Abstract
Background
While there is substantial support in the literature for an increased prevalence of cannabis use in cigarette smokers, emerging studies allude to the possibility that cannabis users may, in turn, be at significantly elevated risk for rapid transitions in their cigarette smoking trajectories. If there is evidence in its favor, the increased rates of cigarette smoking in cannabis users may prove to be the most significant public health problem associated with cannabis use.
Methods
In a sample of 3,787 female twins (age range 18–29 years), we examined, using discrete-time survival analyses, whether women who reported cannabis use were at increased risk for regular cigarette smoking and progression to nicotine dependence.
Results
After controlling for a large number of potential covariates, we found that women who used cannabis were at 4.4 and 2.8 increased hazards for transitioning from initiation to regular smoking and from regular smoking to nicotine dependence respectively.
Conclusions
Cannabis use is associated with transitions to more involved stages of cigarette smoking in women. This is a source of public health concern, first due to the high mortality associated with cigarette smoking and second, due to the high prevalence of cannabis use in the general population.
Keywords: Cannabis, Tobacco, Women, Survival Model, Epidemiology
1. Introduction
1.1. Tobacco Use and Women
Tobacco use is the leading cause of preventable death in the United States (Centers for Disease Control (CDC), 2002). Even though national statistics report that fewer women than men are current smokers (18.5 vs 23.4%, in 2004)(Centers for Disease Control (CDC), 2004), this gender difference continues to diminish. For instance, thirty-day cigarette smoking is more frequently reported by U.S. girls than boys in the 8th and 10th grade, and unlike young men, rates of current smoking have not significantly declined in young women (Johnston et al., 2006). Furthermore, relative to male smokers, women who smoke are now at significantly greater risk for lung-cancer – while the relative risks for smoking-related morbidity has increased modestly over the last 5 decades, there has been a nearly four-fold increase in relative risks of lung-cancer related deaths in women (Smoking and Tobacco Control Program, 1997). Therefore, young women are emerging as a population that is exceptionally vulnerable to the long-term consequences of cigarette smoking and reducing rates of tobacco use and modifying risk factors that may exacerbate a woman’s likelihood of becoming dependent on nicotine are key public health concerns (U.S. Department of Health and Human Services, 2000).
1.2 The stages of tobacco use
Tobacco-related behaviors consist of multiple stages that are contingent on each other. Initiation and experimentation with cigarettes, including smoking a few puffs or a whole cigarette may be considered the first. Only in those that have ever attempted to smoke cigarettes, can the liability to regularly smoke be assessed. Similarly, the risk for heavy smoking and nicotine dependence is contingent upon smoking cigarettes on a regular basis, although in adolescents, the threshold may be lower (DiFranza et al., 2007). There are several points along this trajectory where a risk factor may have an impact – for instance, Kendler et al (1999) have demonstrated that certain risk factors are only associated with early stages of smoking (e.g. history of divorce) while others may correlate with both earlier and later (dependence) stages although to a varying degree (e.g. problem drinking and alcohol dependence). One might argue however, that along with individual-specific risk and protective factors, including other substance use and psychopathology, social/contextual factors (e.g. taxation, smoking policy) may also facilitate transitions in cigarette smoking. However, persuasive evidence suggests that individual factors are more predictive of transitions than are social/contextual influences (Kandel et al., 2004).
1.3 The effects of cannabis use on tobacco use
While associations between tobacco and cannabis are well established (e.g. adolescents who smoke cigarettes are 9–15 times more likely to use cannabis (Johnston et al., 2005; Mathers et al., 2006)), the earlier age of onset for tobacco use has meant that the bulk of this literature has focused on the extent to which tobacco use may be a risk factor for cannabis use. Only recently has there developed a considerable interest in the possible “reverse” influence of cannabis use on tobacco-related trajectories. For instance, Highet (2004) found that in boys aged 13–15, cannabis use encouraged their tobacco smoking and that participants reported difficulty quitting tobacco smoking due to the use of tobacco in their marijuana joints even when they were attempting to quit tobacco use. Another study found that cannabis use significantly impeded self-reported attempts to quit smoking cigarettes, despite the acknowledgement that cigarette smoking was harmful (Amos et al., 2004). In a clinical sample, Gourlay et al (1994), reported that cannabis use was negatively associated with sustained smoking cessation subsequent to an intervention involving transdermal nicotine and behavioral counseling.
In addition to these qualitative evaluations, some epidemiological studies have also shown an association between prior cannabis use and cigarette smoking. Patton et al (2005), for instance, found that teenagers who had never used tobacco but had used cannabis on a weekly basis were 8.4 times more likely to initiate tobacco use when compared to their counterparts who never used cannabis. These authors further reported that even after accounting for level of tobacco use and other socio-demographic characteristics, daily cannabis users who were not tobacco dependent at age 20 were 3.6 times more likely to develop nicotine dependence by age 24. In comparable analyses, Ford et al (2002) found that, among tobacco smokers, past month cannabis use was associated with a 1.9 greater odds of reporting continued tobacco smoking compared to those who never used cannabis. Recently Timberlake et al (2007) reported that frequent (>10 times) and recent (past month) cannabis use increased odds of transitioning to daily smoking and to nicotine dependence, while later-onset use (> 13 years of age at first use) significantly decreased risk of subsequent nicotine dependence.
This growing evidence suggests that nicotine dependence and persistent cigarette smoking may be the leading, and most alarming, public health consequence of cannabis use. A revealing new study demonstrated that those who smoked cigarettes alone were at increased odds for COPD (chronic obstructive pulmonary disease), but this risk was exacerbated in those who smoked both cigarettes and cannabis. In fact, when compared to non cigarette smokers, there was a nearly 18 fold increase for COPD risk in cigarette and cannabis users (Tan et al., 2007), which also reflected a significant increase over those who smoked cigarettes alone. As women have consistently outnumbered men in deaths attributable to COPD (Centers for Disease Control and Prevention, 2003), the study of the risk for cigarette smoking attributable to cannabis use is particularly important in women.
1.4 Goals
In this study, we examine whether cannabis use plays a critical role in the transition from cigarette smoking initiation to regular cigarette smoking and subsequently, on the transition from regular smoking to nicotine dependence. Using a sample of 3,787 young adult twin women from the Missouri Adolescent Female Twin Study (MOAFTS), aged 18–29 years, we examined whether women with a prior history of cannabis use were at increased risk for transitioning from cigarette smoking initiation to regular smoking (RS), and also for the subsequent transition from RS to DSM-IV nicotine dependence (ND).
2. MATERIALS AND METHODS
2.1 Sample
Interview data on 3,787 young adult women (mean age 22 years, range 18–29) and questionnaire data on 3,656 of these women were collected during a follow-up phase of the Missouri Adolescent Female Twin Study (MOAFTS) conducted during 2002-2005 (Heath et al., 1999; Heath et al., 2002). MOAFTS consists of a cohort of female same-sex twin pairs born between July 1st 1975 – June 30th 1985 who were identified from birth records (Heath et al., 1999; Slutske et al., 2004). Twins were eligible to participate if both members of the twin pair had survived past infancy, were not adopted at birth and if their biological mother was a resident of the state at the time of their birth. Using a cohort-sequential sampling design, twins and their parents were invited to participate in the baseline interviews with at least one biological parent (the biological mother whenever possible), during 1994–1999, when the twins were 13, 15, 17 or 19 years old. Recruitment of 13 year olds continued over a two-year period as twins became age-eligible. A telephone diagnostic interview was administered, first to the parents, and subsequently, after obtaining parental permission, to the twins (minors). Of the 2,369 pairs that were identified as live-born, 95.6% were located. The final sample of twins interviewed at baseline for each cohort comprised 1633 pairs (72.5% of pairs targeted), including 579, 291, 367 and 373 pairs aged 13, 15, 17 and 19 respectively. About 13% reported African-American ancestry and 40% of the participants came from rural residences. Further details regarding sample recruitment and characteristics are given elsewhere (Heath et al., 1999; Knopik et al., 2005). Subsequently, participants were invited to (a) complete a mailed questionnaire, (b) participate in a mini follow-up telephone interview, (c) participate in the first full-length follow-up telephone interview and, (d) complete an accompanying mailed questionnaire to (c). Data for our analyses stem from (c) and (d). A total of 3,060 women from the baseline interview were re-interviewed, in 2002–2005 for (c), along with data collection on 728 women, from the baseline sampling frame, who had previously not been interviewed. To minimize sampling bias, all twins were invited to participate, provided that they had not previously indicated an unwillingness to participate in future studies, and provided that neither they nor their parents had not previously indicated unwillingness for their families to participate in future research. Data from these follow-up interviews, along with data from the questionnaires that accompanied them were utilized in the present study. Despite having a prior wave of interview data, these analyses cannot utilize the longitudinal design of data collection as questions on illicit drugs were not asked of the younger participants at baseline. Hence, these analyses are conducted ‘within wave’ and do not utilize baseline data, except to correct inconsistent response patterns (i.e. a participant reporting cannabis use at baseline but not at follow-up). Informed consent, as approved by the institutional review board at Washington University School of Medicine was obtained from participants for each wave of assessment.
2.2 Measures
2.2.1 Lifetime Cannabis Use
As our aim is to investigate the influence of cannabis use on stages of cigarette smoking, the primary independent variable was a lifetime history of cannabis use, coded dichotomously as the response to a question on whether the participant had ever used cannabis.
2.2.2 Smoking outcomes
There were two primary outcomes of interest that were contingent on each other:
Regular Cigarette Smoking (RS) was defined as smoking a 100 or more cigarettes lifetime. Those who had smoked 20–99 cigarettes were also, and only, included as regular smokers if they had smoked as frequently as once a week for a period of 2 months or longer. Those who had never smoked a cigarette (not even a puff) were coded as missing for the RS measure,
Nicotine Dependence (ND), was defined using the 7 DSM-IV (American Psychiatric Association, 1994) dependence criteria with 3+ symptoms clustering within the same 12-month period; any individual who did not report a lifetime history of RS was coded as missing for the ND measure. The latter was motivated by the structure of the interview, where those not reporting RS were not queried about their ND symptoms, and based on prior epidemiological work (Breslau et al., 2001; Centers for Disease Control (CDC), 1993).
2.2.3 Other aspects of cannabis use
In addition to lifetime cannabis use (used even once), we included in a series of subsequent models, other aspects of cannabis involvement:
(a) Lifetime frequent use was defined as a lifetime history of using cannabis 40 or more times.
(b) Daily cannabis use was defined as using cannabis every day during the period of peak use.
(c) 3 or more instances, in their lifetime, on which they had stayed under the influence of cannabis use for a whole day.
(d) Cannabis abuse/dependence problems measured by endorsement of a lifetime history of either 1 or more of 4 DSM- IV abuse or 1 or more of 6 DSM-IV dependence criteria.
2.2.4 Potential Covariates
As the relationship between cannabis and onset of RS, and subsequently ND, may be mediated by aspects of behavioral disinhibition (e.g. conduct problems), negative affect regulation (e.g. major depressive disorder) and other risk factors, we included several measures of psychopathology as control measures in our study. In addition to ethnicity (being African-American or Caucasian) and zygosity (being the member of an MZ or DZ twin pair), we included:
From the behavioral disinhibition spectrum:
(a) Conduct Problems: coded dichotomously as having 3 or more self-reported DSM-IV conduct problems before age 18;
(b) Inattentiveness and Hyperactivity: Coded using maternal reports of DSM-IV symptoms of attention deficit hyperactivity disorder (ADHD) - 6 or more inattention or hyperactivity symptoms, occurring prior to age 7, was defined as inattentiveness and/or hyperactivity respectively;
(c) Regular alcohol use: Using alcohol once a month for any 6 month period or longer;
(d) Personality traits of antagonism, impulsivity and aggression: Coded from self-reported questionnaire data. Antagonism was coded by reversing scoring, and averaging 12 items assessing agreeableness from the NEO-PI(Costa et al., 1985). Impulsivity was also constructed as a continuous scale by reverse coding and averaging 12 items assessing control from the Multidimensional Personality Questionnaire (MPQ)(Tellegen, 1982); Also, from the MPQ, 12 items assessing aggression were utilized.
From the negative affect regulation spectrum:
(e) Major depressive disorder: Coded using self-reported DSM-IV diagnostic criteria;
(f) Social phobia: Coded using self-reported DSM-IV criteria of social anxiety disorder;
(g) Panic attacks: Having four or more symptoms of panic attacks, with problems getting worse in the first 10 minutes of at least two or more attacks;
(h) Suicide attempt: Self-reported attempt to take one’s own life (independent of the context of diagnosing major depression);
(i) Personality trait of neuroticism: Assessed by summing 12 items from the neuroticism sub-scale of the NEO-PI;
Parent-child relationships (as reported by the participant) were included as covariates as follows:
(j) Parent-child conflict: Self-reported history of having frequent unpleasant disagreements or conflicts with a parent figure (mother or father) between the ages of 6 and 13 years;
(k) Low parent-child closeness: Self-reported lack of closeness between the participant and either parent figure during the ages of 6–13 years;
Two abuse-related covariates were also included:
(l) Childhood sexual abuse: Self-reported forced sexual contact prior to age 16 with a family member or with someone outside the family who was 5 years older than the participant;
(m) Childhood physical abuse: Self-report of physical injury or being hurt on purpose by an adult relative prior to age 16;
All covariates, with the exception of the personality measures, were collected from the telephone interviews and coded dichotomously. The personality scales were derived from a self-report mailed questionnaire that followed the interviews and scales were standardized to have a mean of 0 and a standard deviation of 1.0.
2.3 Sample Characteristics
Our sample consisted of 2,024 MZ (964 pairs and 96 twins from incomplete pairs) and 1,763 DZ twins (810 full pairs and 143 twins from incomplete pairs). Of the 3,787 women (median age 22 years, range 18–29 years) who participated in the telephone interviews, 72% reported ever having tried cigarettes. Approximately 48% of those who had tried cigarettes (or 35% of the entire sample) also reported a lifetime history of regular smoking. About 50% of the regular smokers (25% of those who had tried cigarettes or 17.4% of the full sample) met criteria for a lifetime history of DSM-IV ND. Mean age of initiation of cigarette smoking, RS and ND were 14.4 [range 4–26 years], 15.5 [range 8–26 years] and 18.4 [range 10–26 years].
Of these 3,787 women, 44% reported cannabis use, with a mean age of initiation of 16.6 [range 5–27 years]. About 32% and 9% of the cannabis users reported cannabis use after initiation of regular smoking and nicotine dependence respectively while 26% and 13% reported cannabis initiation and regular smoking and nicotine dependence respectively in the same year. Of those who reported cannabis use (N=1673), 23% reported frequent use (i.e. 40+ times lifetime), 12.9% reported daily use during peak usage, 11.5% reported 3+ instances of staying under the influence for a whole day while 22% reported 1 or more symptoms of DSM-IV abuse or dependence. Only 8% of the sample met criteria for either DSM-IV cannabis abuse or dependence. About 18% of the women reported cannabis use within a month of the interview, 22% reported using cannabis prior to the past month but within the last year while 60% had not used cannabis in the year preceding the interview. The prevalence of other covariates is presented in Table 1 for the full sample.
Table 1.
Sample characteristics of 3,787 young adult women participating in the first full-length follow-up interview of the Missouri Adolescent Female Twin Study
| Prevalence / Mean | |
|---|---|
| Age (mean) | 21.7 years [18–29] |
| Ethnicity (Caucasian) | 86.8% |
| Zygosity (Member of Monozygotic Pair) | 52.5% |
| Ever smoked cigarettes (initiation) | 71.8% |
| Outcomes | |
| Regular cigarette smoking | 34.7% |
| DSM-IV nicotine dependence | 17.4% |
| Main Predictor | |
| Cannabis Use | 44.2% |
| Behavioral Disinhibition | |
| Conduct Problems | 4.2% |
| Inattentiveness | 5.0% |
| Hyperactivity | 2.2% |
| Regular Alcohol use | 47.9% |
| Antagonism# | 0.31 [0.0–0.85] |
| Impulsivity# | 0.41 [0.15–0.75] |
| Aggression# | 0.26 [0.0–0.96] |
| Negative Affect Regulation | |
| Major Depressive Disorder | 20.8% |
| Social Phobia | 14.2% |
| Panic Attacks | 12.5% |
| Suicidal attempt | 5.2% |
| Neuroticism# | 0.43 [0.0–0.98] |
| Parent-child relationship | |
| Parent-child conflict | 68.7% |
| Lack of parent-child closeness | 3.2% |
| Physical/Sexual Abuse | |
| Childhood sexual abuse | 7.3% |
| Childhood Physical abuse | 6.0% |
Mean for continuous measure shown on unstandardized variable – variable was converted to z-score (mean = 0, SE = 1.0) for analyses;
2.4 Statistical Analyses
We used survival analyses to examine whether young adult women who had used cannabis were at increased risk for transitioning from initiation of cigarette smoking to RS, and subsequently from RS to ND. The advantage of a survival model over the more frequently used logistic regression is its ability to utilize data from individuals who may not have passed the period of risk for onset of the outcome measure. For instance, in our sample which is aged 18–29 years, some women may not be past the age of risk for onset of ND. These individuals would have been uninformative in the regression framework but would contribute to survival models. Also, as age of onset of both RS and ND was assessed in years (“how old were you when…?”), we opted to use a discrete-time model (Jenkins, 1995), which assumes that events of interest occur in intervals that are not continuous (e.g. grouped-continuous) and uniform in width (a year) across the entire period of study. Our independent variable, cannabis use, and all covariates (except the continuous personality traits) were time varied with respect to onset of RS and ND (age at clustering of 3+ symptoms of dependence). In other words, if an individual reported initiating cannabis use after the age of onset of RS, they were coded as 0 for cannabis use in models predicting RS. This was done separately for each outcome (RS and ND). Data were coded in person-years format, such that each individual in the dataset had as many observations as (a) age till event of interest occurs or (b) age at completion of interview if censored. A robust variance estimator was used to account for clustering of family data. A piece-wise baseline hazard (with a constant) was defined for the baseline hazard function (duration1≤17, duration 2=18–25, duration 3= 25 and older) and the complementary log-log function, which makes the assumption of a continuous distribution underlying the piece-wise discrete-time hazards, was used for estimation. While a fully non-parametric baseline hazard is preferred, it is infeasible for behaviors that have distinct periods of risk (i.e. typical ranges for ages of onset of substance use). Our piece-wise baseline hazards were defined by examining the frequency of onset of regular smoking and nicotine dependence in all eligible person years (range 1–29 years) and collapsing across smaller intervals. To demonstrate the robustness of our results to definition of the piece-wise constant, we also re-ran our full models with a log-transformed measure of time to event (i.e. age of onset for the smoking behaviors).
The primary models examined the influence of a lifetime history of cannabis use on RS and ND. In a series of secondary models, we examined the influence of more involved stages of cannabis use, such as frequent use, weekly use, frequently staying under the influence and cannabis problems. As we did not have ages associated with each of these measures, there was the possibility that the age of onset of these behaviors was subsequent to onset of RS or ND. As a consequence, we used the proportional odds logit model for these secondary analyses which should only be viewed as illustrative.
All survival modeling was conducted in STATA (Jenkins, 2001; Stata Corp, 2003). Along with discrete-time models, we also used STATA to derive the cumulative hazards plots of transition from initiation of cigarette smoking to RS and from RS to ND, stratified by cannabis use, by computing life tables for each participant. We also used a forward stepwise selection procedure, with a conservative entry criterion of p-value ≤0.005 to examine whether cannabis use and the remaining covariates in the model were retained in the multivariate context.
3. RESULTS
3.1. Transitions to RS and ND
Risk for transitioning from initiation of cigarette smoking to RS and from RS to ND was significantly elevated in women with a prior history of cannabis use (Figure 1). Without covariate adjustments, those women who used cannabis were at 5.2 and 3.5 increased hazards for transitioning from initiation to RS and from RS to ND respectively. Even after controlling for ethnicity (with African-American women at a reduced hazard for transition from initiation to RS) and zygosity (no effect), and other covariates from the behavioral disinhibition and negative affect regulation spectrum, women with a prior history of cannabis use were 4.4 times more likely to transition from initiation to RS, and 2.8 times more likely to transition from RS to ND (Table 2). Also shown in Table 2 are the hazards associated with the other covariates. For instance, in addition to cannabis use, the strongest correlates of the transition from initiation to RS, included conduct problems, parent-child conflict, impulsivity and antagonism and panic attacks. On the other hand, correlates of the transition from RS to ND included major depressive disorder, social phobia and childhood physical abuse. We also fitted a forward stepwise model – for the transition to RS, along with cannabis use, measures of conduct disorder, parent-child conflict, impulsivity and antagonism, and panic attacks were retained in the model. For the transition from RS to ND, cannabis use, along with major depressive disorder, social phobia, regular alcohol use and aggression were significant at p < 0.005.
Figure 1.
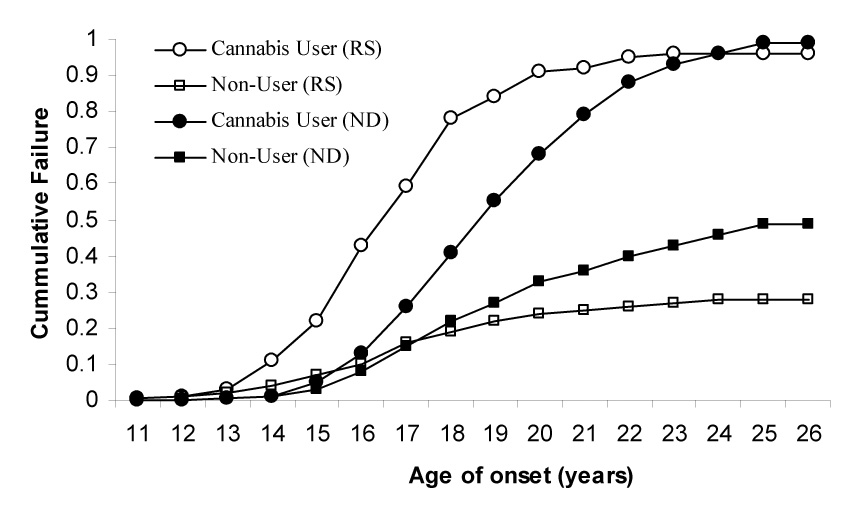
Likelihood of transitioning from initiation of cigarette smoking to regular smoking (RS) and from RS to DSM-IV nicotine dependence (ND) in 3,787 women, stratified by their lifetime history of cannabis use.
Table 2.
Hazards ratios (HR) with their 95% confidence limits depicting the association between cannabis use and transitions from initiation of cigarette smoking to regular smoking (RS) and from RS to DSM-IV nicotine dependence (ND), while controlling for covariates of behavioral disinhibition and negative affect regulation.
| Risk Factor | Regular | Smoking (RS) | Nicotine | Dependence (ND) |
|---|---|---|---|---|
| H.R. | 95% C.I. | H.R. | 95% C.I. | |
| Cannabis Use | 4.41* | 3.57−5.44 | 2.80* | 1.84−4.26 |
| Ethnicity | 0.19* | 0.13−0.29 | 0.60 | 0.38−0.97 |
| Zygosity | 0.94 | 0.80−1.11 | 1.13 | 0.92−1.40 |
| Behavioral Disinhibition | ||||
| Conduct Problems | 1.94* | 1.47−2.57 | 1.08 | 0.74−1.58 |
| Inattentiveness | 1.20 | 0.84−1.70 | 0.85 | 0.52−1.39 |
| Hyperactivity | 0.98 | 0.57−1.68 | 1.25 | 0.75−2.10 |
| Regular Alcohol use | 1.13 | 0.90−1.42 | 2.10* | 1.59−2.79 |
| Antagonism | 3.30* | 1.32−8.25 | 2.26 | 0.62−8.30 |
| Impulsivity | 3.96* | 1.46−10.75 | 1.59 | 0.37−6.76 |
| Aggression | 1.82 | 0.97−3.43 | 1.04 | 0.37−6.76 |
| Negative Affect Regulation | ||||
| Major Depressive Disorder | 1.24 | 0.94−1.64 | 1.51* | 1.14−2.00 |
| Social Phobia | 1.23 | 0.96−1.58 | 1.35* | 1.05−1.83 |
| Panic Attacks | 1.51* | 1.08−2.09 | 1.00 | 0.69−1.45 |
| Suicidal attempt | 1.07 | 0.65−1.76 | 1.37 | 0.88−2.16 |
| Neuroticism | 1.22 | 0.69−2.13 | 0.78 | 0.36−1.67 |
| Parent-child relationship | ||||
| Parent-child conflict | 1.32* | 1.10−1.58 | 0.99 | 0.77−1.28 |
| Lack of parent-child closeness | 0.80 | 0.51−1.23 | 0.89 | 0.57−1.38 |
| Physical/Sexual Abuse | ||||
| Childhood sexual abuse | 1.22 | 0.65−2.30 | 0.78 | 0.37−1.37 |
| Childhood Physical abuse | 1.73 | 1.19−2.52 | 1.83 | 1.24−2.69 |
Transition to RS includes data on 1462 MZ and 1259 DZ twins;
Transition from RS to ND includes data on 660 MZ and 655 DZ twins
H.R. in bold reflect those significant at p ≤ 0.05 in the multivariate survival model;
reflects those covariates that were retained with a p-value of 0.005 or less in a forward selection model.
3.2 Secondary Analyses
To determine the robustness of our results to analytic strategy, we also tested some additional models. First, we tested a log-transformed time to event as the baseline hazard. The hazards ratios for RS and ND were 4.5 [95% C.I. 3.6–6.2] and 2.3 [95% C.I. 1.6–3.1] respectively. Thus, our analyses were robust to the parameterization of the baseline hazard function. Next, we examined whether ties (e.g. onset of RS and cannabis use in the same year) had induced spurious associations. Ties did influence the effect size of the hazards of RS in cannabis users, which continued to be significant but was reduced to 1.8 [95% C.I. 1.5–2.2]. As expected, there were fewer instances of onset of cannabis use and nicotine dependence in the same year, and consequently this hazard was unchanged. In the logit framework, the proportional adjusted odds for RS and ND in lifetime cannabis users was 4.9 [95% C.I. 3.5–6.2] and 3.7 [95% C.I. 2.7–5.1] respectively. These estimates were comparable to the hazards ratios in Table 2. Secondary analyses revealed that more involved stages of cannabis use were strongly associated with increased likelihood of RS and ND. Individuals who were lifetime frequent users were at 1.9 and 1.6 odds of RS and ND respectively. These odds-ratios were largely unchanged when the definition of frequent use was modified (e.g. used 11+ times in their lifetime). Those who were daily users were at 3.3 and 2.1 increased odds of RS and ND. Often staying under the influence of cannabis for a whole day was also associated with RS (O.R. 1.9) and ND (O.R. 2.0) as was a lifetime history of cannabis problems (OR with RS 1.9, OR with ND 1.9).
4. DISCUSSION
Our study demonstrates that prior cannabis use is associated with significantly elevated hazards of both regular smoking (RS) and nicotine dependence (ND), even after accounting for a wide selection of potential covariates. This risk of increased cigarette involvement attributable to cannabis-related behaviors has been investigated in a number of observational, clinical and epidemiological studies (Amos et al., 2004; Ford et al., 2002; Gourlay et al., 1994; Highet, 2004; Patton et al., 2005; Sartor et al., 2007; Timberlake et al., 2007). Our findings closely parallel these results, demonstrating that a self-reported lifetime use of cannabis that precedes RS and/or ND is significantly related to transitions from initiation to RS and from RS to ND, even after controlling for covariates from the behavioral disinhibition and negative affect regulation spectrum. In both instances, cannabis use was the most potent risk factor associated with the hazards of RS and ND. In secondary analyses, we found evidence for an association between more involved stages of cannabis use (frequent use, daily use, staying under the influence and DSM-IV problems) with RS and ND. A majority of the risk factors for the early transition from initiation to RS were aspects of behavioral disinhibition, whereas the latter transition from RS to ND was associated with aspects of negative affect regulation.
4.1 The reverse gateway
Patton and colleagues (2005) have termed the influence of cannabis use on cigarette smoking a “reverse gateway”. The traditional “gateway” hypothesis (Kandel, 1975), applies to the increased risk of other illicit drug use in individuals using cannabis. There are 3 key features of an association hypothesized to represent a “gateway” effect. First, the age of onset of the gateway drug must precede the onset of the outcomes – this refers to sequence. Second, exposure to the gateway drug must be independently associated with an increased risk of the outcome – this is termed association. Third, and most controversially, exposure to the gateway drug has a causal impact on the outcome. Cannabis has been noted in numerous epidemiological studies to demonstrate sequence and association with other illegal substances, however, in most samples the assumption of sequence is reversed when examining cannabis and initiation of cigarette smoking, hence the term “reverse gateway”. In our sample of young adult women as well, only 6.8% reported smoking their first cigarette after using cannabis for the first time. However, our results demonstrate considerable support for both sequence and association between cannabis use and later stages of cigarette smoking such as RS and ND.
It is essential to note that the third assumption of a “gateway” mechanism, causation, is virtually impossible to verify within the human experimental paradigm. Even though our results and those by Patton et al (2005) have demonstrated an increment in rates of cigarette smoking in adolescents who used cannabis frequently, this effect may alternatively be attributed to a general process of socialization into deviant peer groups or by a general predisposition to non-normative behavior (e.g. delinquency). Indeed, we find that women who consumed alcohol regularly, reported conduct problems and frequent conflicts, as children, with their parents, and reported higher scores for aggression and impulsivity, were also at increased hazards for onset of RS. This suggests that the association between cannabis use and RS may potentially be a facet of a more general predisposition to problem behavior. In contrast, the correlates of the transition from RS to ND, in addition to cannabis use, were regular alcohol consumption, major depressive disorder and social phobia, with limited evidence for elements of general behavioral disinhibiton. We may speculate that the association between cannabis use and ND may be more representative of a general self-medication model, whereby women smoke cigarettes and use cannabis for negative affect regulation. Therefore, while cannabis use predicts transitions from initiation to RS and from RS to ND, the correlated risk factors underlying these mechanisms appear to be somewhat unique.
However, we cannot rule out the possibility that cannabis use has a causal influence on cigarette smoking. For instance, the increased hazard of RS and ND in cannabis users may be a consequence of the two substances sharing a common route of administration (i.e. most commonly smoked in cigarette/joint) (A. Agrawal and M. T. Lynskey, personal communication). Indeed, participants of several focus group studies (Amos et al., 2004; Highet, 2004) reported that using common paraphernalia to smoke cannabis and tobacco make the two activities inseparable. Smoking blunts has also recently gained popularity (Golub et al., 2005; Golub et al., 1999; Kelly, 2005). Blunts, or cannabis packed in inexpensive cigar paper, is more common in young males. While it may not have affected our analyses of a Midwestern female sample, blunts represent another level of commonality between cannabis and tobacco smokers. Therefore, further research that disentangles causal and correlated risks is needed.
4.2 Limitations
Our study may be viewed with the following limitations in mind: First, as discussed above, this is a sample of young adult women, and these conclusions may not apply to men or to samples of other demographic characteristics. Second, we cannot be certain of the precise temporal spacing between cannabis initiation and transition to RS or to ND – our assessment in years is crude, but comparable to other studies. While we opted to include ties (initiation of cannabis use and RS in the same year) as contributing to risk, it is possible that concomitant cannabis-tobacco users are at even greater risk for prolonged tobacco and cannabis use than those who have onsets separated by months or even a few weeks. Third, we did not have data on the extent of cigarette use during the year in which participants initiated cannabis use, or in the subsequent year. This would have allowed us to test whether cannabis use reinforces pre-existing cigarette smoking. We did note (not shown) that while a younger age at initiation of cigarette smoking was significantly associated with RS, it did not mediate the risk attributable to cannabis use. Fourth, retrospective recall may have biased self-reports of substance use, although only modestly as most twins were no more than 3–5 years past reported age of initiation. Finally, our sample is not suitable for the study of two related hypotheses: the risk of initiation of cigarette smoking in cannabis users and successful smoking cessation in tobacco users. Therefore, the possibility that cannabis users may be at increased risk for persistent smoking cannot be ruled out.
4.3 Conclusions
Epidemiological studies are a vital tool informing public health policy surrounding substance use. For instance, Chen and Kandel (1995) using a longitudinal sample of men and women reported that regular and persistent cigarette smoking, unlike alcohol and cannabis use, neither declined in adulthood nor quantitatively decreased, making it “one of the most serious drug-related health problems in the population”. As noted by Robins (1995), such empirical findings can aid in the formulation of public health policy rooted in science. Therefore, given the impact of cannabis use on cigarette smoking, and consequently on the health of women (and men), we urge further scientific inquiry into this public health challenge for the 21st century.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edition, Revised edn. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Amos A, Wiltshire S, Bostock Y, Haw S, McNeill A. 'You can't go without a fag…you need it for your hash'--a qualitative exploration of smoking, cannabis and young people. Addiction. 2004;99:77–81. doi: 10.1111/j.1360-0443.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- Breslau N, Johnson EO, Hiripi E, Kessler R. Nicotine dependence in the United States: prevalence, trends, and smoking persistence 3. Arch Gen Psychiatry. 2001;58:810–816. doi: 10.1001/archpsyc.58.9.810. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) MMWR. Vol. 42. 1993. Behavioral Risk Factor Surveillance System -- Michigan, 1987–1991; pp. 692–695. [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) MMWR. Vol. 51. 2002. Annual smoking-attributable mortality, years of potential life lost, and economic costs-United States; pp. 300–303. [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Morbidity and Mortality Weekly. Vol. 54. 2004. Cigarette smoking among adults - United States, 2004; pp. 1121–1124. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2003. Deaths Final Mortality Statistics. [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health. 1995;85:41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. The NEO Personality Inventory Manual Psychological Assessment Resources. Odessa, FL: 1985. [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, Pbert L, O'Loughlin J, McNeill AD, Ockene JK, Friedman K, Hazelton J, Wood C, Dussault G, Wellman RJ. Susceptibility to nicotine dependence: the Development and Assessment of Nicotine Dependence in Youth 2 study. Pediatrics. 2007;120:e974–e983. doi: 10.1542/peds.2007-0027. [DOI] [PubMed] [Google Scholar]
- Ford DE, Vu HT, Anthony JC. Marijuana use and cessation of tobacco smoking in adults from a community sample. Drug Alcohol Depend. 2002;67:243–238. doi: 10.1016/s0376-8716(02)00066-2. [DOI] [PubMed] [Google Scholar]
- Golub A, Johnson BD, Dunlap E. The growth in marijuana use among American youths during the 1990s and the extent of blunt smoking. J Ethn.Subst Abuse. 2005;4:1–21. doi: 10.1300/J233v04n03_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub AL, Johnson BD. Cohort changes in illegal drug use among arrestees in Manhattan: from the Heroin Injection Generation to the Blunts Generation. Subst Use Misuse. 1999;34:1733–1763. doi: 10.3109/10826089909039425. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Prospective study of factors predicting outcome of transdermal nicotine treatment in smoking cessation. BMJ. 1994;309:842–846. doi: 10.1136/bmj.309.6958.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Howells W, Bucholz KK, Glowinski AL, Nelson EC, Madden PA. Ascertainment of a mid-western US female adolescent twin cohort for alcohol studies: assessment of sample representativeness using birth record data. Twin.Res. 2002;5:107–112. doi: 10.1375/1369052022974. [DOI] [PubMed] [Google Scholar]
- Heath AC, Madden PA, Grant JD, McLaughlin TL, Todorov AA, Bucholz KK. Resiliency factors protecting against teenage alcohol use and smoking: influences of religion, religious involvement and values, and ethnicity in the Missouri Adolescent Female Twin Study. Twin.Res. 1999;2:145–155. doi: 10.1375/136905299320566013. [DOI] [PubMed] [Google Scholar]
- Highet G. The role of cannabis in supporting young people's cigarette smoking: a qualitative exploration. Health Educ Res. 2004;19:635–643. doi: 10.1093/her/cyg089. [DOI] [PubMed] [Google Scholar]
- Jenkins SP. Easy estimation methods for discrete-time duration models. Oxford Bulletin of Economics and Statistics. 1995;57:129–138. [Google Scholar]
- Jenkins SP. Stata Technical Bulletin STB 39. 2001;39 [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Ann Arbor, MI: University of Michigan News and Information Services; 2005. Teen drug use down but progress halts among youngest teens. [On-line]. Available: www.monitoringthefuture.org; accessed 05/05/06‥. [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Ann Arbor, MI: University of Michigan News and Information Services; 2006. Decline in daily smoking by younger teens has ended. [On-line]. Available: www.monitoringthefuture.org, accessed 06-25/07. [Google Scholar]
- Kandel D. Stages in adolescent involvement in drug use. Science. 1975;190:912–914. doi: 10.1126/science.1188374. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Kiros GE, Schaffran C, Hu MC. Racial/ethnic differences in cigarette smoking initiation and progression to daily smoking: a multilevel analysis. Am J Public Health. 2004;94:128–135. doi: 10.2105/ajph.94.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BC. Bongs and blunts: notes from a suburban marijuana subculture. J Ethn.Subst Abuse. 2005;4:81–97. doi: 10.1300/J233v04n03_04. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol.Med. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Sparrow EP, Madden PA, Bucholz KK, Hudziak JJ, Reich W, Slutske WS, Grant JD, McLaughlin TL, Todorov A, Todd RD, Heath AC. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: a female twin study. Psychol Med. 2005;35:625–635. doi: 10.1017/s0033291704004155. [DOI] [PubMed] [Google Scholar]
- Mathers M, Toumbourou JW, Catalano RF, Williams J, Patton GC. Consequences of youth tobacco use: a review of prospective behavioural studies. Addiction. 2006;101:948–958. doi: 10.1111/j.1360-0443.2006.01438.x. [DOI] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Sawyer SM, Lynskey M. Reverse gateways? Frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction. 2005;100:1518–1525. doi: 10.1111/j.1360-0443.2005.01220.x. [DOI] [PubMed] [Google Scholar]
- Robins LN. The natural history of substance use as a guide to setting drug policy. Am J Public Health. 1995;85:12–13. doi: 10.2105/ajph.85.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Xian H, Scherrer JF, Lynskey M, Duncan AE, Haber R, Grant JD, Bucholz K, Jacob T. Psychiatric and Familial Predictors of Transition Times Between Smoking Stages:Results From an Offspring-of-Twins Study. Addict Behav. 2007 doi: 10.1016/j.addbeh.2007.09.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Hunt-Carter EE, Nabors-Oberg RE, Sher KJ, Bucholz KK, Madden PA, Anokhin A, Heath AC. Do college students drink more than their non-college-attending peers? Evidence from a population-based longitudinal female twin study. J.Abnorm.Psychol. 2004;113:530–540. doi: 10.1037/0021-843X.113.4.530. [DOI] [PubMed] [Google Scholar]
- Smoking and Tobacco Control Program; National Cancer Institute. Monograph 8: Changes in Cigarette-Related Disease Risks and Their Implications for Prevention and Control. Bethesda, MD: 1997. [Google Scholar]
- Stata Corp. STATA. College Station, TX: 2003. [Google Scholar]
- Tan WC, Wang E, Tan J, Fitzgerald M, Hogg R, Sin D, Wang W, Jong A, Gao S, Lo C. The Impact of Cigarette and Marijuana Smoking in Chronic Obstructive Lung Disease Study in Vancouver. Canada: 2007. http://www.thoracic.org/sections/publications/press-releases/conference/articles/2007/abstracts/The_Impact_of_Cigarette_and_Marijuana_Smoking_in_Chronic_Obstructive_Lung_Disease_Study_in_Vancouver_Canada_681.pdf . [Google Scholar]
- Tellegen A. Brief Manual for the Multidimensional Personality Questionnaire University of Minnesota. Minneapolis, MN: 1982. [Google Scholar]
- Timberlake DS, Haberstick BC, Hopfer CJ, Bricker J, Sakai JT, Lessem JM, Hewitt JK. Progression from marijuana use to daily smoking and nicotine dependence in a national sample of U.S. adolescents. Drug Alcohol Depend. 2007;88:272–281. doi: 10.1016/j.drugalcdep.2006.11.005. [DOI] [PubMed] [Google Scholar]
- U.S.Department of Health and Human Services. Understanding and Improving Health U.S. Government Printing Office. Washington, D.C: 2000. Healthy People 2010. [Google Scholar]


