [Google Scholar]
- 21.Rawlings D J, Saffran D C, Tsukada S, Largaespada D A, Grimaldi J C, Cohen L, Mohr R N, Bazan J F, Howard M, Copeland N G, Jenkins N A, Witte O N. Science. 1993;261:358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 22.Thomas J D, Sideras P, Smith C I E, Vorechovsky I, Chapman V, Paul W E. Science. 1993;261:355–361. doi: 10.1126/science.8332900. [DOI] [PubMed] [Google Scholar]
- 23.Hitoshi Y, Sonoda E, Kikuchi Y, Yonehara S, Nakauchi H, Takatsu K. Int Immunol. 1993;9:1183–1190. doi: 10.1093/intimm/5.9.1183. [DOI] [PubMed] [Google Scholar]
- 24.Koike M, Kikuchi Y, Tominaga A, Takaki S, Akagi K, Miyazaki J-I, Yamamura K-I, Takatsu K. Int Immunol. 1995;7:21–30. doi: 10.1093/intimm/7.1.21. [DOI] [PubMed] [Google Scholar]
- 25.Scher I. Immunol Rev. 1982;64:117–136. doi: 10.1111/j.1600-065x.1982.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 26.Santos-Argumedo L, Lund F E, Heath A W, Solvason N, Wu W W, Grimaldi J C, Parkhouse R M E, Howard M. Int Immunol. 1995;7:163–170. doi: 10.1093/intimm/7.2.163. [DOI] [PubMed] [Google Scholar]
- 27.Bolen J B, Rowley R B, Spana C, Tsygankov A Y. FASEB J. 1992;6:3403–3409. doi: 10.1096/fasebj.6.15.1281458. [DOI] [PubMed] [Google Scholar]
- 28.Burkhardt A L, Brunswick M, Bolen J B, Mond J J. Proc Natl Acad Sci USA. 1991;88:7410–7414. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamanashi Y, Mori S, Yoshida M, Kishimoto T, Inoue J, Yamamoto T, Toyoshima K. Proc Natl Acad Sci USA. 1989;86:6538–6542. doi: 10.1073/pnas.86.17.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell M-A, Sefton B M. Mol Cell Biol. 1992;12:2315–2221. doi: 10.1128/mcb.12.5.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren C L, Morio T, Fu S M, Geha R S. J Exp Med. 1994;179:673–680. doi: 10.1084/jem.179.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefanova I, Corcoran M L, Horak E M, Wahl L M, Bolen J B, Horak I D. J Biol Chem. 1993;268:20725–20728. [PubMed] [Google Scholar]
- 33.Eisenmann E, Bolen J B. Nature (London) 1992;355:78–80. [Google Scholar]
- 34.Corey S J, Equinoa A, Puyana-Theall K, Bolen J B, Cantly L, Mollinedo F, Jacjson T R, Hawkins P T, Stephens L R. EMBO J. 1993;12:2681–2690. doi: 10.1002/j.1460-2075.1993.tb05929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pazdrak K, Schreiber D S, Forsythe P, Justement L, Alam R. J Exp Med. 1995;181:1827–1834. doi: 10.1084/jem.181.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng G, Ye Z S, Baltimore D. Proc Natl Acad Sci USA. 1994;91:8152–8155. doi: 10.1073/pnas.91.17.8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satterthwaite A B, Witte O N. Curr Opin Immunol. 1996;6:454–458. doi: 10.1016/s0952-7915(96)80029-x. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi N, Hitoshi Y, Mita S, Hosoya Y, Murata Y, Kikuchi Y, Tominaga A, Takatsu K. Int Immunol. 1990;2:181–187. doi: 10.1093/intimm/2.2.181. [DOI] [PubMed] [Google Scholar]
- 39.Yagi T, Aizawa S, Tokunaga T, Shigetani Y, Takeda N, Ikawa Y. Nature (London) 1993;366:742–745. doi: 10.1038/366742a0. [DOI] [PubMed] [Google Scholar]
- 40.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 41.Hibbs M L, Tarlinton D M, Armes J, Grail d, Hodgson G, Maglitto R, Stacker S A, Dunn A R. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 42.Aoki Y, Isserbacher K J, Pillai S. Proc Natl Acad Sci USA. 1994;91:10606–10609. doi: 10.1073/pnas.91.22.10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Koizumi T, Watanabe T. J Exp Med. 1996;184:831–838. doi: 10.1084/jem.184.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Appleby M W, Gross J A, Cooke M P, Levin S D, Qian X, Perlmutter R M. Cell. 1992;70:751–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- 45.Mahajan S, Fargnoli J, Burkhardt A L, Kut S A, Saouaf S J, Bolen J B. Mol Cell Biol. 1995;15:5304–5311. doi: 10.1128/mcb.15.10.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rawlings D J, Scharenberg A M, Park H, Wahl M I, Lin S, Kato R M, Fluckinger A-C, Witte O N, Kinet J-P. Science. 1996;271:822–825. doi: 10.1126/science.271.5250.822. [DOI] [PubMed] [Google Scholar]