Abstract
A critical link between hemostatic factors and atherosclerosis has been inferred from a variety of indirect observations, including the expression of procoagulant and fibrinolytic factors within atherosclerotic vessels, the presence of fibrin in intimal lesions, and the cellular infiltration of mural thrombi leading to their incorporation into developing plaques. To directly examine the role of the key fibrinolytic factor, plasminogen, in atherogenesis, plasminogen-deficient mice were crossed to hypercholesterolemic, apolipoprotein E-deficient mice predisposed to atherosclerosis. We report that the loss of plasminogen greatly accelerates the formation of intimal lesions in apolipoprotein E-deficient animals, whereas plasminogen deficiency alone does not cause appreciable atherosclerosis. These studies provide direct evidence that circulating hemostatic factors strongly influence vessel wall disease in the context of a disorder in lipid metabolism.
Keywords: fibrinolysis, apolipoprotein E
Fibrin has been recognized as a significant component of atherosclerotic lesions for more than a century (1), and the location, level, forms, and biological activities of fibrin(ogen) and its degradation products within atherosclerotic tissues have been characterized in detail (2, 3). In the context of a persistent or recurring vascular challenge, the local formation and degradation of fibrin matrices may have a profound impact on the formation of atherosclerotic lesions. Consistent with this general hypothesis, microscopic analyses of atherosclerotic human vessels strongly suggest that mural thrombi formed as a consequence of plaque fissure, if not fatal, are readily incorporated into growing intimal lesions (4, 5). Fibrin formed on and within the vessel wall may provide a provisional matrix that supports the local adhesion, migration, and proliferation of the primary cells participating in the formation of atherosclerotic lesions, including platelets, endothelial cells, inflammatory cells, and smooth muscle cells (6, 7). An implicit extension of this concept is that local plasminogen (Plg) activation leading to the cellular infiltration and clearance of fibrin-rich matrices may be a critical feature of atherogenesis. In this regard, it is notable that Plg has been shown to be critical to cellular infiltration and resolution of fibrin-rich skin wounds (8).
Although fibrin clearance may be important in lesion progression, Plg activation may also influence vessel wall disease through several distinct mechanisms. For example, plasmin-mediated proteolysis could conceivably have a profound impact on lesion progression through either the activation of latent growth factors [e.g., transforming growth factor β (TGF-β)], the generation of biologically active fibrin degradation products (FDPs), the degradation of common extracellular matrix components, and the activation of protease zymogens (e.g., procollagenases), or some combination of these processes (9, 10). The interplay between plasmin and TGF-β activation is particularly intriguing because this growth factor is a known autocrine inhibitor of smooth muscle cell (SMC) proliferation and migration, and the inhibition of plasmin-mediated TGF-β activation in the vessel wall has been proposed to promote lesion formation in mice (11, 12).
To definitively establish the role of plasmin-mediated proteolysis in atherogenesis, we have examined the impact of Plg deficiency on the development of vessel wall disease in apolipoprotein E (ApoE)-deficient mice that develop hypercholesterolemia and spontaneous atherosclerotic lesions (13, 14). We report that Plg deficiency alone does not lead to appreciable atherosclerotic lesions in mice, but the absence of Plg accelerates lesion development in both the proximal and distal aorta of animals lacking ApoE. These results provide a direct demonstration that circulating hemostatic factors strongly influence the progression of vessel wall disease driven by a defect in lipid metabolism.
MATERIALS AND METHODS
Generation of Double-Deficient Mice.
Plg-deficient mice (20) were interbred with ApoE-deficient mice (13) to generate control, Plg−/−, ApoE−/−, and ApoE−/−/Plg−/− mice of a similar, but hybrid (129/NIH Black Swiss/C57BL/6) genetic background. To exclude the possible influence of secondary genetic modifiers, comparative analyses were done in paired studies using littermates. Mice of all genotypes were housed together and were maintained on a standard low fat mouse chow containing 6.5% fat (ether extraction) and 0.028% cholesterol (Formulab Diet 5008; Purina Mills).
Genotype Analyses.
The genotypes of mice were established by PCR analysis using tail or ear biopsy DNA. The wild-type ApoE allele was detected using primers complementary to sequences within the 5′-flanking region of the mouse ApoE gene (ApoE primer 1, 5′-GCTCCTGAAGGAACTGGAGCACGTCCCAGC-3′; ApoE primer 2, 5′-GGTACTGGGCACTGAGAACCGCTCCTTCCC-3′; ref. 15). ApoE primer-2 is complementary to a sequence deleted in the disrupted ApoE allele (13). Together, ApoE primer 1 and ApoE primer 2 generate a 220-bp PCR product. The targeted ApoE allele was detected using ApoE primer 1 (see above) and a primer complementary to a sequence within the inserted neomycin resistance gene (Neo primer 1: 5′-CATGAGAGCAGCCGATTGTCTGTTGTGCCC-3′). Together, ApoE primer 1 and Neo primer 1 generate a 570-bp product. The genotypes for the Plg gene were established as described (8).
Lipid Analysis.
Plasma lipid analyses were done using mice fasted for at least 6 h. Cholesterol and triglycerides were measured enzymatically using a Boehringer Mannheim/Hitachi 717 analyzer. Lipid profile data were compared by least squares analysis using a general linear model procedure.
Histological Analysis.
Mouse tissues were perfusion-fixed using 10% neutral buffered formalin (Sigma). For qualitative analysis, hearts and aortic arches were embedded in paraffin, sectioned at 4 μm thickness, and stained with either hematoxylin/eosin, elastic Van Gieson, or Gomori’s trichrome. For quantitative analyses, tissues were processed and evaluated using a modification of the methods described by Paigen et al. (16) and Lawn et al. (12). Briefly, hearts were cut in half along a plane below the two atria and the top half was embedded in OCT compound (Miles). Sections (10 μm thick; cut perpendicular to the aorta) were prepared from the aortic sinus to the ascending aorta. The sections were mounted on slides and stained with oil red-O and hematoxylin, and the surface area of oil red-O-stained lesions were determined using a calibrated microscope eyepiece by an investigator unaware of animal genotype. Mean lesion area per section was calculated using the values obtained from five sections each separated by 70 μm. To consistently score the same region within the aortae of individual animals, the first, most-proximal section evaluated was at the point that the aorta became rounded with the cusps of the valves barely discernible. Mean lesion areas from ApoE−/− and ApoE−/−/Plg−/− mice were compared using a nonparametric median scores test.
Immunohistochemical Analyses.
Macrophages were stained with a rat anti-mouse Mac-2 monoclonal antibody (Boehringer Mannheim). Fibrin(ogen) was localized using rabbit anti-mouse fibrin(ogen) serum. Immunohistochemistry was performed with a Vector Elite ABC kit (Vector Laboratories) using a peroxidase detection system and 3-amino-9-ethyl-carbazole (AEC) as a chromogen (Biomeda, Foster City, CA).
In Situ Hybridization.
Fixed tissues were cryosectioned and hybridized with 35S-labeled RNA probes complementary to mouse urokinase-type Plg activator (uPA) and Plg activator inhibitor 1 (PAI-1) mRNA as described (17). The template plasmid for sense and antisense mouse uPA probes contained a XbaI–PstI fragment of the mouse uPA cDNA [nt 37–428 in the numbering system of Belin et al. (18)]. The template plasmid for mouse PAI-1 probes contained a PstI fragment of the mouse PAI-1 cDNA [nt 6–341 in the numbering system of Prendergast et al. (19)].
RESULTS
Generation of Mice with Combined Plg and ApoE Deficiency.
Plg-deficient mice (20) were interbred with ApoE-deficient mice (13) to generate mice with single (Plg−/−, ApoE−/−) and combined (ApoE−/−/Plg−/−) deficiencies in Plg and ApoE. All mice generated appeared normal at birth and displayed normal neonatal weight gain and survival, regardless of genotype. However, beyond about 2 months of age, Plg−/− mice began to deviate from mice of other genotypes in both body weight and survival, phenotypes shown earlier to depend on circulating fibrinogen and likely to be related to the systemic impairment of fibrinolysis (8). At 20 weeks of age, the average body weight of ApoE−/− mice was similar to control animals [31 ± 5 g (n = 102), 32 ± 4 g (n = 10), respectively], whereas the average weight of Plg−/− mice was about 25% lower [24 ± 5 g (n = 16)]. When ApoE deficiency was superimposed on Plg deficiency, an additional reduction in average body weight was observed [19 ± 3 g (n = 59)]. Consistent with the general health liability of Plg deficiency (8), only about 70% of Plg−/− (cohort of 44 tracked) and 50% of ApoE−/−/Plg−/− (cohort of 76 tracked) mice survived beyond 20 weeks of age, whereas >95% of ApoE−/− and control mice survived beyond this period. Thus, ApoE deficiency poses an additional challenge to the overall health of Plg−/− mice.
As expected from earlier studies (13, 14), 5- to 8-month-old ApoE−/− mice displayed pronounced hypercholesterolemia, elevated plasma triglycerides, and a small decrease in high density lipoprotein (HDL) cholesterol (Table 1). Plg deficiency did not significantly alter total plasma cholesterol or triglyceride levels in either ApoE+/− or ApoE−/− mice. However, the loss of Plg was associated with a small reduction in HDL cholesterol in ApoE−/− mice, a parameter that may be linked to the low body weights of Plg-deficient mice.
Table 1.
Cholesterol and triglyceride levels in ApoE- and Plg-deficient mice
| Genotype | Total cholesterol, mg/dl | Triglyceride, mg/dl | HDL cholesterol, mg/dl |
|---|---|---|---|
| ApoE+/−/Plg+/− | 120 ± 20 (n = 17) | 30 ± 20 (n = 17) | 90 ± 20 (n = 14) |
| ApoE+/−/Plg−/− | 100 ± 20 (n = 6) | 20 ± 20 (n = 6) | 50 ± 20 (n = 4) |
| ApoE−/−/Plg+/− | 860 ± 350* (n = 44) | 120 ± 70* (n = 44) | 30 ± 10 (n = 44) |
| ApoE−/−/Plg−/− | 870 ± 390*† (n = 17) | 50 ± 30‡ (n = 17) | 10 ± 5§ (n = 12) |
Number of mice analyzed indicated in parentheses.
P < 0.0001 as compared to ApoE+/−/Plg+/− and ApoE+/−/Plg−/− mice.
P = 0.92 as compared to ApoE−/−/Plg+/− mice; not significantly different.
P = 0.10 as compared to ApoE−/−/Plg+/− mice; not significantly different.
P < 0.001 as compared to ApoE−/−/Plg+/− mice.
Plg Deficiency Accelerates Atherosclerotic Lesion Development in the Aortic Arch.
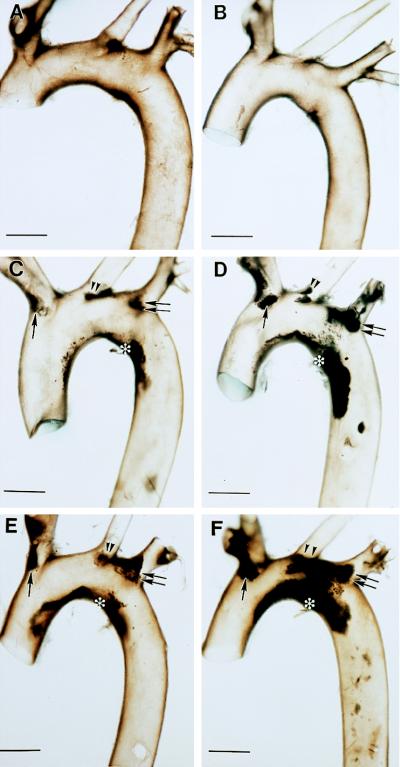
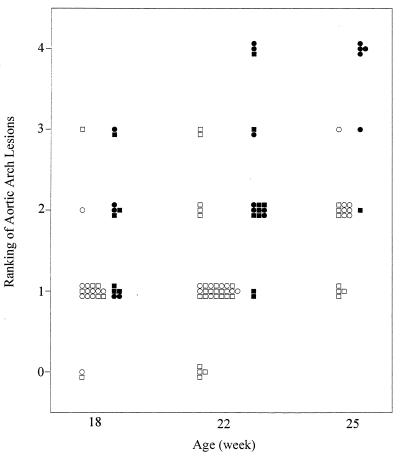
To initially evaluate the impact of Plg deficiency on the development and progression of atherosclerosis, the aortic arches from control, Plg−/−, ApoE−/−, and ApoE−/−/Plg−/− mice were grossly examined (see representative data in Fig. 1) and scored for lesion severity (see below and Fig. 2), using animals ranging from 18 to 25 weeks of age. The aortae from 8 control and 9 Plg−/− mice contained no visible lesions in the arch (see representative data in Fig. 1 A and B). In contrast, 57 of 63 ApoE−/− mice examined displayed aortic lesions (typically in the lesser curvature of the arch and at carotid branch points) and these became advanced with age (Fig. 1 C and E). However, more extensive lesions were observed in the aortae of ApoE−/− mice lacking Plg than in age-matched mice lacking ApoE alone (for representative examples, see Fig. 1 C–F). To systematically compare the arch lesions in ApoE−/− and ApoE−/−/Plg−/− mice, the gross appearance of lesions was ranked by an investigator unaware of animal genotype using a scoring system in which the absence of lesions was graded as 0, extensive lesions encompassing all three carotid branch points and the entire lesser curvature was graded as 4, and the severity of intermediate lesions was graded from 1 to 3. Consistent with the known age-dependence of atherosclerotic disease in ApoE−/− mice (21, 22), the lesions were more extensive in 25-week-old mice relative to 18- or 22-week-old animals of the same genotype (Fig. 2). However, the lesions were significantly more extensive in ApoE−/−/Plg−/− mice relative to ApoE−/− mice at every age examined (Fig. 2). For example, at 22 weeks of age, 14 of 16 (88%) ApoE−/−/Plg−/− mice were assigned a lesion score of ≥2, and 3 were assigned the maximum score of 4, whereas only 5 of 31 (16%) ApoE−/− mice were assigned a score of ≥2, and none rated a score of 4.
Figure 1.
Gross appearance of aortic arches of ApoE- and Plg-deficient mice. Brightfield view of the aortic arches collected from 26-week-old control (A) and Plg−/− (B) mice. Comparable views of typical aortic arches collected from 22-week-old (C and D) and 25-week-old (E and F), ApoE−/− (C and E) and ApoE−/−/Plg−/− (D and F) mice. Note the dark-appearing atherosclerotic lesions along the lesser curvature (asterisk), brachiocephalic trunk (single arrow), left common carotid (double arrowheads), and left subclavian (double arrows). Under darkfield, the lesions appear as opaque, cream-colored deposits. (Bars = 1 mm.)
Figure 2.
Lesion severity scored in the aortic arches of ApoE−/− (open symbols) and ApoE−/−/Plg−/− mice (filled symbols) at 18, 22, and 25 weeks of age. The extent of lesion development was scored according to the ranking system described in the text. Males and females are indicated with squares and circles, respectively. Twenty-four of 33 (73%) ApoE−/−/Plg−/− mice evaluated were analyzed along with ApoE−/− littermates. Statistical comparisons of the distributions within each genotype using a nonparametric median scores test indicated the lesion scores were significantly larger in older animals (P < 0.02 for 18-week-old mice vs. 25-week-old mice). In unpaired statistical analyses, the lesion scores were significantly larger in ApoE−/−/Plg−/− mice relative to ApoE−/− mice at each age examined (P < 0.01 at 18 weeks, P < 0.0001 at 22 weeks, P < 0.001 at 25 weeks). The difference between ApoE−/− and ApoE−/−/Plg−/− mice was also highly significant in paired littermates using Poisson regression analysis (P < 0.001, 56 pairs from 17 litters). Gender was not a significant variable at 22 weeks where sufficient data was available for comparison.
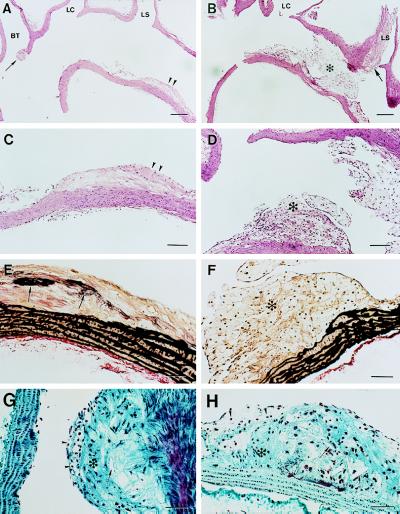
Histological analyses of the aortic arches from three 22-to 25-week-old ApoE−/− and three ApoE−/−/Plg−/− mice (serial sectioned longitudinally) provided further evidence of differences in lesion development in animals with and without Plg (see representative data in Fig. 3). Consistent with the gross observations described above, the lesions were generally more extensive in the ApoE−/− mice lacking Plg, often occluding a major portion of aortic lumen (Fig. 3 B and D). The thick and occlusive cellular deposits along the lesser curvature of ApoE−/−/Plg−/− mice were primarily macrophage-derived foam cells based on their histologic appearance (Fig. 3 D and F) and immunostaining with a Mac-2 antibody (data not shown). Complex fibrous lesions were also observed within the arches of mice of both genotypes (Fig. 3 G and H). However, in our qualitative analysis, advanced lesions were more frequently encountered in the mice lacking Plg.
Figure 3.
Histology of the atherosclerotic lesions within the aortic arches of ApoE−/− and ApoE−/−/Plg−/− mice. Representative longitudinal sections through the aortic arch of 25-week-old ApoE−/− (A, C, E, and G) and ApoE−/−/Plg−/− (B, D, F, and H) mice. The sections shown were cut in the plane of the branch points of the brachiocephalic trunk (BT), left common carotid (LC), and left subclavian (LS). (A) Low magnification view of hematoxylin/eosin-stained section showing an ApoE−/− vessel that is largely free of lesions other than a small, but complex, lesion at the entrance into the BT (arrow) and a fibrofatty lesion in the lesser curvature (double arrowheads). (B) Low magnification view of hematoxylin/eosin-stained section showing an ApoE−/−/Plg−/− vessel that has extensive foam cell-rich lesions that appear to span across the aorta from the lesser curvature to the LS branch point (asterisk). A more complex and highly occlusive lesion is present in the LS (arrow). (C) High magnification view of the fibrofatty lesion shown in A. (D) High magnification view of the foam cell-rich lesion (asterisk) shown in B (parallel section). (E) High magnification view of the fibrofatty lesion shown in A and C (parallel section) stained with elastic Van Gieson. Note the apparent elastic fiber deposition in intima (arrows). (F) High magnification view of the foam cell-rich lesion (asterisk) shown in B and D (parallel section) stained with elastic Van Gieson. Note the apparent distortion of the elastic fibers in the medial layer. (G) High magnification view of the complex BT lesion shown by arrow in A (parallel sections) stained with Gomori’s trichrome. Note the obvious cholesterol clefts (arrow), collagen-rich matrix deposition (asterisk), and surface foam cells (arrowheads). (H) High magnification view of a complex carotid lesion from an ApoE−/−/Plg−/− mouse with comparable features to those highlighted in G. (Bars: A and B = 200 μm; C and D = 100 μm; E–H = 50 μm.)
Plg Deficiency Enhances Atherosclerosis in the Proximal Aorta.
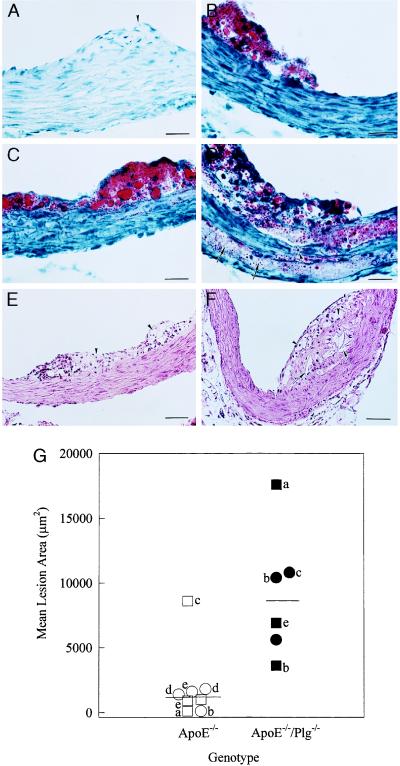
The proximal aorta is particularly prone to the development of intimal lesions in both ApoE−/− mice (13, 14, 21, 22) and other mouse models of atherosclerosis (23), and this region has been favored for quantitative and qualitative analyses of atherosclerosis because the cusps of the valves provide a valuable positional guidepost in comparative studies of sectioned tissue (16). To quantitatively examine the impact of Plg deficiency on the development of atherosclerotic lesions in the proximal aorta, mean lesion areas were measured in oil red-O-stained tissue sections following the well-established experimental approach described by Paigen et al. (16). Eight 20-week-old ApoE−/− mice were evaluated in parallel with 6 ApoE−/−/Plg−/− mice (5 of 6 being littermates of test ApoE−/− animals), 5 Plg−/− mice, and 8 control animals. Intimal lesions were not detected in any of the 20-week-old control (data not shown) or Plg−/− (Fig. 4A) mice evaluated. Furthermore, in separate experiments, no lesions were detected in any of 11 control and Plg−/− mice analyzed microscopically at 21 to 44 weeks of age. However, oil red-O-positive lesions were detected in every 20-week-old ApoE−/− mouse evaluated, and these were typically fatty streak lesions (median, 1,200 μm2; range, 130–8,600 μm2; Fig. 4 B, E, and G). Far more extensive lesions were observed in the ApoE−/−/Plg−/− mice examined in parallel (median, 8,600 μm2; range, 3,600–18,000 μm2; Fig. 4 D, F, and G), and these ranged in appearance from foam cell-rich fatty streaks (Fig. 4C) to more complex lesions (Fig. 4 D and F) with occasional disruption and/or distortion of the media (Fig. 4D). Because the lesions within the proximal aortae of 20-week-old animals were primarily fatty streaks, the quantitative differences observed between ApoE−/− and ApoE−/−/Plg−/− mice suggests that the accelerated vessel wall disease observed in mice lacking Plg begins at a relatively early stage of lesion development.
Figure 4.
Quantitative and qualitative analyses of lesions within the proximal aortae of ApoE- and Plg-deficient mice. (A) Typical oil red-O stained section from a 20-week-old Plg−/− mouse showing a normal vessel wall in the region around a valve cusp (arrowhead). (B) Section from a 22-week-old ApoE−/− mouse showing a small fatty streak lesion. (C and D) Sections from 22-week-old ApoE−/−/Plg−/− mice displaying a fatty streak (C) and an advanced fibroproliferative lesion (D). Note the substantial lipid deposits deep in the medial layer (arrows) and apparent disruption (arrowhead) of elastic lamina in D. (E) Typical appearance of a proximal aorta lesion in a 25-week old ApoE−/− mouse stained with hematoxylin/eosin. (F) Complex lesion in the proximal aorta of a 20-week-old ApoE−/−/Plg−/− mouse. (G) Mean lesion areas in the proximal aortae of 20-week-old ApoE−/− mice (open symbols) and ApoE−/−/Plg−/− mice (filled symbols). Littermate pairs are indicated by letters. Males and females are indicated by squares and circles, respectively. The median values for both ApoE−/− and ApoE−/−/Plg−/− mice are indicated by bars. The difference in mean lesion areas in the ApoE−/− and ApoE−/−/Plg−/− groups was highly significant using a nonparametric median scores test (P < 0.004). (Bars: A–D = 25 μm; E and F = 50 μm.)
uPA and PAI-1 Are Expressed in the Atherosclerotic Lesions.
The finding that Plg is a powerful modifier of atherosclerosis in ApoE−/− mice would imply that the factors controlling Plg activation (e.g., uPA and PAI-1) are expressed in the developing lesions of these animals. Like human atherosclerotic lesions (24, 25), in situ hybridization studies showed that both uPA and PAI-1 mRNA were highly expressed in advanced lesions within the arches and proximal aortae of both ApoE−/− and ApoE−/−/Plg−/− mice (data not shown). The expression of these factors was patchy within the intima and appeared to be associated with a subset of both macrophages and SMC, a pattern reminiscent of that observed in human lesions. Little expression of either uPA or PAI-1 mRNA was observed in SMC located within unaltered medial tissue, macrophage foam cells within early fatty streaks, and normal appearing vessels (data not shown).
Fibrin(ogen) Deposits in the Atherosclerotic Lesions.
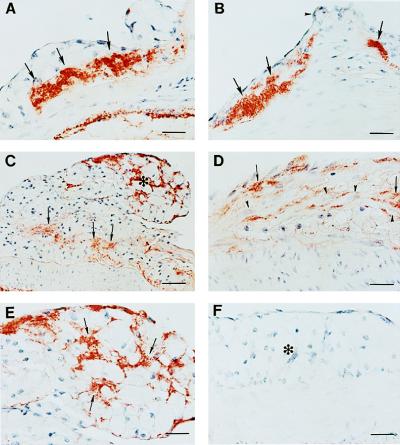
Because fibrin(ogen) and FDPs are well-documented components of human atherosclerotic plaques (2, 3), we tested immunologically for the presence of fibrin(ogen) deposits within the lesions of ApoE−/− and ApoE−/−/Plg−/− mice. As shown in Fig. 5, fibrin(ogen) was present within both foam cell-rich fatty streak and more complex lesions of ApoE−/− and ApoE−/−/Plg−/− mice. In early lesions, fibrin(ogen) appeared to be a significant component of the matrix surrounding foam cells (Fig. 5 C and E), possibly supporting cell adhesion. In more complex lesions, fibrin(ogen) was widely distributed, including diffuse deposits within the fibrous portions of lesions (Fig. 5 C and D). These data show that fibrin(ogen) deposition is a conspicuous feature in atherosclerotic lesions of ApoE−/−/Plg−/− mice, but any quantitative differences in fibrin(ogen) or its derivatives within comparable lesions of ApoE−/−/Plg−/− and ApoE−/− mice remains to be established.
Figure 5.
Fibrin(ogen) deposition in early intimal lesions. Sections from the proximal aorta of a 25-week-old ApoE−/−/Plg−/− mouse (A) and a littermate ApoE−/− mouse (B) showing fibrin deposits at the base of the lesions (arrows). The cusp of the aortic valves are indicated by arrowhead in B. (C) Section from the aortic arch of the same mouse as in A displaying a large, foam cell-rich lesion. Note fibrin(ogen)-positive staining around the foam cells (asterisk), and the diffuse fibrin(ogen)-positive region at the base of the lesion (arrows). (D) Section from the aortic arch of the same mouse as in B showing spindle-shaped smooth muscle cells (arrowheads) intermingling with fibrin(ogen) deposits (arrows). (E) High magnification view of the foam cell-rich region in C. Note the intense fibrin(ogen)-positive stain surrounding the foam cells (arrows). (F) Foam cell-rich lesion (asterisk) from a 6-month-old ApoE−/−/Fib−/− mouse demonstrating the specificity of the rabbit anti-mouse fibrinogen serum. (Bars: A, B, and D–F = 25 μm; C = 50 μm.)
DISCUSSION
The studies discussed here show that loss of a key hemostatic factor, Plg, greatly accelerates the development of atherosclerotic lesions in a disease model driven by a disorder in lipid metabolism. The aggravating effect of Plg deficiency on lesion development is observed widely in the aortic tree, including the lesser curvature of the aortic arch, the carotid branch points, and the proximal aorta. Because Plg deficiency alone does not cause appreciable atherosclerosis in animals, it appears that plasmin(ogen) deficiency is a powerful amplifier of atherosclerotic disease that is expressed only in the context of other underlying challenges. However, it should be noted that a deficit in Plg activation may have a significant impact on atherosclerosis in the context of challenges far less severe than ApoE deficiency, such as a simple high fat diet. A comparison has not yet been made of the atherosclerotic tendency of Plg−/− and control mice when maintained on a high fat diet, but it is interesting that transgenic mice expressing apolipoprotein (a) [Apo(a)], a known inhibitor of Plg activation and fibrinolysis, develop severe atherosclerosis when challenged with a cholesterol-rich, high fat diet (ref. 12 and see further discussion below). The finding that Plg deficiency accelerates atherosclerosis provides direct evidence that fibrinolytic factors influence the progression of vessel wall disease, and emphasizes the potential importance of hemostatic factors as a whole in atherosclerosis.
Individual arterial lesions of both ApoE−/− and ApoE−/−/Plg−/− mice varied in morphological features. Animals of both genotypes carried intimal lesions with the qualitative appearance of early, intermediate, and advanced disease, including fibrous plaques containing necrotic cores, cholesterol clefts, macrophage foam cells, SMC, and deposits of elastic fibers and collagen. Therefore, Plg deficiency does not appear to block the progression of lesions to any specific stage. There was also no obvious genotype-specific difference in the local expression of uPA or PAI-1 within comparable lesions. However, detailed analyses focusing on specific cell markers and selected matrix components will be useful in further defining any genotype-dependent features of lesions.
A detailed understanding of the mechanism(s) by which Plg deficiency influences atherosclerosis might provide valuable insights into the pathogenesis of human disease and also suggest therapeutic strategies to prevent or slow atherosclerosis. Interestingly, Plg deficiency may have opposing effects that individually favor or hinder lesion development, but, in the balance, accelerate atherosclerosis. The accumulation of mural and intimal fibrin due to loss of plasmin-mediated fibrinolysis might be the dominant proatherogenic factor. Histological analyses of developing human atheromas suggest that mural thrombi are readily organized by inflammatory cells and SMC, and these are ultimately assimilated into the growing intimal mass (4, 5). Consistent with these findings, fibrin(ogen) is known to support the adhesion, migration, and/or proliferation of the major cell types contributing to plaque development, including macrophages and SMC (6, 26, 27). Fibrin(ogen) has recently been identified as an important bridging molecule between endothelial cells and leukocytes, an interaction that is mediated in part by the endothelial cell adhesion molecule ICAM-1 (26, 27). In addition, fibrin-immobilized platelets are likely to promote the further binding of leukocytes of all types through the platelet adhesion molecule P-selectin (28).
Although increased amount or persistence of fibrin at the vessel wall may be a potent driving force in lesion progression, several other mechanisms may contribute to the acceleration of atherosclerosis in Plg−/− mice. First, a systemic up-regulation of inflammatory pathways in Plg−/− mice, triggered by widespread fibrin deposition and organ damage, may contribute to the local deposition or activity of inflammatory cells at the vessel wall. Second, atherosclerotic lesion development might be accelerated in Plg−/− mice as a result of reduced HDL cholesterol levels and a subsequent decrease in reverse cholesterol transport. However, this is probably not a major factor in accelerated lesion progression based on the observation that HDL cholesterol is rendered almost undetectable in ApoE−/− mice that also lack ApoAI, yet lesion development in ApoE−/−/ApoAI−/− and ApoE−/− mice is not significantly different (29). A final potential mechanism whereby Plg deficiency might promote atherosclerosis is through the loss of plasmin-mediated TGF-β activation in the vessel wall. Active TGF-β is an inhibitor of SMC migration and proliferation (30), and a reduction of TGF-β activation in the vessel wall in the absence of plasmin might unleash SMC proliferation, inflammation, and intimal thickening. This appealing hypothesis has been proposed to explain the proatherogenic phenotype of transgenic mice expressing Apo(a) (11, 12). A pathway linking Apo(a), Plg activation, and latent TGF-β activation, also provides an attractive explanation for the atherogenic risk previously associated with high plasma levels of lipoprotein (a) (31).
Although the loss of Plg appears to favor atherosclerotic disease, this may be despite possible impediments to lesion progression presented by Plg deficiency. For example, the ability of inflammatory cells and SMC to proteolytically dissect their way through the extracellular matrix, particularly fibrin-rich matrices, could be significantly hampered in the absence of plasmin-mediated proteolysis. This scenario would be analogous to the impediment in keratinocyte migration observed within wound fields in Plg-deficient mice, which can be ameliorated by fibrinogen deficiency (8). A similar impediment in SMC migration might be anticipated in Plg−/− mice based on the pattern of expression of fibrinolytic factors in intimal lesions (24, 25).
Another consequence of Plg deficiency that might reduce atherogenic potential is the loss of biologically active FDPs. Specific plasmin-generated FDPs have been reported to have a variety of biological properties, including the ability to stimulate cell proliferation, chemotaxis, and angiogenesis (3). FDPs can be readily detected and extracted from human atherosclerotic lesions, and some of these atheroma-derived fibrin derivatives are angiogenic (2, 3). The biological importance of FDPs in vessel wall disease has yet to be clearly established in vivo, but the development of complex atherosclerotic plaques in ApoE−/−/Plg−/− mice suggests that plasmin-derived FDPs are not essential for lesion progression.
Defining the impact of fibrin(ogen)-deficiency (32) on atherosclerosis in ApoE−/− and ApoE−/−/Plg−/− mice will be useful in distinguishing between a fibrinolytic and nonfibrinolytic basis for accelerated lesion development in Plg-deficient animals. If the impact of Plg deficiency on lesion development is primarily a consequence of impaired fibrinolysis or the loss of plasmin-generated FDPs, then one might anticipate that lesion development would be slowed in ApoE−/−/Plg−/− mice by eliminating fibrinogen. However, if the impact of Plg deficiency is primarily related to a nonfibrinolytic activity, such as plasmin activation of latent TGF-β, then the loss of fibrinogen would not be expected to slow atherosclerosis in ApoE−/−/Plg−/− mice. The increasing availability of mice with deficits in coagulation and fibrinolytic factors, cytokines, growth factors, matrix components, and cell adhesion molecules should be useful in defining the interplay between these factors in both atherosclerosis and restenosis.
Acknowledgments
We thank Avinne Overton, Kathleen Ware, Kathy Saalfeld, and Pamela Groen for their technical assistance. We also thank Susan Wert and Sherri Profitt for their help with histological and morphometric analyses. Finally, we are very grateful to Keith Kombrinck for critically reading the manuscript. This work was supported by a grant from the National Institutes of Health (HL47826).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: Plg, plasminogen; uPA, urokinase-type Plg activator; PAI-1, Plg activator inhibitor 1; FDPs, fibrin degradation products; ApoE, apolipoprotein E; Apo(a), apolipoprotein (a); SMC, smooth muscle cells; TGF-β, transforming growth factor-β; HDL, high density lipoprotein.
References
- 1.Rokitansky C. A Manual of Pathological Anatomy. IV. London: Sydenham Society; 1852. , Part II, pp. 261–275. [Google Scholar]
- 2.Bini A, Kudryk B J. Ann NY Acad Sci. 1995;748:461–471. doi: 10.1111/j.1749-6632.1994.tb17342.x. [DOI] [PubMed] [Google Scholar]
- 3.Smith E B. Thromb Res. 1994;75:329–335. doi: 10.1016/0049-3848(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 4.Fuster V, Badimon L, Badimon J J, Chesebro J H. N Engl J Med. 1992;326:242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz C J, Valente A J, Kelley J L, Sprague E A, Edwards E H. Semin Thromb Hemostasis. 1988;14:189–195. doi: 10.1055/s-2007-1002775. [DOI] [PubMed] [Google Scholar]
- 6.Naito M, Funaki C, Hayashi T, Yamada K, Asai K, Yoshimine N, Kuzuya F. Atherosclerosis (Dallas) 1992;96:227–234. doi: 10.1016/0021-9150(92)90069-s. [DOI] [PubMed] [Google Scholar]
- 7.Stirk C M, Kochhar A, Smith E B, Thompson W D. Atherosclerosis (Dallas) 1993;103:159–169. doi: 10.1016/0021-9150(93)90259-w. [DOI] [PubMed] [Google Scholar]
- 8.Bugge T H, Kombrinck K W, Flick M J, Daugherty C C, Danton M J, Degen J L. Cell. 1996;87:709–719. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- 9.Odekon L E, Blasi F, Rifkin D B. J Cell Physiol. 1994;158:398–407. doi: 10.1002/jcp.1041580303. [DOI] [PubMed] [Google Scholar]
- 10.Werb Z, Mainardi C L, Vater C A, Harris E D., Jr N Engl J Med. 1977;296:1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- 11.Grainger D J, Kemp P R, Liu A C, Lawn R M, Metcalfe J C. Nature (London) 1994;370:460–462. doi: 10.1038/370460a0. [DOI] [PubMed] [Google Scholar]
- 12.Lawn R M, Wade D P, Hammer R E, Chiesa G, Verstuyft J G, Rubin E M. Nature (London) 1992;360:670–672. doi: 10.1038/360670a0. [DOI] [PubMed] [Google Scholar]
- 13.Plump A S, Smith J D, Hayek T, Aalto-Setala K, Walsh A, Verstuyft J G, Rubin E M, Breslow J L. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S H, Reddick R L, Piedrahita J A, Maeda N. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 15.Horiuchi K, Tajima S, Menju M, Yamamoto A. J Biochem (Tokyo) 1989;106:98–103. doi: 10.1093/oxfordjournals.jbchem.a122828. [DOI] [PubMed] [Google Scholar]
- 16.Paigen B, Morrow A, Holmes P A, Mitchell D, Williams R A. Atherosclerosis (Dallas) 1987;68:231–240. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- 17.Witte D P, Aronow B J, Stauderman M L, Stuart W D, Clay M A, Gruppo R A, Jenkins S H, Harmony J A. Am J Pathol. 1993;143:763–773. [PMC free article] [PubMed] [Google Scholar]
- 18.Belin D, Vassalli J D, Combepine C, Godeau F, Nagamine Y, Reich E, Kocher H P, Duvoisin R M. Eur J Biochem. 1985;148:225–232. doi: 10.1111/j.1432-1033.1985.tb08829.x. [DOI] [PubMed] [Google Scholar]
- 19.Prendergast G C, Diamond L E, Dahl D, Cole M D. Mol Cell Biol. 1990;10:1265–1269. doi: 10.1128/mcb.10.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bugge T H, Flick M J, Daugherty C C, Degen J L. Genes Dev. 1995;9:794–807. doi: 10.1101/gad.9.7.794. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima Y, Plump A S, Raines E W, Breslow J L, Ross R. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 22.Reddick R L, Zhang S H, Maeda N. Arterioscler Thromb. 1994;14:141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- 23.Breslow J L. Science. 1996;272:685–688. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- 24.Lupu F, Heim D A, Bachmann F, Hurni M, Kakkar V V, Kruithof E K. Arterioscler Thromb Vasc Biol. 1995;15:1444–1455. doi: 10.1161/01.atv.15.9.1444. [DOI] [PubMed] [Google Scholar]
- 25.Schneiderman J, Sawdey M S, Keeton M R, Bordin G M, Bernstein E F, Dilley R B, Loskutoff D J. Proc Natl Acad Sci USA. 1992;89:6998–7002. doi: 10.1073/pnas.89.15.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Languino L R, Plescia J, Duperray A, Brian A A, Plow E F, Geltosky J E, Altieri D C. Cell. 1993;73:1423–1434. doi: 10.1016/0092-8674(93)90367-y. [DOI] [PubMed] [Google Scholar]
- 27.Sriramarao P, Languino L R, Altieri D C. Blood. 1996;88:3416–3423. [PubMed] [Google Scholar]
- 28.Kirchhofer D, Riederer M A, Baumgartner H R. Blood. 1997;89:1270–1278. [PubMed] [Google Scholar]
- 29.Zhang S H, Reddick R L, Avdievich E, Surles L K, Jones R G, Reynolds J B, Quarfordt S H, Maeda N. J Clin Invest. 1997;99:2858–2866. doi: 10.1172/JCI119479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grainger D J, Kirschenlohr H L, Metcalfe J C, Weissberg P L, Wade D P, Lawn R M. Science. 1993;260:1655–1658. doi: 10.1126/science.8503012. [DOI] [PubMed] [Google Scholar]
- 31.Howard G C, Pizzo S V. Lab Invest. 1993;69:373–386. [PubMed] [Google Scholar]
- 32.Suh T T, Holmbäck K, Jensen N J, Daugherty C C, Small K, Simon D I, Potter S, Degen J L. Genes Dev. 1995;9:2020–2033. doi: 10.1101/gad.9.16.2020. [DOI] [PubMed] [Google Scholar]