Abstract
The development of methods for efficient gene transfer to terminally differentiated retinal cells is important to study the function of the retina as well as for gene therapy of retinal diseases. We have developed a lentiviral vector system based on the HIV that can transduce terminally differentiated neurons of the brain in vivo. In this study, we have evaluated the ability of HIV vectors to transfer genes into retinal cells. An HIV vector containing a gene encoding the green fluorescent protein (GFP) was injected into the subretinal space of rat eyes. The GFP gene under the control of the cytomegalovirus promoter was efficiently expressed in both photoreceptor cells and retinal pigment epithelium. However, the use of the rhodopsin promoter resulted in expression predominantly in photoreceptor cells. Most successfully transduced eyes showed that photoreceptor cells in >80% of the area of whole retina expressed the GFP. The GFP expression persisted for at least 12 weeks with no apparent decrease. The efficient gene transfer into photoreceptor cells by HIV vectors will be useful for gene therapy of retinal diseases such as retinitis pigmentosa.
Inherited retinal degenerations define a genetically and clinically heterogeneous group of eye diseases. Retinitis pigmentosa is the most common form of retinal degeneration, affecting ≈1 in 3,500 people. Symptoms include progressive loss of visual field, night blindness, and the deposition of pigment in the retina. Although increasing numbers of genes responsible for retinal degenerative diseases have recently been identified, the underlying mechanism of retinal degeneration is not well understood and there are no adequate therapies for these diseases at present. Therefore, an efficient method of gene transfer into the retina provides a useful tool for both the investigation and treatment of retinal degeneration.
Many techniques have been tested for in vivo gene transfer into the retina. Nonviral methods, such as liposomes, are inefficient and only achieve transient expression of the transgene (1, 2). The majority of cells in the mature retina, including photoreceptor cells, are terminally differentiated and are incapable of proliferation. In this regard, vectors derived from retroviruses such as Moloney murine leukemia virus (MLV) are not useful because they require proliferation of the target cells for integration and stable expression. Consequently, the use of retroviral vectors are limited to ex vivo gene transfer (3, 4). Several other viral vectors, including herpes simplex virus, adenovirus and adeno-associated virus vectors, have been developed for gene delivery to nondividing cells such as neurons. However, transduction of photoreceptor cells or other neuronal retinal cells by means of these viral vectors has been inefficient, and sustained long-term expression has not been observed (5–12). Recently, Flannery et al. (13) have reported that the use of a rhodopsin promoter in adeno-associated virus vectors allowed efficient photoreceptor-targeted gene expression. Successful gene transfer to photoreceptor cells is important for gene therapy of retinal degenerative diseases, because degeneration primarily affects photoreceptor cells and most of the identified genes causing retinal degeneration are expressed specifically in photoreceptor cells (14).
We have described previously (15) a lentiviral vector system based on the HIV that can transduce nondividing cells in vitro and in vivo. Subsequently, we have shown efficient gene transfer and sustained long-term expression of the transgene in terminally differentiated neurons of the adult rat brain with HIV vectors (16, 17). In the present study, we have evaluated the ability of an HIV vector, which contains the green fluorescent protein (GFP) reporter gene under the control of the cytomegalovirus (CMV) promoter, to transduce retinal cells of rats. In addition, an HIV vector with the rhodopsin promoter was designed to direct photoreceptor-specific expression of the reporter gene. We report here efficient transduction of retinal cells using HIV vectors.
MATERIALS AND METHODS
Plasmid Constructs and Preparation of Viral Vectors.
pEGFP-C1 B/S was constructed by inserting a 13-bp SpeI linker at the BglII site of pEGFP-C1 (Clontech) to introduce two in-frame stop codons just upstream of XhoI site. A humanized, red-shifted GFP gene fragment, driven by the CMV promoter, was obtained from pEGFP-C1 B/S by digesting with AseI, blunt-ending with the Klenow fragment of DNA polymerase I, and digesting with XhoI. This fragment was subcloned into the EcoRV and XhoI sites of pBluescript KS(+) (Stratagene), resulting in pKS-CMV-GFP. The HIV vector with the CMV promoter, pHR′-CMV-GFP, was constructed by inserting the 1,340-bp BamHI-XhoI fragment containing the CMV promoter and the GFP gene from pKS-CMV-GFP into the same sites of pHR′ vector (15). The HIV vector with the rhodopsin promoter, pHR′-Rho-GFP, was constructed by replacing the CMV promoter fragment in pHR′-CMV-GFP with the bovine rhodopsin promoter fragment from −2174 rhodopsin-lacZ fusion construct (a kind gift from D. J. Zack, ref. 18). The HIV-based vectors were generated as described (16). The MLV-based vector, pCLNCG, was derived from a pCL vector (19) and contains the same CMV-GFP gene expression cassette as pHR′-CMV-GFP and additionally the neomycin resistance gene. Cloning details are available upon request. The MLV-based vector was generated by the same procedure as the HIV-based vectors except using 293 gp/bsr cells, that stably express MLV gag and pol under the control of the CMV promoter. The titer of pHR′-CMV-GFP and pCLNCG vectors was determined by infection of 208F rat fibroblasts, seeded in six-well plates at 1 × 105 cells per well the day before infection, with serial dilutions of concentrated vector stocks in the presence of 8 μg/ml of Polybrene (Aldrich). After overnight incubation, cell culture medium was changed and the cells further incubated for 2 days, and the number of GFP-positive cells was scored by fluorescence microscopy and/or fluorescence-activated cell sorting analysis to quantify the titer. After concentration by ultracentrifugation, titers of 3–5 × 108 infectious units/ml on 208F cells were usually obtained. The titer of pHR′-Rho-GFP vector was normalized for HIV-1 p24 antigen level, that was determined by ELISA (DuPont).
In Vivo Delivery of Vectors.
Adult Fischer 344 rats (age 4 weeks) or pups (age 2 to 5 days) were anesthetized by i.p. injection of ketamin/acepromazine/xylazine or chilling on ice for 5 min, respectively. For pups, the eyeball was exposed by an incision in the eyelid, parallel to the future edge of the open eyelid. Subretinal injections were performed under an operating microscope. A small incision was made in sclera, and 0.5–1.5 μl of vector suspension (≈5 × 105 infectious units) was injected through the incision into the subretinal space using a glass capillary connected with a 5-μl syringe (Hamilton). For adult rats, a total of eight eyes were injected with the pHR′-CMV-GFP vector, eight with the pHR′-Rho-GFP vector, 10 with the pCLNCG vector, and 10 with saline. For pups, a total of 44 eyes were injected with the pHR′-CMV-GFP, 24 with the pHR′-Rho-GFP, and 44 with the pCLNCG.
Detection of GFP Expression.
Rats were transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer at 2, 6, or 12 weeks after injection. Eyes were enucleated and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 6–8 h at 4°C followed by cryoprotection by soaking in 30% sucrose at 4°C overnight. Eyes were frozen in O.C.T. compound (Tissue-Tek) on dry ice and 20-μm-thick cryostat sections were cut parallel to the vertical meridian of the eye. GFP expression were analyzed by confocal laser scanning microscopy (Bio-Rad). The images were generated electronically with Adobe photoshop. Fluorescent signals were collected under the same conditions and no modification on intensity was made, so that fluorescent intensity of each section can be compared. Cell nuclei were counterstained with 4′,6′ diamino-2-phenylindole and observed with a UV filter. The same fields were examined with ×40 objective lens equipped with grid to determine the number of GFP-positive cells and the total number of photoreceptor cells. At least 2,000 cells in four separate fields of the most efficiently transduced eye in each group were scored.
RESULTS
To study gene transfer and expression in vivo, we used a humanized, red-shifted GFP gene as a reporter under the control of the CMV promoter or the rhodopsin promoter in an HIV-based vector system (15). Since the virus particles are pseudotyped with the vesicular stomatitis virus G glycoprotein, the vector can be concentrated to high titer and transduce a broad range of tissues. The titer of HIV vector with the CMV promoter was determined by infecting dividing 208F rat fibroblast cells. However, the titer of HIV vector with the rhodopsin promoter was difficult to determine, because rhodopsin promoter activity is extremely low in established nonretinal cell lines and even in Y79 retinoblastoma cells. Therefore, equal amounts of recombinant viruses normalized for HIV-1 p24 antigen level were used. For comparison, vesicular stomatitis virus G glycoprotein pseudotyped MLV-based vector with the CMV promoter, matched for transducing activity on 208F cells, was used. Approximately 5 × 105 infectious units of vector suspension were injected into the subretinal space of both eyes of 2- to 5-day-old pups or 4-week-old adult rats (Fig. 1). At serial time points 2 to 12 weeks postvector delivery, rats were sacrificed and eye tissue was cryosectioned and analyzed for GFP expression by fluorescence microscopy.
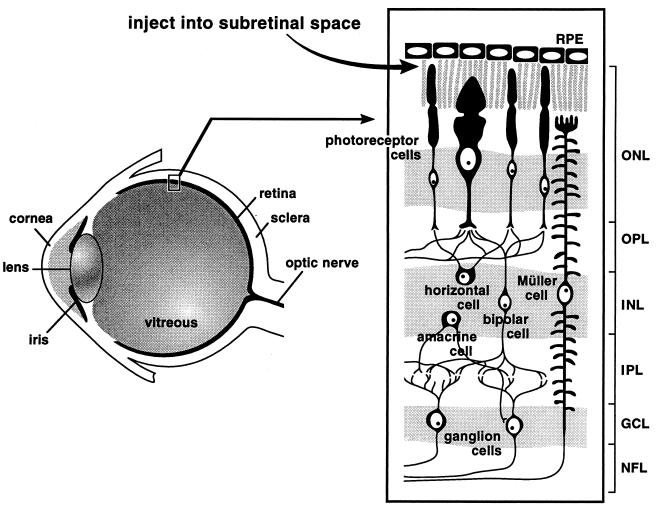
Figure 1.
Schematic diagram of the eye and retina. The site of subretinal injection is indicated. ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; NFL, nerve fiber layer.
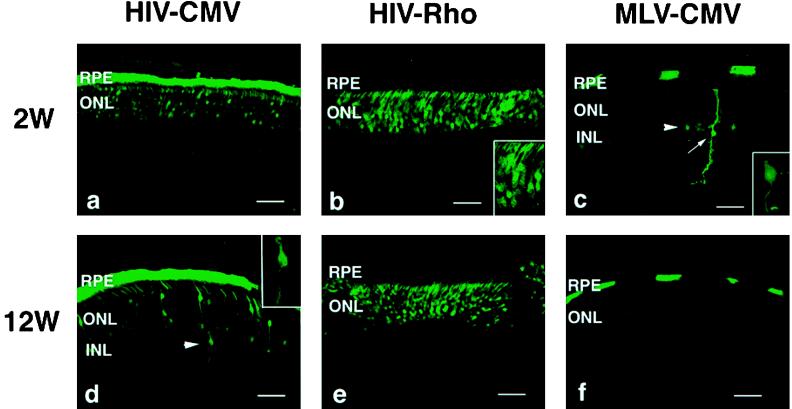
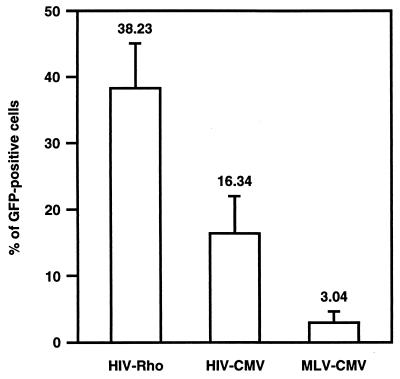
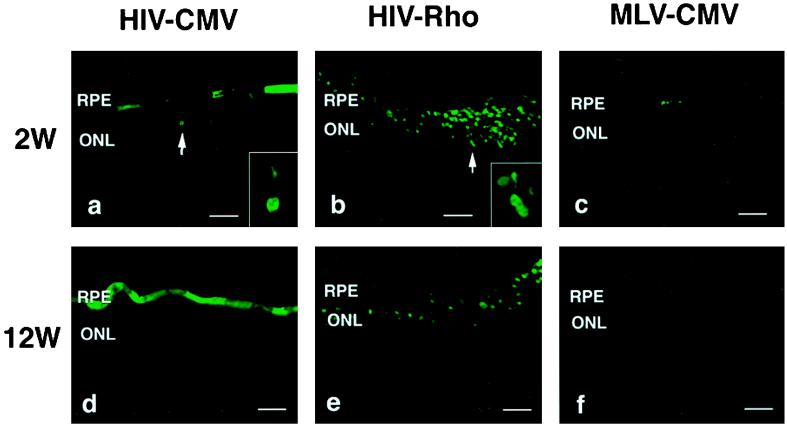
Fig. 2 shows retinal sections from pups 2 and 12 weeks postinjection. In eyes injected with the HIV vector with the CMV promoter, the GFP gene was intensely expressed in a very high proportion of RPE cells as well as in a large number of photoreceptor cells (Fig. 2 a and d). In addition, a few GFP-positive cells were unambiguously identified as bipolar cells (see arrowhead in Fig. 2d) on the basis of morphological characteristics, and no positive cells were seen in other types of retinal cells. No green fluorescence was detected in the eyes of control animals that received only saline (data not shown). In contrast, the MLV vector with the CMV promoter transduced RPE and photoreceptor cells inefficiently (Fig. 2 c and f). Additionally, small numbers of positive cells were found in the neural retina, including bipolar cells and mostly Müller cells (see Fig. 2c), around the injection site. For the MLV vector, the intensity of the green fluorescence in photoreceptor cells was much less than that transduced with HIV vectors. Injection of the HIV vector with the rhodopsin promoter resulted in GFP expression only in photoreceptor cells (Fig. 2 b and e). Note that unlike the expression in nonretinal cells in vitro, GFP expression with the rhodopsin promoter in photoreceptor cells was more efficient than that with the CMV promoter. Although the number of GFP-positive cells varied highly in each eye and even in the different sections from the same eye, most successfully transduced eyes showed that photoreceptor cells in >80% of the whole area of the retina viewed through multiple sections expressed GFP (representative example is shown in Fig. 3). The pattern and the total number of GFP-positive cells did not change appreciably for at least 12 weeks after injection of HIV vectors or MLV vector (Fig. 2 a–f). Several inflammatory cells such as macrophages were observed in the eyes surrounding the injection site at 2 weeks after injection of vectors. This inflammation was likely due to the surgical damage during injection, because a similar phenomenon was observed in control animals that had saline injections (data not shown). No inflammation was observed at 6 and 12 weeks postinjection. To compare the transduction efficiency of photoreceptor cells, sections of eyes were stained with 4′,6′ diamino-2-phenylindole and were then examined by fluorescence microscopy to determine the percentage of GFP-positive photoreceptor cells (Fig. 4). The difference between HIV and MLV vectors with the CMV promoter is greater than 5-fold (≈16% vs. 3% for HIV and MLV vectors, respectively). The HIV vector with the rhodopsin promoter transduced about 2.5 times more photoreceptor cells than the HIV vector with the CMV promoter. The increased efficiency may be due to high levels of expression of the transgene driven by the photoreceptor-specific rhodopsin promoter, because equal amounts of HIV vectors were injected per eye.
Figure 2.
Expression of GFP in the retina of rat pups 2 and 12 weeks after injection. (a and d) HIV vector with the CMV promoter. Arrowhead in d indicates GFP-positive bipolar cell. (Inset) Higher magnification of GFP-positive bipolar cell. (b and e) HIV vector with the rhodopsin promoter. Note that only photoreceptor cells are GFP positive. (Inset) Higher magnification of GFP-positive photoreceptor cells. (c and f) MLV vector with the CMV promoter. Arrowhead in c indicates GFP-positive bipolar cell. Arrow in c indicates GFP-positive Müller cell. (Inset) Higher magnification of GFP-positive bipolar cell. Original magnification, ×400. Bars = 50 μm.
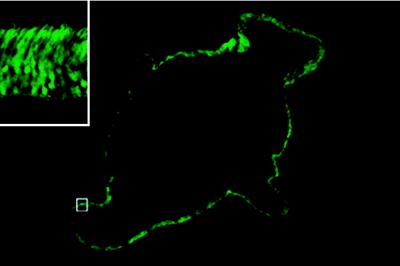
Figure 3.
Extent of GFP expression in the retina. Transverse section of the pup eye 2 weeks after injection of the HIV vector with the rhodopsin promoter. Note that photoreceptor cells in whole area of retinal section express GFP. Original magnification, ×40. (Inset) Higher magnification of the area indicated by □.
Figure 4.
Proportion of GFP-positive photoreceptor cells in the outer nuclear layer 2 weeks after injection into the eyes of pups. HIV-Rho, HIV vector with the rhodopsin promoter; HIV-CMV, HIV vector with the CMV promoter; MLV-CMV, MLV vector with the CMV promoter. At least 2,000 cells in 4 separate fields of the most efficiently transduced eye in each group were scored. Data represent means ± SD. P values are calculated by Student’s t test. P = 0.0180, HIV-Rho vs. HIV-CMV. P = 0.0008, HIV-Rho versus MLV-CMV. P = 0.0141, HIV-CMV vs. MLV-CMV.
We next evaluated the ability of HIV vectors to transduce cells in the adult rat eye. Fig. 5 shows that the pattern of GFP expression in adult rat eyes injected with HIV vectors is similar to that of pups but the transduction efficiency is decreased. With the CMV promoter, the distribution of GFP-positive cells in RPE was more restricted than that in pups, and only photoreceptor cells around the injection site expressed GFP (Fig. 5 a and d). With the rhodopsin promotor, GFP-positive cells were also seen only in the outer nuclear layer, where photoreceptors are located, surrounding the injection site (Fig. 5 b and e). There were no significant differences in the pattern and the percentage of GFP-positive cells between 2 and 12 weeks postinjection of HIV vectors. In contrast, the MLV vector transduced only a few RPE cells, and no GFP-positive cells were found in the neural retina, presumably because the majority of the retinal cells are terminally differentiated in adult eyes (Fig. 5c). Little or no expression of GFP was detected at 12 weeks postinjection (Fig. 5f).
Figure 5.
Expression of GFP in the adult retina 2 and 12 weeks after injection. (a and d) HIV vector with the CMV promoter. (b and e) HIV vector with the rhodopsin promoter. (c and f) MLV vector with the CMV promoter. Arrows in a and b indicate GFP-positive photoreceptor cells. Insets in a and b, higher magnification of GFP-positive photoreceptor cells. Original magnification, ×400. Bars = 50 μm.
DISCUSSION
The present study demonstrates that HIV-based vectors can efficiently transfer and express a transgene in retinal cells, especially in RPE and photoreceptor cells. In adult rats, expression of the transgene was restricted around the injection site. One possible explanation for this is that the interstitial space between photoreceptor cells is more tight in adults than in pups, and HIV vector particles may not diffuse into the interphotoreceptor and subretinal space. To transduce a larger number of retinal cells in the adult, multiple subretinal injections are needed. An alternative way of delivery is intravitreous injection with high numbers of vector particles.
Expression of the transgene persisted for at least 12 weeks with no apparent decrease. Since outer segments of photoreceptor cells continuously turn over by phagocytosis of RPE cells, long-term expression of the transgene in photoreceptor cells is presumably due to stable integration of the transgene into the genome of cells with self-renewing properties. This idea is supported by our previous study (16) using an HIV vector with mutant integrase, in which we found that expression of the transgene in rat brain depends on its ability to integrate. Recently, we have also demonstrated long-term expression of the transgene in liver and muscle using HIV vectors (T. Kafri, U. Blömer, F.H.G., and I.M.V., unpublished work). Therefore, long-term expression in the retina as well as the brain cannot be explained by the relative immune privilege in these tissues.
MLV requires proliferation of target cells for integration and expression of the transgene (20). Hence the ability of MLV vectors to infect neuronal retinal cells in pups is likely due to a small proportion of retinal cells capable of proliferation and differentiation at the time of injection. In fact, autoradiographic studies with 3H-thymidine have shown that 20–30% of retinal cells still proliferate in the early postnatal period (21). In addition, lineage analysis using MLV vectors to mark clones has shown the existence of proliferating multipotential progenitor cells in postnatal rodent retina (22).
Several other virus-based vectors, including those of herpes simplex virus, adenovirus and adeno-associated virus, have been tested for gene delivery to retinal cells. The transduction frequency of these vectors to photoreceptor cells was extremely low and expression of transgenes was transient or diminished over long terms (5–12). However, these vectors can transduce RPE cells with relatively high efficiency (5–9, 11, 12). Our studies here also show efficient and sustained transduction in RPE cells with HIV and MLV vectors. RPE cells may efficiently take up viral particles by their intrinsic phagocytic activity.
It was of interest to evaluate the specificity and efficiency of transgene expression using a cell-type specific promoter in the HIV-based vector delivery system. We chose the rhodopsin promoter as a cell-type specific promoter to drive the expression of the reporter gene, because rhodopsin is the most abundant photoreceptor-specific protein (4 × 107 rhodopsins per rod) (23). Additionally, it has been shown in transgenic mouse experiments that the DNA fragment of the rhodopsin promoter we used in this study can direct photoreceptor-specific expression (18). The use of a rhodopsin promoter in the HIV vector led not only to photoreceptor-specific expression but also to higher expression levels than with the CMV promoter. Recently, Flannery et al. (13) have also reported that adeno-associated virus vectors with the rhodopsin promoter can achieve efficient transduction of photoreceptor cells.
In vivo gene delivery to photoreceptor cells is important for gene therapy of inherited retinal degenerative diseases such as retinitis pigmentosa, because most of the identified genes responsible for retinal degeneration so far are expressed specifically in photoreceptor cells (14). Our results suggest that the HIV vector is a promising vehicle for delivering therapeutic genes to photoreceptor cells. This can be tested in mouse models of retinitis pigmentosa, including the spontaneous mutants rd and rds (24–26), and mice carrying a targeted disruption of the rhodopsin gene or the γ subunit of rod cGMP phosphodiesterase gene (27, 28). Transgenic experiments in rd and rds mice have shown that introduction of wild-type genes, the β subunit of rod cGMP phosphodiesterase and peripherin genes, respectively, can rescue retinal degeneration (29, 30). However, it is not yet clear when and how many photoreceptor cells have to be rescued from degeneration to maintain minimal visual function. Hence, the HIV-based vector delivery system offers substantial promise for both the investigation and treatment of retinal diseases.
Acknowledgments
We are grateful to D. J. Zack for generously providing the bovine rhodopsin promoter construct and N. Somia for critical reading of the manuscript. We thank members of the Verma and Gage laboratories for helpful suggestions. H.M. was supported by the Human Frontier Science Program Fellowship, and M.T. was supported by the Nippon Eye Bank Association Fellowship. This work was supported by grants from the National Institutes of Health, American Paralysis Association, and Frances Berger Foundation. I.M.V. is an American Cancer Society Professor of Molecular Biology.
ABBREVIATIONS
- CMV
cytomegalovirus
- MLV
Moloney murine leukemia virus
- GFP
green fluorescent protein
- RPE
retinal pigment epithelium
References
- 1.Masuda I, Matsuo T, Yasuda T, Matsuo N. Invest Ophthalmol Visual Sci. 1996;37:1914–1920. [PubMed] [Google Scholar]
- 2.Hangai M, Kaneda Y, Tanihara H, Honda Y. Invest Ophthalmol Visual Sci. 1996;37:2678–2685. [PubMed] [Google Scholar]
- 3.Dunaief J L, Kwun R C, Bhardwaj N, Lopez R, Gouras P, Goff S P. Hum Gene Ther. 1995;6:1225–1229. doi: 10.1089/hum.1995.6.9-1225. [DOI] [PubMed] [Google Scholar]
- 4.Kido M, Rich K A, Lang G, Barrón E, Kohn D B, Al-Ubaidi M R, Blanks J C. Curr Eye Res. 1996;15:833–844. doi: 10.3109/02713689609017624. [DOI] [PubMed] [Google Scholar]
- 5.Li T, Breakefield X O, Garber D, Knipe D, Roof D, Mukai S, Berson E L, Dryja T P. Invest Ophthalmol Visual Sci. 1993;34:1370. (1993). [Google Scholar]
- 6.Bennett J, Wilson J, Sun D, Forbes B, Maguire A. Invest Ophthalmol Visual Sci. 1994;35:2535–2542. [PubMed] [Google Scholar]
- 7.Li T, Adamian M, Roof D J, Berson E L, Dryja T P, Roessler B J, Davidson B L. Invest Ophthalmol Visual Sci. 1994;35:2543–2549. [PubMed] [Google Scholar]
- 8.Jomary C, Piper T A, Dickson G, Couture L A, Smith A E, Neal M J, Jones S E. FEBS Lett. 1994;347:117–122. doi: 10.1016/0014-5793(94)00512-5. [DOI] [PubMed] [Google Scholar]
- 9.Mashhour B, Couton D, Perricaudet M, Briand P. Gene Ther. 1994;1:122–126. [PubMed] [Google Scholar]
- 10.Bennett J, Tanabe T, Sun D, Zeng Y, Kjeldbye H, Gouras P, Maguire A M. Nat Med. 1996;2:649–654. doi: 10.1038/nm0696-649. [DOI] [PubMed] [Google Scholar]
- 11.Ali R R, Reichel M B, Thrasher A J, Levinsky R J, Kinnon C, Kanuga N, Hunt D M, Bhattacharya S S. Hum Mol Genet. 1996;5:591–594. doi: 10.1093/hmg/5.5.591. [DOI] [PubMed] [Google Scholar]
- 12.Zolotukhin S, Potter M, Hauswirth W W, Guy J, Muzyczka N. J Virol. 1996;70:4646–4654. doi: 10.1128/jvi.70.7.4646-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flannery J G, Zolotukhin S, Vaquero M I, LaVail M M, Muzyczka N, Hauswirth W W. Proc Natl Acad Sci USA. 1997;94:6916–6921. doi: 10.1073/pnas.94.13.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dryja T P, Li T. Hum Mol Genet. 1995;4:1739–1743. doi: 10.1093/hmg/4.suppl_1.1739. [DOI] [PubMed] [Google Scholar]
- 15.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 16.Naldini L, Blömer U, Gage F H, Trono D, Verma I M. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blömer, U., Naldini, L., Kafri, T., Trono, D., Verma, I. M. & Gage, F. H. (1997) J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 18.Zack D J, Bennett J, Wang Y, Davenport C, Klaunberg B, Gearhart J, Nathans J. Neuron. 1991;6:187–199. doi: 10.1016/0896-6273(91)90355-4. [DOI] [PubMed] [Google Scholar]
- 19.Naviaux R K, Costanzi E, Haas M, Verma I M. J Virol. 1996;70:5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller D G, Adam M A, Miller A D. Mol Cell Biol. 1990;10:4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanks J C, Bok D. J Comp Neur. 1977;174:317–328. doi: 10.1002/cne.901740208. [DOI] [PubMed] [Google Scholar]
- 22.Turner D L, Cepko C L. Nature (London) 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- 23.Nathans J. Biochemistry. 1992;31:4923–4931. doi: 10.1021/bi00136a001. [DOI] [PubMed] [Google Scholar]
- 24.Pittler S J, Baehr W. Proc Natl Acad Sci USA. 1991;88:8322–8326. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Travis G H, Sutcliffe J G, Bok D. Neuron. 1991;6:61–70. doi: 10.1016/0896-6273(91)90122-g. [DOI] [PubMed] [Google Scholar]
- 26.Connell G, Bascom R, Molday L, Reid D, Mclnnes R R, Molday R S. Proc Natl Acad Sci USA. 1991;88:723–726. doi: 10.1073/pnas.88.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphries M M, Rancourt D, Farrar G J, Kenna P, Hazel M, Bush R A, Sieving P A, Sheils D M, McNally N, Creighton P, Erven A, Boros A, Gulya K, Capecchi M R, Humphries P. Nat Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- 28.Tsang S H, Gouras P, Yamashita C K, Kjeldbye H, Fisher J, Farber D B, Goff S P. Science. 1996;272:1026–1029. doi: 10.1126/science.272.5264.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lem J, Flannery J G, Li T, Applebury M L, Farber D B, Simon M I. Proc Natl Acad Sci USA. 1992;89:4422–4426. doi: 10.1073/pnas.89.10.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Travis G H, Groshan K R, Lloyd M, Bok D. Neuron. 1992;9:113–119. doi: 10.1016/0896-6273(92)90226-4. [DOI] [PubMed] [Google Scholar]