Abstract
In eukaryotic cells, DNA polymerase β (polβ) carries out base-excision repair (BER) required for DNA maintenance, replication, recombination, and drug resistance. A specific deletion in one allele in the coding sequence of the polβ gene occurs in colorectal and breast carcinomas. The 87-bp deleted region encodes amino acid residues 208–236 in the catalytic domain of the enzyme. Here, we report evidence for expression of the wild-type (WT) and the truncated polβ proteins in colorectal tumors. To elucidate the potential functional consequences of polβ truncation, stable HeLa cell lines were established from cloned WT and variant polβΔ208–236. Cells expressing the variant protein exhibited substantially decreased BER activity. To test our hypothesis that truncated polβ may disrupt the function of the WT enzyme, we stably transfected mouse embryonic fibroblast 16.3 cells with polβΔ208–236 cDNA. Reverse transcription–PCR and Western blot analyses showed that the new cell line, 16.3ΔP, expresses the WT and the truncated polβ mRNA and proteins. BER and binding activities were undetectable in these cells. Furthermore, in vivo the 16.3ΔP cells were more sensitive to N-methyl-N′-nitro-N-nitrosoguanidine than the 16.3 cells. On adding increasing amounts of 16.3ΔP protein extracts, the BER and DNA binding activities of extracts of the parent 16.3 cell line progressively declined. These results strongly suggest that truncated polβ acts as a dominant negative mutant. The defective polβ may facilitate accumulation of mutations, leading to the expression of a mutator phenotype in tumor cells.
Keywords: expression in tumors, base-excision repair, DNA binding
Oncogenesis is a consequence of accumulated mutations in multiple genes. The presence of a mutator phenotype facilitates the early stages of tumor growth and progression (1). This phenotype includes alterations of gene products involved in DNA replication and repair as well as in maintaining genomic stability and integrity. Genes encoding enzymes involved in DNA replication are potential targets for mutations leading to cancer. DNA polymerase β (polβ), a single-copy gene, is involved in base-excision repair (BER) for DNA maintenance. It also may be involved in replication, recombination, and drug resistance in eukaryotic cells (2–8). An excision activity of polβ during the repair process also was documented (9). Recently, evidence that polβ has a role in mammalian meiosis was reported (10). Furthermore, a double knockout mutation of the polβ gene in the germ line is lethal in mice (11). An embryonic fibroblast cell line with homozygous deletion of the polβ gene in the germ line is reported to be defective in uracil-mediated BER (5).
The DNA polβ gene, which encodes a 39-kDa protein, consists of 335 amino acids with two distinct functional domains of 8 kDa and 31 kDa, separated by a short protease-sensitive region (12). The N-terminal 8-kDa polypeptide (75 residues) has a strong affinity for single-stranded DNA and is devoid of nucleotidyltransferase activity (12). The polymerase activity of the polβ enzyme resides in the C-terminal 31-kDa domain (≈260 residues). The 31-kDa domain may bind to a double-stranded DNA (13). We provided evidence that a specific 87-bp deletion encoding amino acid residues 208–236 exists in the cDNA of the polβ gene in primary colorectal and breast adenocarcinomas (14, 15). Additionally, we found that in the polβ gene a single T deletion in prostate adenocarcinoma causes premature termination within the gene at codon 181 (15). A recent report shows point mutations in the polβ gene in bladder cancer (16).
In our previous studies, all tumors with alterations in the polβ gene were associated with heterozygous mutations (14, 15). Here we report expressions of both wild-type (WT) and trucated polβ proteins in a specific primary colorectal tumor. Corresponding normal mucosa expresses the WT polβ protein only. In all tumors previously studied, amino acids are truncated in the catalytic domain of the polβ enzyme whereas the NH2-terminal binding domain remains unaltered (14–16). Consequently, the polβ variant retains its affinity for the damaged cognate DNA template. However, it is likely that the catalytic activity of the polβ enzyme is impaired, owing to the truncation, which would result in a defect in its DNA repair function. Identifying such a defect would allow us to evaluate potential functional consequences of polβ truncation. We have undertaken a study to determine the functional consequences of such truncation of polβ by directly measuring the repair activities of the variant enzyme in the cell. We have generated stable HeLa cell lines transfected with WT and variant polβΔ208–236 cDNAs. HeLa cells were chosen for these studies because they are proficient at repair and polβ expression is low (2, 17). Biochemical properties of the polβΔ208–236 cells were studied with respect to BER activity. These studies showed that function of polβΔ208–236 cells decreases significantly when compared with the functions of the WT enzyme. Furthermore, to elucidate whether the truncated polβ has a role in inhibiting the WT enzyme, we examined an embryonic fibroblast cell line expressing WT polβ (5). We took advantage of the fact that the amino acid sequence of polβ is highly homologous (>95%) in various species (18–20). Thus, stable cell lines of embryonic fibroblasts transfected with polβΔ208–236 cDNA were established. Our findings indicate that the variant form of polβ has markedly decreased BER activity compared with the activity of the WT enzyme and it abrogates the BER function of the WT enzyme.
MATERIALS AND METHODS
Tumor Tissue Extracts.
Cell extracts were prepared from primary colorectal tumors and normal matched mucosa. Tumors selected showed an 87-bp deletion in cDNA (14, 15).
Stable HeLa Cell Lines.
Both WT and polβΔ208–236 cDNA were amplified from normal mucosa and colorectal tumor mRNAs, respectively (14, 15) and further subcloned in a sense orientation in the vector pcDNAIneo (Invitrogen) at the BamHI restriction site. This expression vector contains a strong cytomegalovirus promoter and a simian virus 40 origin for replication in eukaryotic cells. The plasmids were purified by CsCl gradient. The orientation and the sequence of the clones were confirmed by sequencing before transfecting the plasmids into the HeLa cells. Plasmids were used for stable transfection into HeLa cells were: (i) vector-pcDNAIneo (HeLaV), (ii) WT polβ, and (iii) the variant, polβΔ208–236. Cells were plated at 1.2 × 106 in 100-mm dishes and allowed to attach for 24 hr before transfection. The calcium phosphate coprecipitation method with 5 μg of the appropriate plasmids was used for transfection. Clones were selected in DMEM medium containing 300 μg/ml of G418 (GIBCO/BRL). Initially, 10 individual clones were grown and RNA was isolated to monitor the level of polβ expression by Northern blot analysis (data not shown).
A mouse embryonic fibroblast cell line (16.3) expressing WT polβ was grown following the method of Sobol et al. (5). The polβΔ208–236 plasmid was used for stable transfection into 16.3 cells as for HeLa cells. Colonies were selected after growing in DMEM with 80 μg/ml hygromycin B and 700 μg/ml G418 for 30 days and expanded. Stable cell lines were established and stored at −190°C. To obtain average activity of these cell lines, a pooled cell line (named 16.3ΔP) was prepared by mixing an equal number of cells from 28 individual cell lines.
Western Blot Analysis.
Proteins of cell extracts made from tumors, stable HeLa, 16.3, and 16.3ΔP cell lines were analyzed by Western blot using a purified polyclonal anti-polβ antibody (5).
polβ Activity.
The polβ activity was measured in the cell extracts using a gel activity assay (10) altered as follows. Protein (50 μg) from HeLa, HeLaV, WT, and polβΔ208–236 cells were separated on a 12% SDS/PAGE gel containing 144 μg/ml activated gapped salmon sperm DNA. After electrophoresis, SDS was removed from the gel. The proteins were renatured and incubated at 37°C for 4 hr in DNA polymerase reaction mixture containing [α-32P]dCTP. The gel was washed, dried, and autoradiographed at −80°C.
BER and Product Analysis.
The BER activities in nuclear extracts (50 μg protein) were determined using a sequence of 51-bp double-stranded DNA containing a G/U mismatch (5). The reaction conditions for the BER assay were as those described by Dianov et al. (3), with a few modifications: after the BER reaction, the 51-bp product was run on a 15% polyacrylamide gel, and a corresponding 51-bp band was excised from of the gel and incubated with an elution buffer (0.5 M ammonium acetate/10 mM MgCl2/1 mM EDTA/0.1% SDS, pH 6.8) overnight at 37°C. After purification the BER product was digested with 40 units of SalI or XbaI at 37°C for 3 hr. The digested products were separated by 15% urea PAGE.
PCR Amplification of mRNA.
Total RNA from 16.3 and 16.3ΔP cells were reverse-transcribed and amplified using primers that would amplify the entire coding sequence of human polβ cDNA (14, 15). The PCR products were separated on a 1% agarose gel.
Gel Mobility Shift Assays.
Gel mobility shift assays in nuclear extracts from the 16.3 and 16.3ΔP cell lines were performed (21) with modifications. A 51-bp double-stranded DNA and 1 μg of polyinosinic-polycytidylic acid in a volume of 10 μl was incubated for 10 min. The reaction buffer as used in the BER assay was used in this assay as binding buffer.
Binding of 16.3ΔP Protein to dsDNA Cellulose.
The binding capability of both 16.3 and 16.3ΔP proteins was determined by DNA cellulose chromatography. Forty milligrams of 16.3 and 16.3ΔP nuclear proteins were loaded on 1.5 × 1-cm columns of native DNA-cellulose (Pharmacia). The columns were preequilibrated with a buffer (25 mM Tris HCl, pH 7.5/1 mM DTT/1 mM EDTA/1 mM phenylmethylsulfonyl fluoride) containing 150 mM NaCl (22). These were washed with 5 ml of the same buffer and eluted with 5 ml of buffer containing 1 M NaCl. Fractions of 0.75 ml were collected on ice. Trichloroacetic acid-precipitated fractions of 200 μl were dissolved in 15 μl of water and separated by a 12.5% SDS/PAGE. Initially the gel was stained with Coomasie blue. Fractions showing stained bands were pooled as pool 1 containing flow-through and washings. Pool 2 contained the eluents. These two pools were concentrated using Centricon-10 (Amicon) and analyzed by Western blot.
Survival Assay.
The survival of 16.3 and 16.3ΔP cells was determined by colony forming assay. Two hundred 16.3 or 16.3ΔP cells were seeded in each 60-mm dish overnight. A stock solution of N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) (Aldrich, 99%+ pure) was prepared in dimethyl sulfoxide (DMSO). Serial dilutions were made with DMEM containing hygromycin and G418 immediately before use. Cells were treated with MNNG (1 nM to 500 nM) in serum-free medium for 30 min and washed with PBS to remove the MNNG completely. Fresh medium was added, and the cells were allowed to grow for 5 days with one change of medium. Cells were fixed in 70% methanol, stained with Giemsa, washed with water, air-dried, and scored for surviving colonies. Colonies containing a minimum of 100 cells were scored. The same protocol was followed for control cells in medium containing DMSO.
RESULTS
Western Blot Analysis of polβ Protein in Tumors.
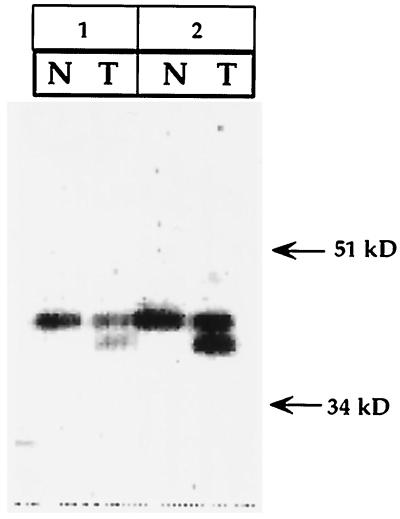
To determine a potential expression of the truncated polβ protein in tumors, tissue extracts were made from two primary colorectal tumors exhibiting the 87-bp deletion in cDNA (14, 15). Results of Western blot analysis are shown in Fig. 1. The polβ antibody distinctly recognized two proteins in tumors and one protein in normal matched mucosa. In both tumors, the slow-moving protein is 39 kDa, an expected size for polβ. In addition, a relatively faster-moving protein also expressed in these tumors is ≈36 kDa. The molecular size of the only protein expressed in normal mucosa is 39 kDa.
Figure 1.
Western blot analysis of polβ protein in tumors showing expression of truncated polβ protein in two colorectal tumors and in its matched normal mucosa. Twenty micrograms protein of tumor (T) and corresponding normal tissue (N) extracts were used in the assay.
Protein Expression in WT and HeLa polβΔ208–236 Cells.
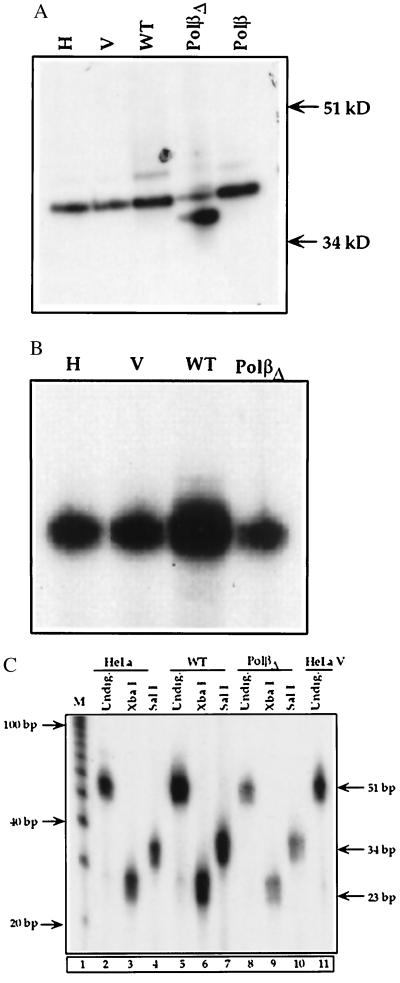
Stable HeLa cell lines overexpressing either WT or polβΔ208–236 were established along with a stable cell line transfected with the vector alone. The polβ expression levels in these cell lines initially were determined by Northern blot analysis using a polβ probe containing 1,050-bp full-length cDNA (23). We selected cell lines based on a substantial expression level of polβ mRNA (data not shown). These selected cell lines were further characterized by Western blot analysis. As shown in lanes WT and polβΔ in Fig. 2A, the polβ and the variant proteins are expressed in cell extracts of the WT and polβΔ208–236 cell lines, respectively. Also, the relative mobilities of the polβ and polβΔ208–236 proteins differ with the latter protein migrating faster; the sizes of the polβ protein were assigned as 39 kDa and ≈36 kDa, respectively. The endogenous level of polβ in the parent HeLa cells (Fig. 2A, lane H) and HeLa cells transfected with the vector alone (Fig. 2A, lane V) and polβΔ are low.
Figure 2.
(A) Western blot analysis of polβ in HeLa cells. Ten micrograms of protein from HeLa cell extract (H in lane 1), HeLaV (V in lane 2), WT (lane 3), and polβΔ208–236 (polβΔ in lane 4) cell extract was used for the analysis. In lane 5, purified human polβ was loaded as a positive control. Proteins were separated by 12.5% SDS/PAGE. Proteins were transferred to immobilon-P membranes, and the immunoblot was probed with a purified polβ polyclonal antibody. (B) Activity gel assay. Activities in HeLa (H), HeLa transfected with pcDNAIneo vector (V), WT, and polβΔ208–236 (polβΔ) cell extracts are shown. (C) BER activity in nuclear extracts of WT and polβΔ208–236 cells. The 51-bp BER products generated by the WT and polβΔ208–236 nuclear extracts are shown in lanes 5 and 8, respectively. The repair products digested with XbaI of WT and polβΔ208–236 (polβΔ) nuclear extracts are shown in lanes 6 and 9, respectively. The size of the XbaI product is 23 bp, indicated at the right. The repaired BER SalI products obtained from WT and polβΔ208–236 nuclear extracts are shown in lanes 7 and 10, respectively. The size of the SalI product is 34 bp. Lanes 1 and 11 show DNA markers and 51 bp product from HeLaV. BER activities in HeLa cells are shown in lanes 2–4 (undigested, digested with XbaI and SalI, respectively). Nuclear extracts from these cells were used in BER assay.
Activity Gel Assay for Polβ Activity.
The activity gel method provides the enzymatic activity of the polβ protein directly in gel and also shows whether the protein is in active form. As Fig. 2B shows, the WT cell extract had enzymatic function and 10-fold greater activity than the control cells, HeLa (Fig. 2B, lane H) and HeLa transfected with vector (Fig. 2B, lane V) as determined by image quantitation. In contrast, using the same amount of protein, polβΔ208–236 variant extracts exhibited poor activity compared with WT cell extracts, indicating that the polβΔ208–236 variant cells have lost substantial activity. It also should be noted that activity shown in lane polβΔ indicates the endogenous activity. No detectable activity was seen in polβΔ208–236 variant protein.
BER Activity of polβΔ208–236 Cells.
To investigate whether the BER function of polβΔ208–236 cells is modified, we measured the BER activity levels in nuclear extracts of both polβΔ208–236 and WT cells. At 10 min of the BER reaction (Fig. 2C), as expected, a 51-bp repair product was formed in both the WT and variant nuclear extracts (Fig. 2C, lanes 5 and 8, respectively). However, when quantified, BER activity in the variant was markedly reduced (6-fold) compared with the WT nuclear extract. Further characterization by digestion with XbaI clearly revealed a 23-bp BER product in the WT nuclear extract (Fig. 2C, lane 6), whereas in polβΔ208–236 nuclear extracts the BER product was 5-fold less (Fig. 2C, lane 9). Similarly, a 34-bp SalI digest product found in the variant nuclear extract (Fig. 2C, lane 10) was at a substantially lower level (5-fold) compared with the WT (Fig. 2C, lane 7). Lanes 2, 3, and 4 in Fig. 2C show considerable activity in HeLa cells undigested and digested with XbaI and SalI, respectively. However, activity in nuclear extract of variant polβΔ cells was 2-fold less than in the nuclear extract of HeLa (Fig. 2C, lanes 2 and 8).
Characterizations of the Transfected Stable Embryonic Fibroblast Cell Line, 16.3ΔP, By Reverse Transcription–PCR (RT-PCR) and Western Blot Analyses.
To elucidate whether truncated polβ can act as a dominant negative mutant, which may disrupt the WT enzyme function, we used a mouse embryonic fibroblast cell line called 16.3 that expressed the WT enzyme (5). The advantages of this cell line are that it is recently established and well defined in regard to genotype, WT polβ protein expression, growth characteristics, and most importantly, for efficient BER activity (5). Stable cell lines were established by transfection with 87-bp deleted polβΔ208–236 cDNA.
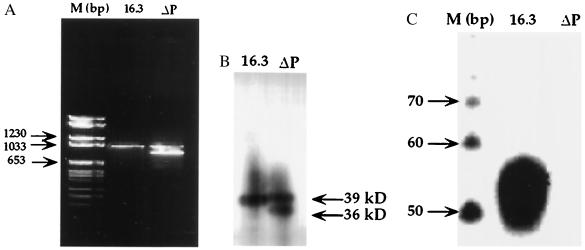
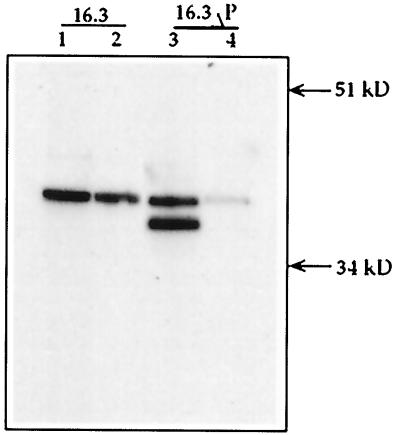
We first assayed polβ mRNA in the new stable line and parent 16.3 cells. RNA from both were amplified by RT-PCR. A PCR product of approximately 1 kb emerged from each of 16.3 and 16.3ΔP cell lines (Fig. 3A). In addition to the 1-kb cDNA, a second mRNA was observed in 16.3ΔP cells (Fig. 3A, lane ΔP). Compared with DNA molecular size markers, the size of the smaller fragment was assigned as approximately 950 bp. To further determine that these mRNAs are expressed as protein, we performed Western blot analysis. As shown in Fig. 3B, the WT (39 kDa) and variant (≈36 kDa) proteins were expressed in the transfected cell line (Fig. 3B, lane ΔP). Only a single polypeptide of 39 kDa (WT) was expressed in the 16.3 parent cell line.
Figure 3.
(A) RT-PCR of RNA from 16.3 and 16.3ΔP cells. Lane M shows the molecular weight mass in base pairs. Lane 16.3 is the RT-PCR product obtained from 16.3 cells. Lane ΔP shows two RT-PCR products obtained from 16.3ΔP cells. (B) Expression of polβ protein of 16.3 and 16.3ΔP cell extracts analyzed by Western blot. Nuclear proteins (20 μg) were separated and immunoblotted as described in Figs. 1 and 2. Lane 16.3 represents the expression of WT polβ protein in 16.3 cell extracts. Lane ΔP shows the expression of both WT and truncated proteins in 16.3ΔP cell extracts. (C) BER activity of 50 μg protein of the 16.3 nuclear extracts is shown in lane 16.3. Lane ΔP shows that BER activity is absent in the presence of 50 μg of 16.3ΔP nuclear extracts. Lane M contains the molecular size markers in base pairs.
BER Activity of 16.3ΔP cells.
To investigate BER activities in these cell lines, we observed that nuclear extract of the 16.3 parent cells exhibited substantial BER activity (Fig. 3C, gel was exposed to x-ray overnight). In contrast, this activity was undetectable in the nuclear extract of 16.3ΔP. Endogenous BER activity in 16.3ΔP could not be detected even when the gel was further exposed to x-ray for 3 days. These results imply that most probably the variant polβ protein inhibits BER activity of the WT protein.
Effect of 16.3ΔP Protein on BER Activity of 16.3 Protein.
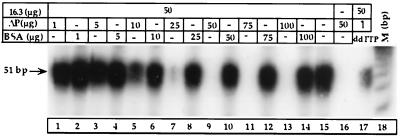
Increasing amounts of nuclear extracts of cells expressing 16.3ΔP were added to a nuclear extract of cells expressing WT polβ (16.3 cell line), and BER activity was measured. Activity in the 16.3 nuclear extract declined progressively when the 16.3ΔP nuclear extract concentration was raised from 1 to 100 μg (Fig. 4). At 25 μg of 16.3ΔP protein, BER activity (Fig. 4, lane 7) markedly declined. At 50 μg of protein, BER activity appeared to be totally lost (Fig. 4, lane 9). To rule out the possibility of a dilution effect, we used BSA as a nonspecific protein. Adding an equivalent amount of BSA, 1–100 μg, to the 16.3 incubation reactions did not alter activity (Fig. 4, lanes 2, 4, 6, 8, 10, 12, and 14).
Figure 4.
BER activity of 16.3 nuclear extracts in the presence of increasing concentration of 16.3ΔP (ΔP) protein (lanes 1, 3, 5, 7, 9, 11, and 13). Lanes 2, 4, 6, 8, 10, 12, and 14 show BER activity of 16.3 nuclear extracts in the presence of various concentrations of BSA. Lane 15 shows BER activity of 16.3 nuclear extracts alone. Lane 16 shows BER activity of 16.3ΔP. Lane 17 shows BER activity of 16.3 nuclear extracts in the presence of 1 μg of 16.3ΔP protein and 40 μM of ddTTP. Lane M is the molecular size marker.
We then examined the effect of ddTTP (3, 4, 6, 24), a specific inhibitor of polβ but not of other DNA polymerases, on the activity of these cells. Adding ddTTP (40 μM, which does not totally inhibit polβ activity) inhibited 87% of BER activity of 16.3 in the presence of 1 μg of 16.3ΔP nuclear extract (Fig. 4, lane 17). In contrast, 1 μg of 16.3ΔP alone reduced the activity by 30% in the nuclear extract of the 16.3 cells (Fig. 4, lane 1). These results indicate that polβ indeed did mediate the BER activity of these cells.
Gel Mobility Shift Assay.
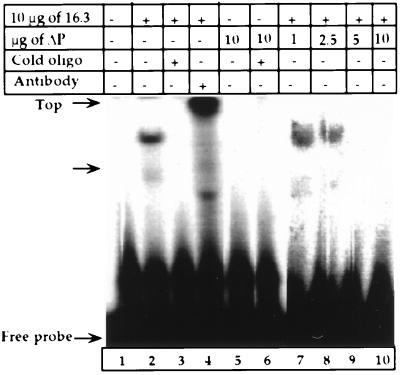
We then determined the DNA binding activity of 16.3ΔP protein using gel mobility shift assay. Shown in Fig. 5, lane 2, 16.3 nuclear protein exhibited binding activity to a 51-bp template. Adding 100-fold excess cold consensus oligonucleotides to the 16.3 reaction mixture totally blocked formation of the binding complex (Fig. 5, lane 3). Furthermore, the bound complex of 16.3 was abolished and supershifted when a purified anti-polβ antibody was added to 16.3 protein reaction mixture (Fig. 5, lane 4). These results indicate that the polβ protein binds to the 51-bp DNA template. However, the binding activity of 16.3ΔP was undetectable in the absence or presence of cold oligonucleotides (Fig. 5, lanes 5 and 6).
Figure 5.
Gel mobility shift assay of the 16.3 and 16.3ΔP nuclear extracts. A 51-bp double-stranded DNA was used as described in Materials and Methods. Lane 1 shows the free probe alone. Lane 2 represents 10 μg protein of 16.3 nuclear extracts. Lane 3 shows effects of cold oligonucleotide on 16.3 mobility. Lane 4 shows a supershift of 16.3 nuclear protein in the presence of polβ antibody. Lane 5 shows results for 16.3ΔP cells. Lane 6 shows activity is undetectable in 16.3ΔP nuclear extracts in the presence of oligonucleotides. Lanes 7–10 represent binding activity of 16.3 nuclear extracts in the presence of 1–10 μg nuclear 16.3ΔP protein.
DNA Binding Activity by DNA Cellulose Chromatography.
To establish further inefficiency of 16.3ΔP protein to bind to DNA, we took a different approach with dsDNA cellulose chromatography. Results in Fig. 6 show Western blot detection of unbound and bound forms of 16.3 and 16.3ΔP nuclear proteins. Fig. 6, lane 2 of 16.3 cells represents protein from eluents with an expected size of WT polβ (39 kDa). In addition, WT polβ protein also was observed in the flow-through and wash fractions (Fig. 6, lane 1). This may be due to overloading the column. By contrast, two distinct proteins with different mobilities were found in the flow-through and wash fractions obtained from 16.3ΔP (Fig. 6, lane 3). The larger protein is the endogenous WT polβ (39 kDa). Lane 4 in Fig. 6 represents a weak expression of endogenous protein from the eluents of 16.3ΔP proteins. The truncated protein (≈36 kDa) was undetectable in these eluents.
Figure 6.
Western blot analysis of fractions obtained from dsDNA cellulose chromatography. Procedures related to loading of the column and elution are described in Materials and Methods. Lane 1 represents a 39-kDa WT polβ protein in flow-through and wash fractions. Lane 2 shows the same protein in the eluent. Proteins in flow-through and wash fractions of 16.3ΔP extract are shown in lane 3. Lane 4 shows a protein in eluents of 16.3ΔP extract.
Effect of 16.3ΔP Protein on DNA Binding Activity of 16.3 Protein.
To assess whether the 16.3ΔP protein interferes with the 16.3 protein’s ability to bind to the 51-bp substrate, we examined the activity of 16.3 protein in the presence of increasing concentrations of 16.3ΔP nuclear protein. At 1 and 2.5 μg of 16.3ΔP nuclear protein, the binding ability of 16.3 decreased substantially, by 61% to 65% (Fig. 5, lanes 7 and 8), compared with 16.3 nuclear protein alone (Fig. 5, lane 2). Initially, the gel was given an overnight exposure to x-ray. This activity was undetectable when the 16.3ΔP nuclear protein concentration was increased to 5 or 10 μg (Fig. 5, lanes 9 and 10), even when the gel was exposed to x-ray for 10 more days. These results suggest that the truncated polβ inhibits the DNA binding activity of the WT protein.
Survival of 16.3ΔP cells exposed to MNNG.
The survival of 16.3ΔP cells treated with MNNG was expressed as relative cloning efficiency, summarized in Table 1. With dose increases of MNNG, 1.0 to 50 nM, the survival of 16.3ΔP cells was decreased from 54% to 22%. When MNNG concentration was raised above 50 nM, it totally blocked survival of these cells. A dose-dependent survival of 16.3 cells treated with equimolar MNNG also was observed (Table 1). However, the 16.3 cell survival rate was significantly better than in 16.3ΔP cells. For example, at 1.0 nM dose, 100% of the 16.3 cells survived, whereas only ≈50% of 16.3ΔP cells survived. None of the 16.3ΔP cells exposed to 100 nM to 500 nM MNNG survived. At the same dose, 16.3 cells survived and formed colonies, although percent survival was lowered with increased concentration of MNNG.
Table 1.
Survival of 16.3 and 16.3ΔP cells exposed to MNNG
| Cells | MNNG, nM | No. of colonies, mean ± SE* | Survival, RCE %† | Significance, P‡ |
|---|---|---|---|---|
| 16.3 | DMSO§ | 93 ± 11 | 100 | |
| 1.0 | 93 ± 4 | 102 ± 8 | <0.001 | |
| 50.0 | 55 ± 2 | 61 ± 8 | <0.001 | |
| 100.0 | 57 ± 3 | 62 ± 5 | <0.001 | |
| 200.0 | 34 ± 2 | 38 ± 5 | <0.001 | |
| 500.0 | 12 ± 2 | 14 ± 4 | <0.001 | |
| 16.3ΔP | DMSO§ | 98 ± 3 | 100 | |
| 1.0 | 53 ± 3 | 54 ± 4 | <0.05 | |
| 10.0 | 36 ± 3 | 37 ± 2 | <0.001 | |
| 50.0 | 21 ± 2 | 22 ± 2 | <0.001 | |
| 100.0 | 0 | 0 | — | |
| 200.0 | 0 | 0 | — | |
| 500.0 | 0 | 0 | — |
The results are the mean of three dishes ± SE; cloning efficiencies of 16.3 and 16.3ΜP cells are 61-85%.
Relative cloning efficiency (RCE) % is expressed as average number of colonies in treated dishes/number of colonies in DMSO control × 100.
Statistical significance was calculated compared with DMSO control using paired t-test.
The highest concentration of DMSO in medium was 0.005%.
DISCUSSION
Our results provide evidence that the truncated form of polβ is expressed as protein in tumors. The tumors express both WT and truncated polβ proteins, whereas the normal mucosa expresses the WT protein only. Furthermore, Southern blot analysis shows an EcoRI and TaqI fragment of the expected sizes with a smaller fragment in these tumors when the matched normal mucosal DNA exhibits the normal allele (25). These data also provide strong evidence that 87-bp deletion encoding 29 amino acids of the WT polβ overexpressed in HeLa cells, result in no activity (activity gel) and inefficient BER activity. This strongly suggests that the polβΔ208–236 cells have lost an important repair function. Furthermore, residual activity in the variant nuclear extract may be attributed to endogenous activity compared with activity shown in the WT overexpressed cell line. Importantly, the endogenous BER activity in the variant nuclear extract seemed to be expressed at a significantly lower level when compared with BER activity in HeLa cells, suggesting that the overexpression of a truncated form of polβ protein in HeLa cells inhibits the endogenous BER function of HeLa cells.
Interestingly, the variant human cDNA was expressed as a protein in a rodent cell line. The RT-PCR assay reveals a deleted form of polβ mRNA in 16.3ΔP variant cells along with the normal mRNA. Results on Western blot analysis demonstrate both WT and truncated proteins are expressed in 16.3ΔP, a stable mouse embryonic fibroblast cell line mimicking the heterozygous polβ expression in tumor cells. More importantly, data presented here suggest that overexpression of truncated polβ in the 16.3 cell line produced a nonfunctional polβ in respect to BER and DNA binding activities. Results from both HeLa variant polβΔ and 16.3ΔP cells strongly indicate that the truncated polβ efficiently inhibits or blocks the BER activity of WT polβ, as our previous studies suggested (14, 15). Because the 31-kDa catalytic domain of the polβ enzyme binds to double-stranded DNA (13), it is conceivable that the 29-aa truncated polβ (in the catalytic domain) would lose its ability to bind to a double-stranded template. Now, with data on gel mobility shift assay and confirmed by DNA cellulose chromatography, we established this assumption. The truncated polβ protein passed through the column (Fig. 6, lane 3). The same protein was not found in eluent fractions (Fig. 6, lane 4), indicating that the 16.3ΔP protein was unable to bind to dsDNA. Conversely, the 16.3 protein efficiently bound to DNA (Fig. 6, lane 2). Thus, the 16.3ΔP nuclear protein lost its binding activity to DNA. Additionally, in vivo the 16.3ΔP cells are noticeably more sensitive to MNNG, a DNA damaging agent, than 16.3 cells. The expression of WT polβ is essential for viability of animals (11). Therefore, our results on survival suggest that overexpression of variant polβ in the 16.3 cells blocks the WT polβ activity. The mechanism(s) underlying this dominant negative effect of the truncated protein is unknown. One possibility is a potential direct interaction of polβ with the XRCC1, a key repair protein, as shown by Kubota et al. (26). We can only speculate that in competition for the binding site on XRCC1 protein, the polβΔP protein may inhibit the BER process of WT polβ. Our future goal is to understand precisely the mechanisms underlying the inhibitory role of the mutant.
The importance of Arginine283 in maintaining the catalytic property of polβ was reported (27, 28). However, the polβ alteration that we observed (truncation of amino acids 208–236) occurs upstream of arginine283. Two other amino acids, lysine230, lysine234 and threonine233 (both localized in the palm subdomain of polβ), bind to backbone phosphates of the template (13). The palm subdomain is also a highly conserved catalytic region. Determining the role of arginine, at amino acid position 228 of polβ as well as that of lysine230, lysine234 and threonine233 in repair activities of polβ will be important. The function of the purified WT polβ enzyme are known (13, 27–29). In a preliminary attempt, we cloned both WT and polβΔ208–236 cDNA in a pET-3a vector and expressed them in Escherichia coli BL-21. A partially purified recombinant variant protein showed no polβ activity. However, efficient polβ activity was associated with the recombinant WT protein (30).
As cellular DNA continuously undergoes alterations, either spontaneously or induced by endogenous and exogenous factors, highly efficient DNA repair systems remove these perturbations. Should polβ, an integral part of the DNA repair machinery, become defective, it could no longer carry out its gap-filling synthesis, because the BER would be ineffective. Consequently the DNA damage would persist. This defect in polβ may lead to accumulation of mutations (perhaps in cancer-related genes like ras, p53, Rb, and others), ultimately generating a mutator phenotype in tumor cells. Results documented here strongly suggest that truncated polβ may function as a dominant negative mutant. Further studies would shed more light on the mechanism of the generation of mutator phenotype in tumor cells. The expression of WT polβ is most likely important in maintaining genetic stability, promoting terminal differentiation, and antagonizing tumor initiation and progression (31).
Acknowledgments
We are indebted to Dr. Samuel H. Wilson, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, for purified human DNA polymerase β, polβ antibody, and a mouse embryonic fibroblast 16.3 cell line, and we are thankful to Dr. Ganes C. Sen, Department of Molecular Biology, The Cleveland Clinic Foundation, for critical comments. We would like to extend our appreciation to Laura Tripepi for preparation of the manuscript and Dorthy Herzberg for editorial assistance.
ABBREVIATIONS
- polβ
DNA polymerase β
- BER
base-excision repair
- MNNG
N-methyl-N′-nitro-N-nitrosoguanidine
- WT
wild type
- RT-PCR
reverse transcription–PCR
- DMSO
dimethyl sulfoxide
References
- 1.Loeb L A. Cancer Res. 1994;54:5059–5063. [PubMed] [Google Scholar]
- 2.Wiebauer K, Jiricny J. Proc Natl Acad Sci USA. 1990;87:5842–5845. doi: 10.1073/pnas.87.15.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dianov G, Price A, Lindahl T. Mol Cell Biol. 1992;12:1605–1612. doi: 10.1128/mcb.12.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammond R A, McClung J K, Miller M R. Biochemistry. 1990;29:286–291. doi: 10.1021/bi00453a039. [DOI] [PubMed] [Google Scholar]
- 5.Sobol R W, Horton J K, Kühn R, Gu H, Singhal R K, Prasad R, Rajewsky K, Wilson S H. Nature (London) 1996;379:183–186. doi: 10.1038/379183a0. [DOI] [PubMed] [Google Scholar]
- 6.Wood R D. Annu Rev Biochem. 1996;65:135–167. doi: 10.1146/annurev.bi.65.070196.001031. [DOI] [PubMed] [Google Scholar]
- 7.Sweasy J B, Loeb L A. J Biol Chem. 1992;267:1407–1410. [PubMed] [Google Scholar]
- 8.Ali-Osman F, Berger M S, Rairkar A, Stein D E. J Cell Biochem. 1994;54:11–19. doi: 10.1002/jcb.240540103. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto Y, Kim K. Science. 1995;269:699–702. doi: 10.1126/science.7624801. [DOI] [PubMed] [Google Scholar]
- 10.Plug A W, Clairmont C A, Sapi E, Ashley T, Sweasy J B. Proc Natl Acad Sci USA. 1997;94:1327–1331. doi: 10.1073/pnas.94.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu H, Marth J D, Orban P C, Mossmann H, Rajewsky K. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 12.Wilson S H, Singhal R K, Kumar A. In: DNA Repair Mechanisms. Bohr V A, Wassermann K, Draemer K H, editors. Vol. 35. Copenhagen: Munksgaard; 1992. pp. 343–360. [Google Scholar]
- 13.Pelletier H, Sawaya M R, Kumar A, Wilson S H, Kraut J. Science. 1994;264:1891–1903. [PubMed] [Google Scholar]
- 14.Wang L, Patel U, Ghosh L, Banerjee S. Cancer Res. 1992;52:4824–4827. [PubMed] [Google Scholar]
- 15.Wang L, Banerjee S. Int J Oncol. 1995;6:459–463. [PubMed] [Google Scholar]
- 16.Matsuzaki J, Dobashi Y, Miyamoto H, Ikeda I, Fujinami K, Shuin T, Kubota Y. Mol Carcinogen. 1996;15:38–43. doi: 10.1002/(SICI)1098-2744(199601)15:1<38::AID-MC6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 17.Risinger J I, Umar A, Boyer J C, Evans A C, Berchuck A, Kunkel T A, Barrett J C. Cancer Res. 1995;55:5664–5669. [PubMed] [Google Scholar]
- 18.Chang L M S, Plevani P, Bollum F J. Proc Natl Acad Sci USA. 1982;79:758–761. doi: 10.1073/pnas.79.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanabe K, Yamaguchi T, Saneyoshi M, Yamaguchi M, Matsukage A, Takahashi T. J Biochem. 1984;96:365–370. doi: 10.1093/oxfordjournals.jbchem.a134846. [DOI] [PubMed] [Google Scholar]
- 20.SenGupta D N, Zmudzka B Z, Kumar P, Cobianchi F, Skowronski J, Wilson S H. Biochem Biophys Res Commun. 1986;136:341–347. doi: 10.1016/0006-291x(86)90916-2. [DOI] [PubMed] [Google Scholar]
- 21.Casas-Finet J R, Kumar A, Karpel R L, Wilson S H. Biochemistry. 1992;31:10272–10280. doi: 10.1021/bi00157a014. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Widen S G, Williams K R, Kedar P, Karpel R L, Wilson S H. J Biol Chem. 1990;265:2124–2131. [PubMed] [Google Scholar]
- 23.Patel U, Chen H-C, Banerjee S. Cell Mol Biol Res. 1994;40:683–691. [PubMed] [Google Scholar]
- 24.Perrino F W, Loeb L A. Mutat Res. 1990;236:289–300. doi: 10.1016/0921-8777(90)90012-t. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee S, Chen H-C, Bhattacharyya N. Cold Spring Harbor Meeting on Cancer Genetics and Tumor Suppressor Genes. Plainview, NY: Cold Spring Harbor Lab. Press; 1996. p. 257. (abstr.). [Google Scholar]
- 26.Kubota Y, Nash R A, Klungland A, Schär P, Barnes D E, Lindahl T. EMBO J. 1996;15:6662–6670. [PMC free article] [PubMed] [Google Scholar]
- 27.Beard W A, Osheroff W P, Prasad R, Sawaya M R, Jaju M, Wood T G, Kraut J, Kunkel T A, Wilson S H. J Biol Chem. 1996;271:12141–12144. doi: 10.1074/jbc.271.21.12141. [DOI] [PubMed] [Google Scholar]
- 28.Werneburg B G, Ahn J, Zhong X, Hondal R J, Kraynov V S, Tsai M-D. Biochemistry. 1996;35:7041–7050. doi: 10.1021/bi9527202. [DOI] [PubMed] [Google Scholar]
- 29.Davies J F, Almassy R J, Hostomska Z, Ferre R A, Hostomsky Z. Cell. 1994;76:1123–1133. doi: 10.1016/0092-8674(94)90388-3. [DOI] [PubMed] [Google Scholar]
- 30.Patel U, Wang L, Banerjee S. Proc Am Assoc Cancer Res. 1993;34:502. (abstr.). [Google Scholar]
- 31.Shadan F F, Villarreal L P. Med Hypotheses. 1996;47:1–9. doi: 10.1016/s0306-9877(96)90033-x. [DOI] [PubMed] [Google Scholar]