Abstract
Background & Aims
Krüppel-like factor 5 (KLF5) is a transcription factor that is highly expressed in proliferating crypt cells of the intestinal epithelium. KLF5 has a pro-proliferative effect in vitro and is induced by mitogenic and stress stimuli. To determine whether KLF5 is involved in mediating proliferative responses to intestinal stressors in vivo, we examined its function in a mouse model of transmissible murine colonic hyperplasia (TMCH), which is triggered by colonization of the mouse colon by the bacterial pathogen, Citrobacter rodentium.
Methods
Heterozygous Klf5 knockout (Klf5+/−) mice were generated from embryonic stem cells carrying an insertional disruption of the Klf5 gene. Klf5+/− mice or wild-type (WT) littermates were infected with C. rodentium by oral gavage. At various time points post-infection (p.i.), mice were sacrificed and distal colons harvested. Colonic crypt heights were determined morphometrically from sections stained with hematoxylin and eosin. Frozen tissues were stained by immunofluorescence using antibodies against Klf5 and the proliferation marker, Ki67, to determine Klf5 expression and numbers of proliferating cells per crypt.
Results
Infection of WT mice with C. rodentium resulted in a 2-fold increase in colonic crypt heights at 14 days p.i. and was accompanied by a 1.7-fold increase in Klf5 expression. Infection of Klf5+/− mice showed an attenuated induction of Klf5 expression, and hyperproliferative responses to C. rodentium were reduced in the Klf5+/− animals as compared to WT littermates.
Conclusion
Our study demonstrates that Klf5 is a key mediator of crypt cell proliferation in the colon in response to pathogenic bacterial infection.
INTRODUCTION
The mammalian gut epithelium is a dynamic tissue that plays an active role in maintaining tissue homeostasis in the face of rapid cell turnover and constant changes in the bacterial milieu. Maintaining the “status quo” requires the stringent regulation of pathways involving proliferation, differentiation, apoptosis and inflammation. This balance becomes even more critical in instances of inflammatory bowel disease (IBD), where dysregulation of these pathways can result in excessive tissue injury, inadequate tissue regeneration and increased risk of developing cancer. While the primary pathway involved in the normal regeneration of the intestinal epithelium has been relatively well characterized, little is known about the molecular events that regulate proliferation during pathogenic bacterial infection or exposure to other stressors.
Krüppel-like factor 5 (KLF5) is a member of a family of zinc finger-containing transcription factors that function in the regulation of diverse cellular processes, including development, proliferation and differentiation 1–6. KLF5 is highly expressed in the intestinal epithelium and is found predominantly in the proliferating cells of the crypt 6–8, suggesting that it has a positive growth regulatory role in the intestinal tissue. Indeed, several in vitro studies support a pro-proliferative role for KLF5 in non-transformed cultured epithelial cells 9–11. In addition, ectopic expression of KLF5 in NIH3T3 cells has been shown to promote proliferation 10. Moreover, KLF5 has been shown to mediate the transforming effects of oncogenic H-RAS 12, 13. Transcriptional targets of KLF5 include a number of genes which encode pro-proliferative components of the cell cycle machinery, including cyclin D1, cyclin B1 and Cdc2 12, 13.
Various external stimuli have been reported to activate KLF5 expression, including addition of fetal bovine serum to serum-deprived cells 10 and treatment of cultured cells with basic fibroblast growth factor (bFGF) 14 and phorbol 12-myristate 13-acetate (PMA) 14, 15. Furthermore, in vivo studies conducted in mouse vascular tissue have shown an increase in KLF5 expression in response to physical stress due to injury 16. Induction of KLF5 has been shown to be downstream of activation of the MAPK/ERK1/2 pathway, with KLF5 expression being driven by the early response gene, EGR-1 14, 17. Recently, our laboratory has reported that KLF5 expression is induced in IEC-6 rat intestinal epithelial cells following exposure to the bacterial component lipopolysaccharide or LPS 18. Thus, given the pro-proliferative activity of KLF5 in vitro and its activation in response to various mitogenic and stress stimuli, we hypothesize that KLF5 may play a role in hyperproliferative responses to external stressors in intestinal tissues.
To address this hypothesis, the current study utilizes a mouse model of hyperproliferation known as transmissible murine colonic hyperplasia (TMCH), to examine the involvement of KLF5 in proliferative changes induced by enteric bacterial pathogenic infection. TMCH is caused by infection with Citrobacter rodentium, a gram-negative, non-invasive bacterial pathogen that colonizes the distal colon of mice by forming attaching and effacing lesions 19. Infection is characterized by dramatic elongation of colonic crypts, hyperproliferation of epithelial cells, goblet cell depletion, and mucosal thickening 20. Over a two-week period, the crypts in the distal colon double in height, reaching a maximum between 14 and 21 days post-infection. Inflammatory responses with C. rodentium infection are minimal, making this model an excellent tool for examining hyperproliferative changes in the colon.
To determine the role of KLF5 in proliferative responses of the mouse colon to C. rodentium infection, we have generated mice with heterozygous knockout of the Klf5 gene (Klf5+/−). Homozygous deletions of Klf5 are embryonic lethal; however heterozygous Klf5 mouse models have been used in other studies to demonstrate key roles for Klf5 in cardiovascular remodeling and adipocyte differentiation 16, 21. In this study, we compare hyperproliferative responses to C. rodentium infection in wild-type (WT) and Klf5+/− mice. Results show that Klf5 in the colonic crypts is induced in response to C. rodentium infection and that hyperproliferative responses are suppressed in the colons of Klf5+/− animals. These findings suggest that induction of KLF5 is a key event that contributes to epithelial cell hyperproliferation following infection with C. rodentium and demonstrate for the first time that KLF5 is an important mediator for colonic hyperproliferation in response to in vivo stressors.
MATERIALS AND METHODS
Animals
C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice strains were bred and housed in the Whitehead Animal Research Facility at Emory University. Animal care and in vivo research complied with all relevant Emory University institutional policies and federal guidelines.
Generation of Klf5+/− Knockout Mice
Mouse embryonic stem (ES) cells containing a disrupted allele for Klf5 (Klf5+/−), clone XB751, were obtained from BayGenomics, Inc. (San Francisco, CA). The disrupted allele was generated by insertion of a gene trap vector into the first intron of the Klf5 gene. The insertional mutation created a fusion transcript containing exon 1 of Klf5 joined to a β-galactosidase/neomycin cassette and followed by a translational stop codon. Chimeras for expression of the disrupted Klf5 allele were generated by the Emory Transgenic Facility by injecting blastocysts with Klf5+/− ES cells and implanting the embryos into pseudo-pregnant females. Chimeric progeny were bred to C57BL/6 animals to produce Klf5+/− founder mice. Male Klf5+/− mice were backcrossed with C57BL/6 females (WT) for four generations to produce Klf5+/− mice on a homogeneous C57BL/6 background. Klf5+/− mice used for the described experiments were F5 generation mice.
Genotyping
A 0.5 cm section of tail was removed and used to prepare genomic DNA with the Extract-N-Amp Tissue PCR kit (Sigma Aldrich) according to manufacturer’s instructions. To identify Klf5+/− mice, PCR analysis was conducted to amplify a portion of the β-galactosidase gene that was part of the insertional mutation. Primers used were: forward: 5′-TTATCGATGAGCGTGGTGGTTATGC-3′ and reverse: 5′-GCGCGTACATCGGGCAAATAATATC-3′.
Transmissible Murine Colonic Hyperplasia (TMCH)
The Citrobacter rodentium strain, Citrobacter rodentium deposited as Citrobacter freundii (Braak) Werkman and Gillen, was obtained from the American Type Culture Collection. Six week-old C57BL/6 WT or Klf5+/− littermates were infected with 100 μl of phosphate-buffered saline (PBS) containing 5 × 108 colony-forming-units (c.f.u.) of C. rodentium by oral gavage. Uninfected controls were given PBS alone. Animals were provided unlimited access to food and water throughout the experiment. Mice were weighed prior to infection to determine baseline weights, and mice were weighed and observed daily. At various time points post-infection (p.i.), mice were euthanized by carbon dioxide asphyxiation and the distal colon removed. Transmissible murine colonic hyperplasia was apparent by the presence of a significantly thickened distal colon and loose stool.
Histology
Sections of distal colon were isolated from the region 3 cm proximal to the anal verge, fixed in 10% formalin solution for 48 h and processed for embedding in paraffin. Transverse sections (5 μm) were stained with hematoxylin and eosin (H&E) for morphological evaluation. Photomicrographs were taken using a Zeiss Axioskop2 plus microscope, and crypt height measurements were determined with AxioVision 4.5 software (Car Zeiss, Inc.). Crypt measurements were conducted in a blinded fashion, taking three separate measurements per tissue sample on well-oriented crypts.
Immunohistochemistry
For Klf5 and Ki67 staining, frozen tissues from distal colon were sectioned, air dried for 1 h and fixed in 4% ultrapure formaldehyde (Biosciences) for 5 m. Slides were washed 3 times in PBS, permeabilized in 1% IgepaI in PBS and incubated in block solution (0.02% Triton X-100, 3% bovine serum albumin or BSA in PBS) for 1 h. Slides were then incubated with primary antibodies against KLF5 (Santa Cruz Biotechnology, H-30, 1:500) or Ki67 (Visionbiosystems, NCL-Ki67p, 1:500) overnight at 4°C in a humidified chamber. For immunofluorescence detection, slides were incubated with either Alexa fluor 488 or Alexa fluor 568 goat anti-rabbit secondary antibodies (Invitrogen), and the nuclei counterstained with Hoechst 33258. Fluorescent staining was viewed with a Zeiss Axioskop2 plus microscope and images from the various samples were captured using identical exposure settings for quantitative purposes. Images were analyzed for intensities of nuclear KLF5 staining using Metamorph Imaging software (Universal Imaging Corp.).
For cyclin D1 staining, paraffin-embedded sections were deparaffinized and antigen retrieval was carried out by incubating slides at 125° C for 10 m in citrate buffer, pH 6.0. Tissue sections were blocked using Avidin/Biotin (Vector Labs, Burlingame, CA) in 2% milk/PBS. Sections were then stained with cyclin D1 polyclonal antibody at a 1:100 dilution (Biocare) and developed using Vectastain Elite ABC kit (Vector Labs) with DAB (Biocare). Sections were then counterstained using Gill’s Hematoxylin (Vector Labs).
Western blot analysis
Whole tissue lysates were prepared by homogenization of colon tissues in Laemmli buffer. Tissue extracts (50 μg protein/lane) were subjected to denaturing polyacrylamide gel electrophoresis and transferred to PVDF membrane. Membranes were blocked with 5% non-fat dried milk in TTBS (20 mM Tris-HCl, 3 mM KCl, 137 mM NaCl, 0.1% Tween 20, pH 7.5) and incubated with the appropriate primary antibodies: KLF5 rabbit polyclonal antibody developed by our laboratory, or β-actin (AC15, Sigma Aldrich) as a loading control.
Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analysis
Total RNA was isolated at 14 days p.i. from mucosal tissue scraped from the distal colon of WT and Klf5+/− mice either uninfected or infected with C. rodentium. Expression of cyclin D1 and cyclin B1 was examined by quantitative RT-PCR using the SYBR Green ER Two-Step qRT-PCR Kit for iCycler (Invitrogen) per manufacturer’s instructions. PCR amplification was conducted with an initial denaturation step at 95°C for 5 min followed by 45 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 45 s and extension at 72°C for 1 min. A final extension was performed at 72°C for 5 min. A melting curve analysis was performed after amplification was completed. Primers used for murine cyclin D1 were as follows: forward--5′-CAGACGTTCAGAACCAGATTC-3′ and reverse—5′-CCCTCCAATAGCAGCGAAAAC-3′. Primers for murine cyclin B1 were: forward—5′CAGTTGTGTGCCCAAGAAGA-3′ and reverse—5′-CTACGGAGGAAGTGCAGAGG-3′. Expression of cyclin D1 and cyclin B1 was normalized to β-actin expression, and relative cyclin D1 and cyclin B1 expression was reported in relation to the WT control.
Statistical Analysis
All data represent an n of 6 mice per condition, except where specifically noted. Data from the experiments are presented as the mean +/− SEM for six mice from each experimental group. Data values for each mouse were determined from the average of multiple measurements as described in the Figure Legends. P values comparing the data for mice in two groups were calculated using a 2-tailed t test. Differences were considered to be significant where the P value was < 0.05.
RESULTS
Since KLF5 has been shown to promote proliferation in vitro, we first examined whether there was a correlation between expression patterns of Klf5 and the proliferation marker, Ki67, in vivo. Immunofluorescence staining of colon tissues from WT adult mice revealed localization of Klf5 to nuclei of epithelial cells extending from the base of the crypt through approximately two-thirds of the crypt height, where active proliferation occurs (Figure 1). Staining of sequential tissue sections with Ki67 showed a strikingly similar pattern to that of Klf5, suggesting that KLF5 participates in proliferative signaling pathways in the colon.
Figure 1. Klf5 localization corresponds to that of the proliferation marker, Ki67.
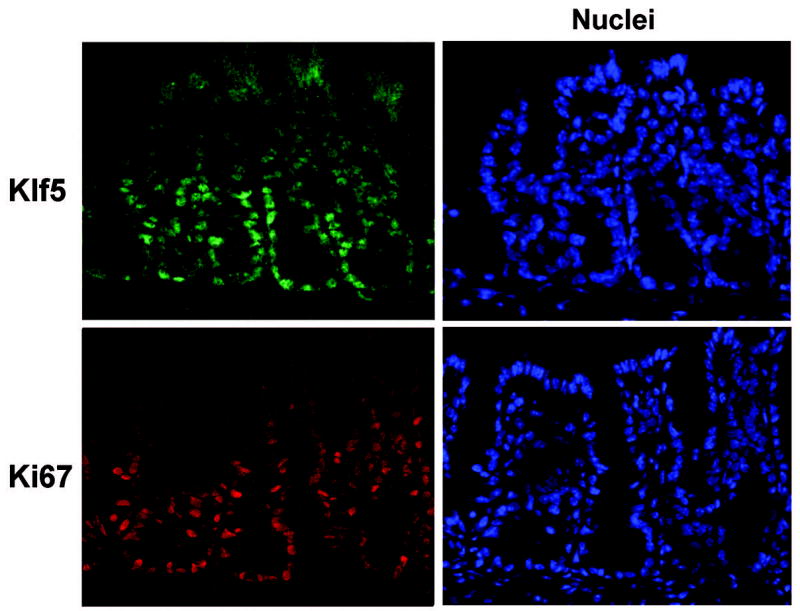
Frozen tissue sections from colon were prepared from C57BL/6 mice and sequential sections were stained by immunofluorescence with antibodies derived against Klf5 (green) or Ki67 (red). Sections were counterstained with Hoechst 33528 (blue) to identify nuclei and visualize tissue morphology.
To examine alterations in Klf5 expression during hyperproliferative responses in colonic tissues, C57BL/6 mice were infected with Citrobacter rodentium to induce TMCH. At days 8 and14 post-infection, crypt heights were increased up to two-fold in response to infection (Figure 2A). With induction of hyperproliferation, total numbers of epithelial cells per crypt increased by 1.8-fold at 14 days p.i. (Figure 2B), and numbers of cells expressing Klf5 visibly increased with crypt heights (Figure 2A). Furthermore, fluorescence staining of Klf5 showed higher intensities of Klf5 staining on a per cell basis in the infected colon (Figures 2A & 2C). Over the time course of infection, Klf5 expression remained confined to the lower two-thirds of the crypt in a pattern similar to that of Ki67 at each time point examined (Figure 2A).
Figure 2. Klf5 is induced in response to infection with C. rodentium.
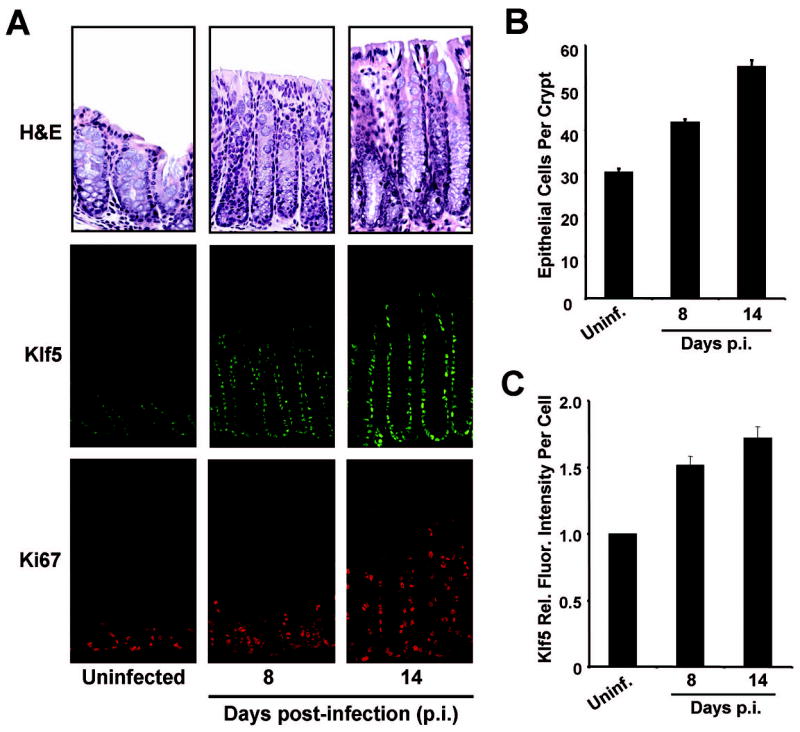
Frozen tissue sections from distal colon were isolated from mice given PBS (uninfected) or infected with C. rodentium and sacrificed 8 or 14 days post-infection (p.i.). (A) Tissues were stained with H& E, or for immunofluorescence using antibodies derived against Klf5 (green) or Ki67 (red). (B) The numbers of epithelial cells per crypt in colons of uninfected or infected mice were measured on the H&E sections. Five crypts per sample were counted. (C) Relative immunofluorescence intensities for Klf5 were determined on a per cell basis by quantitative analysis of Klf5 immunofluorescence images. For each condition, at least 200 cells positive for Klf5 expression were quantified for average fluorescence intensities.
In order to study the direct involvement of Klf5 in hyperproliferative responses triggered by C. rodentium infection, Klf5-heterozygous knockout (Klf5+/−) mice were generated. Mouse embryonic stem (ES) cells with a disruption in one allele of the Klf5 locus were used to produce Klf5+/− mice on a C57BL/6 background. The Klf5+/− ES cell line was acquired from BayGenomics, Inc., and the disrupted allele was generated by random insertion of a gene trap vector into the first intron of the Klf5 gene (Figure 3A). Klf5+/− mice were healthy, fertile, and appeared phenotypically normal. In concordance with previous studies utilizing Klf5 knockout mice, no Klf5−/− mice were acquired, confirming embryonic lethality of the Klf5-homozygous knockout. Initial characterization of colon tissues from Klf5+/− mice revealed that Klf5 protein levels were indeed reduced in the heterozygous animals compared to WT animals as determined by Western blot analysis and immunofluorescence staining of frozen tissue sections (Figure 3B & 3C). Examination of H&E stained colon tissues revealed no overt morphological differences in the Klf5+/− mice compared to WT mice except for a modest reduction in crypt heights as described below. Expression of Klf5 in the Klf5+/− mice were also examined in other regions of the gastrointestinal tract, including the squamous epithelium of the esophagus, the secretory tissues of the stomach, and the mucosa of the small intestine (Supplemental Figure 1). Similar to the colonic tissues, Klf5 levels were significantly reduced in each of these tissues in the Klf5+/− mouse, as determined by immunofluorescence staining.
Figure 3. Klf5 expression is reduced in the colonic epithelium of Klf5+/− mice.
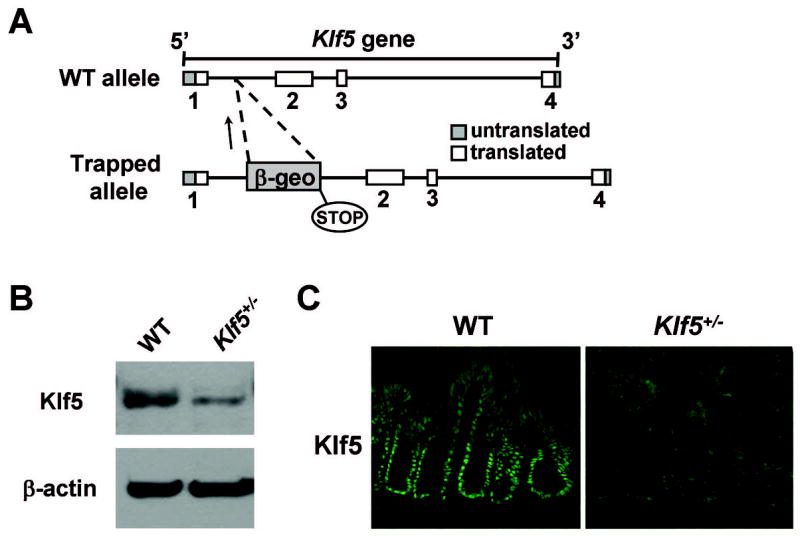
(A) Diagrammatic representation of disruption of the Klf5 gene. The vector insert contains a fusion construct of β-galactosidase and neomycin phosphotransferase II (β-geo). (B) Whole tissue lysates were prepared from colons of adult WT and Klf5+/− mice and subjected to western blot analysis for Klf5, with β-actin shown as a control. (C) Immunofluorescence staining of frozen colon sections from WT and Klf5+/− mice with antibodies derived against Klf5.
To assess the involvement of Klf5 in hyperproliferative effects triggered by colonization of the colon with C. rodentium, crypt heights were examined in both C57BL/6 WT and Klf5+/− littermates at 14 days post-infection (p.i.). In infected WT mice, crypt heights were increased 2-fold compared to uninfected controls (100% ± 5% increase), while this effect was significantly suppressed in the infected Klf5+/− animals with an increase of only 77% ± 5% over control (P < 0.01, n = 6) (Figure 4A). A difference in crypt heights was also seen in the uninfected Klf5+/− mice compared to their WT counterparts (an 11% reduction in crypt heights of Klf5+/− mice compared to WT); however, the impact of reduced Klf5 expression on crypt heights was more pronounced in the infected mice, with crypt heights being 21% lower in the Klf5+/− infected mice compared to WT infected animals (Figure 4B). Similar responses were seen when comparing epithelial cell counts for the various conditions with the exception that the difference between uninfected WT and Kl5+/− animals was not statistically significant (Figure 4C). With infection of WT mice, numbers of epithelial cells per crypt increased by 85% ± 3%, whereas epithelial cells in Klf5+/− mice increased by only 63% ± 2% in response to infection, with these differences being statistically significant (P < 0.01, n = 6).
Figure 4. Increases in crypt heights are reduced in Klf5+/− mice following infection with C. rodentium.
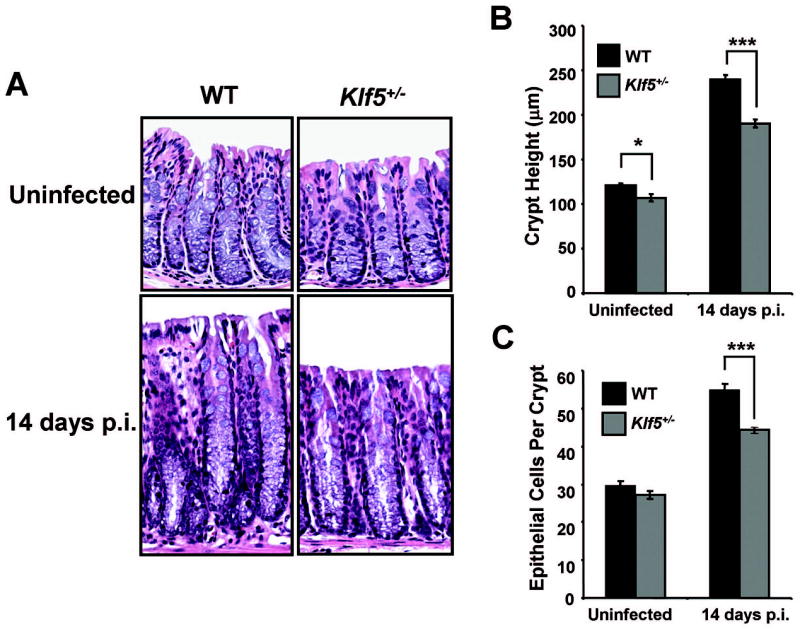
(A) H&E staining of paraffin-embedded distal colon tissues from WT C57BL/6 and Klf5+/− mice either uninfected (PBS controls) or infected with C. rodentium and sacrificed at 14 days p.i. (B) Crypt heights were determined from H & E stained sections measuring well-oriented crypts. Results represent the mean value of six mice, with the value of each mouse being determined from three separate crypt measurements. *P < 0.05, ***P < 0.001; n = 6. (C) Epithelial cells per crypt were counted from colonic crypts of H&E stained sections. Results represent the mean value of six mice, with the value of each mouse being determined by averaging cell counts from five crypts. ***P < 0.001; n = 6. See Supplemental Figures 3A and 3B, respectively, for scatter plots of the individual data values for Figures 4B and 4C.
To confirm that changes in Klf5 levels correlated with hyperproliferation, Klf5 protein expression was examined by immunofluorescence staining of colonic tissues from uninfected and infected C57BL/6 and Klf5+/− mice (Figure 5A). As was quantified in Figure 4B, total numbers of epithelial cells were increased in the crypts of infected WT mice, with epithelial crowding at the base of the crypts. Upon examining intensities of Klf5 staining in colonic epithelial cells of infected mice, both WT and Klf5+/− mice responded to infection by increasing Klf5 levels. In both genotypes, Klf5 levels were induced by 70% over levels in respective uninfected controls (Figure 5B), although this increase was to a lesser absolute level in the Klf5+/− mice due to the absence of one allele. Thus, the single wild-type allele in the Klf5+/− mice responded in a manner comparable to that of the two wild-type alleles, indicating that there were no compensatory changes in Klf5 expression in the infected heterozygous animals due to Klf5 haploinsufficiency. Comparing Klf5 intensities in WT versus Klf5+/− mice following C. rodentium infection, Klf5 staining on a per cell basis was reduced by 45% in the heterozygous animals compared to WT mice. Thus, reduced levels of Klf5 correlated with reduced crypt heights and reduced expansion of epithelial cells in the infected mice. In contrast, the loss of one Klf5 allele in the uninfected animals had a minimal effect on crypt heights and numbers of epithelial cells, suggesting that Klf5 is more critical for responding to external stimuli than for maintaining homeostasis in the crypts.
Figure 5. Induction of Klf5 is reduced in Klf5+/− mice compared to WT mice following C. rodentium infection.
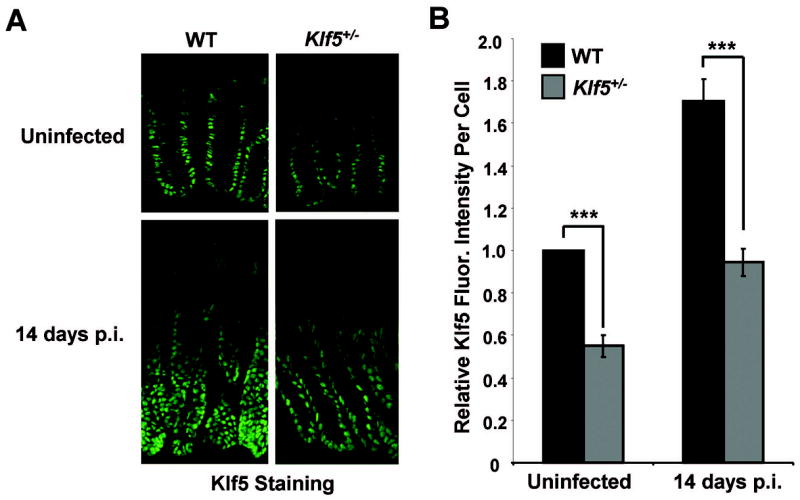
(A) Frozen colonic tissues from uninfected and C. rodentium-infected mice were isolated at 14 days p.i. and stained by immunofluorescence for Klf5 expression. (B) Relative fluorescence intensities per cell were determined by quantitative analysis of images of Klf5 staining. Results represent the mean value of six mice, with the value of each mouse being determined by averaging intensities from at least 200 cells. Results are shown as Klf5 fluorescence intensity per cell relative to intensities in WT mice. ***P < 0.001; n = 6. See Supplemental Figure 3C for a scatter plot of the individual data values.
In examining epithelial cell proliferation in WT mice infected with C. rodentium, immunofluorescence staining of colon tissues from infected mice showed an 81% ± 3% increase in numbers of Ki67-positive epithelial cells per crypt in infected C57BL/6 mice compared to uninfected controls (Figure 6). This hyperproliferative response was significantly reduced in the infected Klf5+/− animals, with an increase of only 54% ± 5% (P < 0.01, n= 6), implicating Klf5 as a mediator of pro-proliferative responses to C. rodentium infection.
Figure 6. Proliferation is reduced in Klf5+/− mice compared to WT mice following infection with C. rodentium.
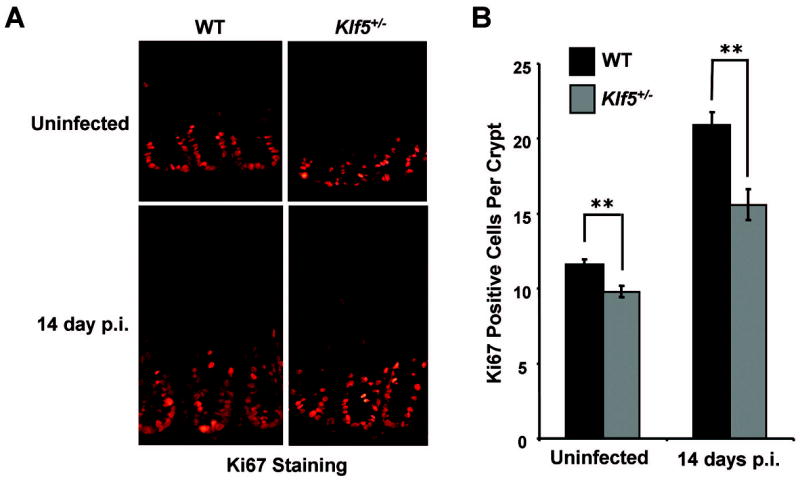
(A) Frozen colonic tissues from uninfected and C. rodentium-infected mice were isolated at 14 days p.i. and stained by immunofluorescence for Ki67 expression. (B) Numbers of Ki67-positive cells were determined on a per crypt basis. Results represent the mean values of six mice, with the value of each mouse being calculated by averaging counts from at least six crypts per mouse. **P < 0.01; n = 6. See Supplemental Figure 3D for a scatter plot of the individual data values for this figure.
Given that Klf5 has been reported to suppress apoptotic responses in certain contexts, it is possible that a reduction in Klf5 levels may also contribute to the suppression of hyperproliferative responses by allowing for increased apoptosis. To test this possibility, colonic tissues from uninfected and infected WT and Klf5+/− mice were stained for caspase-3 expression as a marker for apoptosis (Supplemental Figure 2). Occasional epithelial cells along the luminal surface stained positive for caspase-3 in both uninfected and infected mice, but no substantive differences were seen in numbers of apoptotic cells between WT and Klf5+/− mice under either condition.
Known transcriptional targets of Klf5 that could potentially mediate its contribution to hyperproliferative responses include components of the cell cycle, cyclin D1 and cyclin B1. Thus, we examined the effects of Klf5 heterozygocity on expression of these genes in the absence and presence of C. rodentium infection. Quantitative RT-PCR analysis of cyclin D1 expression showed that cyclin D1 mRNA expression was induced by 1.53-fold over controls in WT mice infected with C. rodentium (Figure 7A). This induction was reduced to 1.25-fold over controls in Klf5+/− infected mice, implicating Klf5 as a mediator of cyclin D1 induction in response to infection. Furthermore, cyclin D1 mRNAs were reduced in uninfected Klf5+/− mice versus WT mice, suggesting the importance of Klf5 in transcriptional regulation of this gene. Similar results were seen with the transcriptional target, cyclin B1, in which this transcript was induced by 2.0-fold with infection in WT animals (Figure 7B). The increase was only 1.75-fold in the Klf5+/− mice following infection, again suggesting the contribution of Klf5 to the regulation of this gene during infection with C. rodentium. Thus, cyclin D1 and cyclin B1 may contribute to hyperproliferative responses to C. rodentium through activation by Klf5. To confirm the changes in cyclin D1 expression seen by RT-PCR, cyclin D1 levels were examined by immunohistochemistry in colonic sections from uninfected and infected WT and Klf5+/− mice (Figure 7C). Protein levels of cyclin D1 correlated with mRNA expression measured by quantitative RT-PCR analysis, confirming the induction of cyclin D1 upon infection with C. rodentium and its reduced expression in the infected Klf5+/− animals.
Figure 7. Increases in cyclin D1 and cyclin B1 levels in response to infection are suppressed in Klf5+/− mice compared to WT mice.
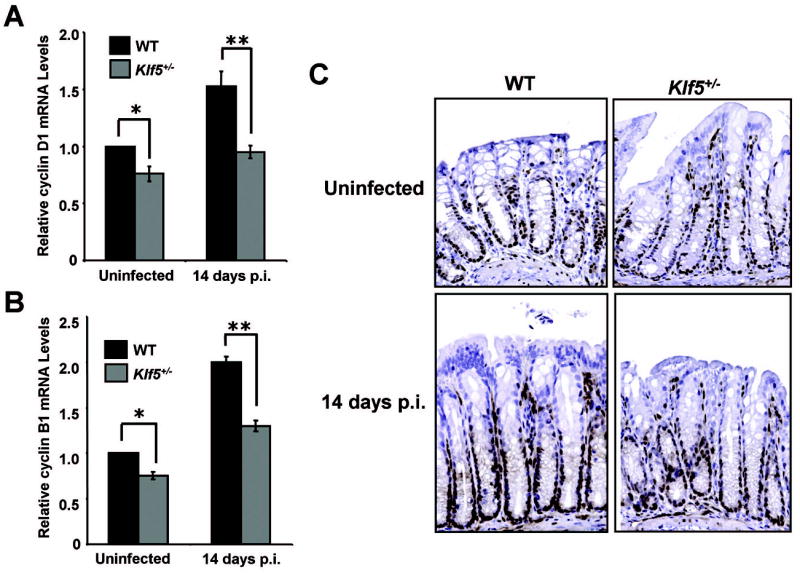
Relative mRNA levels of cyclin D1 (A) or cyclin B1 (B) were examined by quantitative polymerase chain reaction. Analysis was conducted using RNA isolated from the distal colon of WT or Klf5+/− mice that were either uninfected or infected with C. rodentium for 14 days. Expression of cyclin D1 or cyclin B1 was normalized to β-actin, with relative changes in expression being compared to levels in WT mice. *P < 0.005, **P < 0.001, n = 3. (C) Paraffin-embedded sections of distal colon from uninfected or infected WT and Klf5+/− mice isolated at 14 days p.i. were stained for cyclin D1 by immunohistochemistry (brown). Sections were counterstained with hematoxylin (blue). In addition to positive staining in crypt epithelial cells, other cells in the lamina propria also exhibited staining for cyclin D1.
DISCUSSION
The gut epithelium is a rapidly proliferating, self-renewing tissue. In the colon, stem cells reside at the base of the crypt and cycle infrequently to produce actively proliferating transit-amplifying cells that populate the crypt 22. Maintenance of the crypt progenitor compartments is dependent on the Wnt/β-catenin signaling pathway, as disruption of this pathway using various mouse models has been shown to result in loss of proliferative crypts 23–28. While the involvement of the canonical Wnt/β-catenin pathway in maintaining the crypt epithelium is well-characterized, little is known of how proliferation of epithelial cells within the crypts is regulated following exposure to external stressors, such as bacterial pathogenic infection, chronic inflammation or injury. Understanding these responses could aid in developing targeted therapies to manipulate rates of proliferation in diseases where homeostasis of the intestinal epithelium is disrupted. Our studies indicate that Klf5 is induced in mouse colonic tissues in response to bacterial infection with C. rodentium, and that reduction of Klf5 levels results in ameliorated hyperproliferative responses. While Klf5 appears to contribute to some extent in maintaining steady-state levels of colonic epithelial cells in uninfected animals (as evidenced by a slight reduction in crypt heights, numbers of epithelial cells, and numbers of proliferating cells in Klf5+/− mice compared to WT mice), the absence of one allele of Klf5 has a statistically significant effect on measures of hyperproliferation in animals infected with C. rodentium. These results suggest that Klf5 contributes to hyperproliferative responses caused by C. rodentium infection.
In the Citrobacter rodentium infection model, several components of mitogenic signaling pathways are reported to be activated and are likely involved in driving the hyperproliferative response. The epithelial cell mitogen, keratinocyte growth factor (KGF), has been shown to increase with Citrobacter rodentium infection 29. In addition, protein kinase C 30, 31 and the MEK/ERK mitogen-activated protein kinase (MAPK) pathway are activated by colonization with C. rodentium 32. Activation of the MEK/ERK pathway in this model has been shown to induce expression of the early growth response factor 1 (EGR-1) 32. EGR-1 is a transcription factor that participates in proliferative responses to changes in local cell environment, such as exposure to UV, LPS or tissue damage 33, 34. Klf5 may therefore be associated with this signaling pathway during C. rodentium infection, as KLF5 has been shown to be a target of MEK1/ERK/EGR-1 signaling in other systems 12, 14, 17.
In addition to its activation by mitogenic signals, KLF5 has also been shown to be induced by the bacterial component, LPS, in vitro and to enhance inflammatory responses through its effect on NF-κB activity 18. In a similar fashion, other KLF proteins are reported to be induced by exotoxins and other secreted bacterial proteins from a wide variety of bacterial pathogens 35–41. KLF2 and KLF6 are induced by exotoxins from gram negative and gram positive bacteria via activation of Rho protein signaling cascades 41, 42. Furthermore, induction of KLF2 has been shown to suppress inflammatory responses through its modulation of NF-κB and JUN activities 43, 44. Thus, KLF proteins may play a role in coordinating responses to bacterial infection, regulating inflammatory responses as well as proliferative changes. We have not yet explored the effects of KLF5 on inflammation during C. rodentium infection.
Another transcription factor reported to be increased by colonization with C. rodentium is β-catenin 45. As early as 6 days p.i., levels of β-catenin in the colonic epithelium were increased, and β-catenin was redistributed from an insoluble cytoskeletal fraction to a cytoplasmic/nuclear fraction. This relocalization corresponded to early hyperproliferative changes 45. While it was not shown that activation of β-catenin is required for the hyperproliferative response, it is reasonable to assume that increased levels of β-catenin contribute to this response. In reconciling our results to the activation of β-catenin during C. rodentium infection, it is possible that Klf5 and β-catenin may work in concert to drive hyperproliferative responses. β-catenin has been shown previously to interact with KLF4 46, a protein closely related to KLF5 and also expressed in the intestinal epithelium. In those experiments, binding of KLF4 to β-catenin modulates β-catenin transcriptional activity 46. Recent evidence from our laboratory also indicates that KLF5 and β-catenin interact with each other in vitro and that co-expression of KLF5 and β-catenin enhances β-catenin activity (Bialkowska and Yang, unpublished results). Cyclin D1 is a known transcriptional target of both Klf5 and β-catenin, and it is possible that these factors may work together to activate common targets that promote proliferation. Thus, induction of Klf5 during C. rodentium infection may serve to enhance β-catenin activity in order to drive expression of genes involved in hyperproliferative responses. Further experiments to verify this possibility are underway.
In conclusion, these studies provide direct evidence that Klf5 is a key factor in contributing to hyperproliferation of colonic epithelial cells in response to C. rodentium infection. We show that Klf5 is induced by colonization of the mouse colon with C. rodentium and that hyperproliferative responses are suppressed when Klf5 levels are reduced. Thus, induction of Klf5 appears to be an important event for mediating proliferative changes in colonic tissues in response to bacterial pathogenic infection. Given that Klf5 is activated by a number of stress stimuli in vitro, this study prompts the question of whether Klf5 is involved in responses to a wide variety of intestinal stressors, such as various bacterial pathogens, chemical stressors or injury. Future studies will explore these possibilities as well as the importance of Klf5 in restoring tissue homeostasis.
Acknowledgments
This work was in part supported by NIH grants DK52230, DK62899, DK64399, DK76742 and CA84197.
Abbreviations
- c.f.u
colony-forming-units
- ES
embryonic stem
- H & E
hematoxylin and eosin
- KLF5
Krüppel-like factor 5
- PBS
phosphate-buffered saline
- p.i
post-infection
- TMCH
transmissible murine colonic hyperplasia
- WT
wild-type
Footnotes
No conflicts of interest exist for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dang DT, Pevsner J, Yang VW. The biology of the mammalian Krüppel-like family of transcription factors. Int J Biochem Cell Biol. 2000;32:1103–1121. doi: 10.1016/s1357-2725(00)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bieker JJ. Krüppel-like factors: three fingers in many pies. J Biol Chem. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- 3.Kaczynski J, Cook T, Urrutia R. Sp1- and Krüppel-like transcription factors. Genome Biol. 2003;4:206. doi: 10.1186/gb-2003-4-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black AR, Black JD, Azizkhan-Clifford J. Sp1 and Krüppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143–160. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- 5.Ghaleb AM, Nandan MO, Chanchevalap S, et al. Krüppel-like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell Res. 2005;15:92–96. doi: 10.1038/sj.cr.7290271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McConnell BB, Ghaleb AM, Nandan MO, et al. The diverse functions of Krüppel-like factors 4 and 5 in epithelial biology and pathobiology. Bioessays. 2007;29:549–557. doi: 10.1002/bies.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conkright MD, Wani MA, Anderson KP, et al. A gene encoding an intestinal-enriched member of the Krüppel-like factor family expressed in intestinal epithelial cells. Nucleic Acids Res. 1999;27:1263–1270. doi: 10.1093/nar/27.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohnishi S, Laub F, Matsumoto N, et al. Developmental expression of the mouse gene coding for the Krüppel-like transcription factor KLF5. Dev Dyn. 2000;217:421–429. doi: 10.1002/(SICI)1097-0177(200004)217:4<421::AID-DVDY9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Bateman NW, Tan D, Pestell RG, et al. Intestinal tumor progression is associated with altered function of KLF5. J Biol Chem. 2004;279:12093–12101. doi: 10.1074/jbc.M311532200. [DOI] [PubMed] [Google Scholar]
- 10.Sun R, Chen X, Yang VW. Intestinal-enriched Krüppel-like factor (Krüppel-like factor 5) is a positive regulator of cellular proliferation. J Biol Chem. 2001;276:6897–6900. doi: 10.1074/jbc.C000870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chanchevalap S, Nandan MO, Merlin D, et al. All-trans retinoic acid inhibits proliferation of intestinal epithelial cells by inhibiting expression of the gene encoding Krüppel-like factor 5. FEBS Lett. 2004;578:99–105. doi: 10.1016/j.febslet.2004.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nandan MO, Yoon HS, Zhao W, et al. Krüppel-like factor 5 mediates the transforming activity of oncogenic H-Ras. Oncogene. 2004;23:3404–3413. doi: 10.1038/sj.onc.1207397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nandan MO, Chanchevalap S, Dalton WB, et al. Krüppel-like factor 5 promotes mitosis by activating the cyclin B1/Cdc2 complex during oncogenic Ras-mediated transformation. FEBS Lett. 2005;579:4757–4762. doi: 10.1016/j.febslet.2005.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawai-Kowase K, Kurabayashi M, Hoshino Y, et al. Transcriptional activation of the zinc finger transcription factor BTEB2 gene by Egr-1 through mitogen-activated protein kinase pathways in vascular smooth muscle cells. Circ Res. 1999;85:787–795. doi: 10.1161/01.res.85.9.787. [DOI] [PubMed] [Google Scholar]
- 15.Nagai R, Suzuki T, Aizawa K, et al. Phenotypic modulation of vascular smooth muscle cells: dissection of transcriptional regulatory mechanisms. Ann N Y Acad Sci. 2001;947:56–66. doi: 10.1111/j.1749-6632.2001.tb03930.x. discussion 66-67. [DOI] [PubMed] [Google Scholar]
- 16.Shindo T, Manabe I, Fukushima Y, et al. Krüppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat Med. 2002;8:856–863. doi: 10.1038/nm738. [DOI] [PubMed] [Google Scholar]
- 17.Nagai R, Shindo T, Manabe I, et al. KLF5/BTEB2, a Kruppel-like zinc-finger type transcription factor, mediates both smooth muscle cell activation and cardiac hypertrophy. Adv Exp Med Biol. 2003;538:57–65. doi: 10.1007/978-1-4419-9029-7_5. discussion 66. [DOI] [PubMed] [Google Scholar]
- 18.Chanchevalap S, Nandan MO, McConnell BB, et al. Krüppel-like factor 5 is an important mediator for lipopolysaccharide-induced proinflammatory response in intestinal epithelial cells. Nucleic Acids Res. 2006;34:1216–1223. doi: 10.1093/nar/gkl014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luperchio SA, Newman JV, Dangler CA, et al. Citrobacter rodentium, the causative agent of transmissible murine colonic hyperplasia, exhibits clonality: synonymy of C. rodentium and mouse-pathogenic Escherichia coli. J Clin Microbiol. 2000;38:4343–4350. doi: 10.1128/jcm.38.12.4343-4350.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luperchio SA, Schauer DB. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 2001;3:333–340. doi: 10.1016/s1286-4579(01)01387-9. [DOI] [PubMed] [Google Scholar]
- 21.Oishi Y, Manabe I, Tobe K, et al. Krüppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 2005;1:27–39. doi: 10.1016/j.cmet.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–9. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 23.Batlle E, Henderson JT, Beghtel H, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 24.van de Wetering M, Sancho E, Verweij C, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 25.Korinek V, Barker N, Moerer P, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 26.Pinto D, Gregorieff A, Begthel H, et al. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregorieff A, Pinto D, Begthel H, et al. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Muncan V, Sansom OJ, Tertoolen L, et al. Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf-4 target gene c-Myc. Mol Cell Biol. 2006;26:8418–8426. doi: 10.1128/MCB.00821-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins LM, Frankel G, Douce G, et al. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect Immun. 1999;67:3031–3039. doi: 10.1128/iai.67.6.3031-3039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umar S, Sellin JH, Morris AP. Murine colonic mucosa hyperproliferation. II. PKC-beta activation and cPKC-mediated cellular CFTR overexpression. Am J Physiol Gastrointest Liver Physiol. 2000;278:G765–774. doi: 10.1152/ajpgi.2000.278.5.G765. [DOI] [PubMed] [Google Scholar]
- 31.Crane JK, Oh JS. Activation of host cell protein kinase C by enteropathogenic Escherichia coli. Infect Immun. 1997;65:3277–3285. doi: 10.1128/iai.65.8.3277-3285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Grado M, Rosenberger CM, Gauthier A, et al. Enteropathogenic Escherichia coli infection induces expression of the early growth response factor by activating mitogen-activated protein kinase cascades in epithelial cells. Infect Immun. 2001;69:6217–6224. doi: 10.1128/IAI.69.10.6217-6224.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khachigian LM, Collins T. Early growth response factor 1: a pleiotropic mediator of inducible gene expression. J Mol Med. 1998;76:613–616. doi: 10.1007/s001090050258. [DOI] [PubMed] [Google Scholar]
- 34.McMahon SB, Monroe JG. The role of early growth response gene 1 (egr-1) in regulation of the immune response. J Leukoc Biol. 1996;60:159–166. doi: 10.1002/jlb.60.2.159. [DOI] [PubMed] [Google Scholar]
- 35.O’Grady EP, Mulcahy H, O’Callaghan J, et al. Pseudomonas aeruginosa infection of airway epithelial cells modulates expression of Kruppel-like factors 2 and 6 via RsmA-mediated regulation of type III exoenzymes S and Y. Infect Immun. 2006;74:5893–5902. doi: 10.1128/IAI.00489-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hybiske K, Ichikawa JK, Huang V, et al. Cystic fibrosis airway epithelial cell polarity and bacterial flagellin determine host response to Pseudomonas aeruginosa. Cell Microbiol. 2004;6:49–63. doi: 10.1046/j.1462-5822.2003.00342.x. [DOI] [PubMed] [Google Scholar]
- 37.Sauvonnet N, Pradet-Balade B, Garcia-Sanz JA, et al. Regulation of mRNA expression in macrophages after Yersinia enterocolitica infection. Role of different Yop effectors. J Biol Chem. 2002;277:25133–142. doi: 10.1074/jbc.M203239200. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann R, van Erp K, Trulzsch K, et al. Transcriptional responses of murine macrophages to infection with Yersinia enterocolitica. Cell Microbiol. 2004;6:377–390. doi: 10.1111/j.1462-5822.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- 39.Bohn E, Muller S, Lauber J, et al. Gene expression patterns of epithelial cells modulated by pathogenicity factors of Yersinia enterocolitica. Cell Microbiol. 2004;6:129–141. doi: 10.1046/j.1462-5822.2003.00346.x. [DOI] [PubMed] [Google Scholar]
- 40.Moreilhon C, Gras D, Hologne C, et al. Live Staphylococcus aureus and bacterial soluble factors induce different transcriptional responses in human airway cells. Physiol Genomics. 2005;20:244–255. doi: 10.1152/physiolgenomics.00135.2004. [DOI] [PubMed] [Google Scholar]
- 41.Sen-Banerjee S, Mir S, Lin Z, et al. Krüppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720–726. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]
- 42.O’Grady E, Mulcahy H, Adams C, et al. Manipulation of host Kruppel-like factor (KLF) function by exotoxins from diverse bacterial pathogens. Nat Rev Microbiol. 2007;5:337–341. doi: 10.1038/nrmicro1641. [DOI] [PubMed] [Google Scholar]
- 43.SenBanerjee S, Lin Z, Atkins GB, et al. KLF2 is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das H, Kumar A, Lin Z, et al. Krüppel-like factor 2 (KLF2) regulates proinflammatory activation of monocytes. Proc Natl Acad Sci U S A. 2006;103:6653–6658. doi: 10.1073/pnas.0508235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sellin JH, Umar S, Xiao J, et al. Increased beta-catenin expression and nuclear translocation accompany cellular hyperproliferation in vivo. Cancer Res. 2001;61:2899–2906. [PubMed] [Google Scholar]
- 46.Zhang W, Chen X, Kato Y, et al. Novel cross talk of Kruppel-like factor 4 and beta-catenin regulates normal intestinal homeostasis and tumor repression. Mol Cell Biol. 2006;26:2055–2064. doi: 10.1128/MCB.26.6.2055-2064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


