Abstract
In the cycling human endometrium, the expression of interstitial collagenase (MMP-1) and of several related matrix metalloproteinases (MMPs) follows the late-secretory fall in sex steroid plasma concentrations and is thought to be a critical step leading to menstruation. The rapid and extensive lysis of interstitial matrix that precedes menstrual shedding requires a strict control of these proteinases. However, the mechanism by which ovarian steroids regulate endometrial MMPs remains unclear. We report here that, in the absence of ovarian steroids, MMP-1 expression in endometrial fibroblasts is markedly stimulated by medium conditioned by endometrial epithelial cells. This stimulation can be prevented by antibodies directed against interleukin 1α (IL-1α) but not against several other cytokines. Ovarian steroids inhibit the release of IL-1α and repress MMP-1 production by IL-1α-stimulated fibroblasts. In short-term cultures of endometrial explants obtained throughout the menstrual cycle, the release of both IL-1α and MMP-1 is essentially limited to the perimenstrual phase. We conclude that epithelium-derived IL-1α is the key paracrine inducer of MMP-1 in endometrial fibroblasts. However, MMP-1 production in the human endometrium is ultimately blocked by ovarian steroids, which act both upstream and downstream of IL-1α, thereby exerting an effective control via a “double-block” mechanism.
Keywords: extracellular matrix, matrix metalloproteinases, menstruation, epithelial cells
The endometrium is the only human tissue that undergoes extensive cyclic degradation and renewal. Throughout the reproductive years, endometrial tissue proliferates under the influence of estrogens during the follicular phase and differentiates during most of the secretory phase as a result of high levels of progesterone. If no pregnancy occurs, the late-secretory fall of sex steroid plasma concentrations initiates a cascade of events that results in the demarcation of the functional layer of the endometrium and its shedding together with menstrual bleeding (1).
Matrix metalloproteinases constitute a family of enzymes that together can degrade most extracellular matrix proteins (2, 3). Recent work strongly indicates that MMPs are involved in the cyclic alterations of endometrial tissue that occur during the reproductive years (4–11).
Three lines of evidence suggest that MMP-1 plays a crucial role in the lysis of interstitial matrix that initiates menstruation. (i) MMP-1 expression in the endometrium is strictly confined to the perimenstrual phase (5, 11). (ii) It is limited to fibroblasts of the functional layer, where its focal expression matches remarkably well with areas of matrix breakdown. Furthermore, the prominent MMP-1 expression at the periphery of shed fragments marks the planes of tissue cleavage (8). (iii) Whereas culturing endometrial explants in the absence of ovarian steroids leads to MMP-1 expression and extensive tissue lysis, selective pharmacological inhibition of MMPs is sufficient to fully preserve the tissue integrity of such explants (12).
Suppression of MMP-1 expression in endometrial explants by the addition of physiological concentrations of 17β-estradiol and progesterone provides a good explanation why in vivo MMP-1 is exclusively detected just before and during the menstrual phase [i.e., when sex steroid concentrations are lowest (4)]. Furthermore, foci of MMP-1 expression and menstrual breakdown show a clear inverse relation with the occurrence of sex steroid receptors. In areas where endometrial glands are devoid of progesterone receptors, the periglandular distribution of MMP-1-producing cells is particularly striking (8, 13). This observation and the fact that not all stromal cells without detectable estrogen and progesterone receptors express MMP-1 suggest a paracrine interaction between epithelium and adjacent stromal cells.
We have used cultures of epithelial and stromal cells isolated from human endometrium to explore the contribution of paracrine interactions in the regulation of MMP-1 expression by ovarian steroids. Epithelium-derived interleukin 1α (IL-1α) was identified as the key paracrine stimulator of MMP-1 production in endometrial fibroblasts in the absence of ovarian steroids. This stimulation was abolished by physiological concentrations of ovarian steroids. The in vivo relevance of these findings was further supported by an extensive release of IL-1α from short-term cultures of endometrial tissue explants, which occurred only when tissues had been sampled around the menstrual phase.
MATERIALS AND METHODS
Cultures of Endometrial Cells and Tissue Explants.
Essentially pure fibroblasts and highly enriched epithelial cells were isolated from endometrial tissues using techniques previously described (14, 15). In brief, fresh endometrial biopsies were washed in PBS and digested with 400 units/ml of Collagenase A (Sigma) at 37°C for 45 min. Epithelial glands and fibroblasts were then separated by sequential filtration through nylon meshes with pore sizes of 300 μm and 35 μm, respectively. Fibroblasts were directly plated into culture flasks and washed with PBS after 1 h. Epithelial glands were allowed to sediment twice before trypsin treatment, and the resulting suspension of dissociated epithelial cells was plated either in gelatin-coated Millicell-CM inserts (Millipore) or directly in cell culture plates, with comparable results. Purity of cell populations was assessed by immunolabeling of vimentin and cytokeratin. Separated cocultures were allowed to exchange soluble constituents by placing inserts containing epithelial cells on cell culture plates containing confluent monolayers of fibroblasts. Endometrial tissue explants obtained throughout the menstrual cycle from patients with non-endometrial pathologies were histologically dated and cultured in inserts at the interface between air with 5% CO2 and culture medium. Cells were cultured in DMEM/Ham F12 and tissue explants in DMEM. All culture media contained 100 units/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin B, and 0.3 μM 2-hydroxypropyl-β-cyclodextrin (Sigma) and were devoid of serum and phenol red. Where indicated, culture media were supplemented with 100 nM progesterone and 1 nM 17β-estradiol (referred to as “with ovarian steroids” or as “+H”), which were added in water-soluble complexes with 2-hydroxypropyl-β-cyclodextrin (Sigma). Medium conditioned by epithelial cells for 24 h [conditioned medium (CM)] was added to confluent fibroblasts either directly or after preincubation with 10 μg/ml anti-IL-1α, 10 μg/ml anti-tumor necrosis factor α (TNFα), 10 μg/ml anti-IL-1β, 10 μg/ml anti-platelet-derived growth factor B (PDGF-B), or 100 μg/ml anti-epidermal growth factor (EGF) polyclonal antibodies (R & D Systems) for 1 h at 20°C. Recombinant human IL-1α (rhIL-1α) and recombinant human IL-1 receptor antagonist (rhIL-1Ra) were purchased from R & D Systems.
Collagenase Assay.
Total collagenase activity was determined in culture supernatants activated by 2 mM aminophenylmercuric acetate for 2 h at 37°C and measured by the ability to degrade [3H]acetylated collagen in solution at 25°C. One unit of collagenase is defined as the amount of enzyme that degrades 1 μg of soluble collagen per minute (16). Antibodies directed against MMP-1 abolished measurable collagenase activities in media conditioned by endometrial explants, indicating that other collagenases such as MMP-8 and MMP-13 did not contribute to the collagenolytic activity in this assay (E.M., P.L, C.F.S., P.J.C., and Y.E., unpublished data).
RNA Extraction and RNase Protection Assay.
Total RNA was isolated as previously described (17). 32P-labeled probes were synthesized by in vitro transcription according to the manufacturer’s protocol (Promega) using linearized MMP-1 cDNA (gift from H. Nagase, Kansas University, Kansas City, KS) and 36B4 cDNA (gift from K. J. Cullen, Georgetown University, Washington, DC) as a template. Twenty micrograms of total RNA was incubated with 8 × 105 cpm of radiolabeled MMP-1 probe, 1.5 × 105 cpm of radiolabeled 36B4, and 30 μl of hybridization buffer [40 mM PIPES, 0.4 M NaCl, 0.1 M EDTA, and 80% (vol/vol) formamide] for 12 h at 50°C. Remaining single-stranded RNA was digested for 30 min at 25°C with 40 μg/ml RNase A (Sigma), after which the enzyme reaction was stopped by treatment with 0.05% (vol/vol) SDS and 0.25 μg/μl proteinase K. Proteins were subsequently removed by phenol-chloroform extraction. The remaining double-stranded RNA was precipitated with ethanol, dried, and resuspended in a loading buffer containing 80% (vol/vol) formamide. Samples were boiled for 5 min and loaded onto a 6% polyacrylamide sequencing gel. An x-ray film was exposed to the dried gel for 2–4 days. The autoradiogram was scanned and signal intensities were analyzed using nih 1.59 (National Institutes of Health) software.
IL-1α ELISA.
Human IL-1α concentrations were determined in microtiter plates by an immunoassay (R & D Systems). Optical densities were read in a Titertek Multiscan MCC 340 spectrophotometer (Flow Laboratories). The indicated values of added rhIL-1α represent effective concentrations as determined by ELISA. These concentrations required the addition of 10 times higher nominal amounts to the culture medium.
RESULTS
Epithelial Cells Induce MMP-1 Production by Endometrial Fibroblasts via IL-1α-Mediated Paracrine Stimulation.
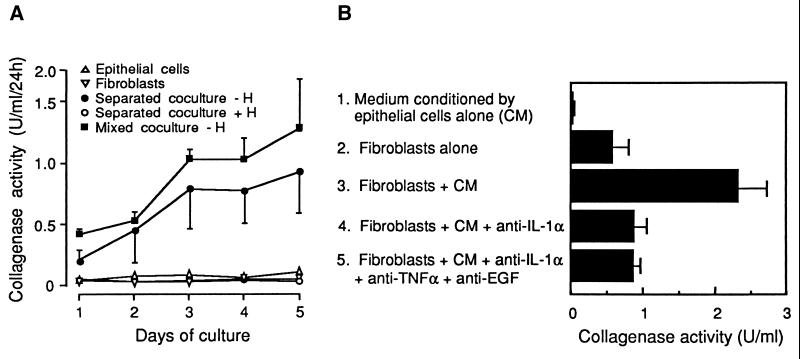
To explore paracrine interactions in the regulation of MMP-1 by ovarian steroids, human endometrial stromal and epithelial cells were purified and maintained in mono- or cocultures. Whereas little or no collagenase activity could be detected in monocultures, mixed cocultures produced considerable collagenase activities in the absence of ovarian steroids. When fibroblasts were physically separated from epithelial cells by a permeable membrane, which permitted free exchange of medium (“separated cocultures”), or when monocultures of fibroblasts were incubated with a medium that had been conditioned by epithelial cells, collagenase was similarly induced. Collagenase induction in separated cocultures was suppressed by addition of physiological concentrations of ovarian steroids (Fig. 1A).
Figure 1.
Collagenase production by endometrial fibroblasts depends on paracrine interactions and is repressed by ovarian steroids. (A) Endometrial epithelial cells (▵) and fibroblasts (▿) were maintained either in monocultures, in mixed cocultures (▪), or in cocultures separated by a permeable membrane. The latter were treated (○, +H) or not (•, −H) by 1 nM 17β-estradiol and 100 nM progesterone. Supernatants were collected daily and replaced by fresh medium. (B) Confluent endometrial fibroblasts were cultured either with fresh medium (lane 2); with medium conditioned by epithelial cells for 24 h (CM, lane 3); with CM that had been preincubated with either 10 μg/ml polyclonal anti-IL-1α antibodies (lane 4), or with a mixture of 10 μg/ml anti-IL-1α, 10 μg/ml anti-TNFα, and 100 μg/ml anti-EGF polyclonal antibodies (lane 5). Collagenase activity was measured in culture supernatants after 24 h. Both A and B are representative of three experiments performed in triplicate on cells obtained by endometrial biopsies from different patients. Values are means ± SEM (n = 3).
The capacity of media conditioned by epithelial cells to induce the production of interstitial collagenase by stromal cells was decreased by more than 80% after preincubation with blocking antibodies against IL-1α, a cytokine expressed in the human endometrium (18). By contrast, antibodies against tumor necrosis factor α, EGF, PDGF-B, or IL-1β, which are cytokines that can stimulate MMP-1 production in cultured fibroblasts or endometrial stromal cells (refs. 19–24; data not shown), were unable to significantly affect MMP-1 induction by epithelial cell-conditioned media (data not shown). Combination of blocking antibodies against IL-1α, tumor necrosis factor α, and EGF did not decrease MMP-1 production further than neutralization of IL-1α alone (Fig. 1B), arguing against a synergistic action of these cytokines.
Regulation of MMP-1 Expression in Endometrial Fibroblasts by IL-1α and Ovarian Steroids.
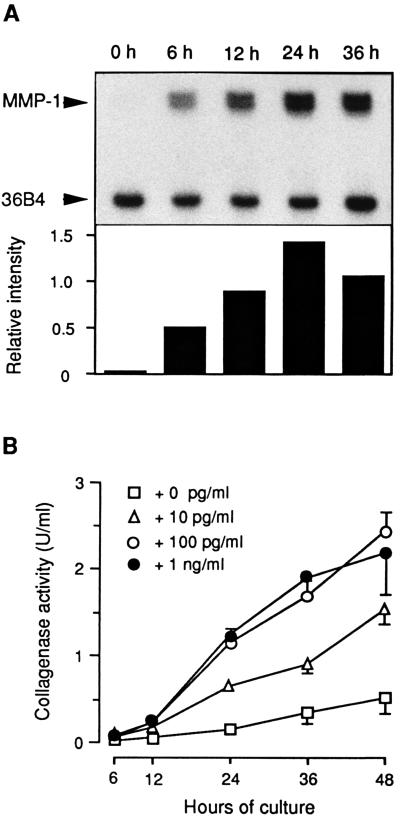
The time course of MMP-1 mRNA and protein expression in response to rhIL-1α was next established by RNase protection assays and by enzyme assays. MMP-1 mRNA expression in endometrial fibroblasts began within 6 h and leveled off after 24 h of exposure to rhIL-1α (Fig. 2A). The rise in enzyme secretion was delayed by 6–12 h. Maximal response was achieved at rhIL-1α concentrations between 10 and 100 pg/ml (Fig. 2B); comparable collagenase activities were elicited by media conditioned by epithelial cells (Fig. 1B). The rhIL-1α-induced MMP-1 production was inhibited by the addition of rhIL-1Ra (25), indicating that the signal was transmitted through the type I IL-1 receptor (data not shown).
Figure 2.
MMP-1 expression in endometrial fibroblasts stimulated by rhIL-1α. (A) Confluent fibroblast monolayers were cultured in the presence of 1 ng/ml rhIL-1α, and total cellular RNA was extracted after the indicated intervals. Twenty micrograms of total RNA were subjected to an RNase protection assay using riboprobes generated from MMP-1 and from 36B4 cDNAs. MMP-1 mRNA expression levels were normalized to 36B4 gene expression as loading control. (B) Cumulative collagenase activity was measured at increasing intervals of culture with the indicated effective concentrations of rhIL-1α. Values are means ± SEM (n = 3).
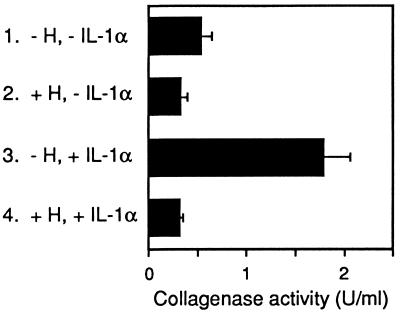
We then investigated whether physiological concentrations of ovarian steroids could suppress MMP-1 production in fibroblasts despite ongoing stimulation by IL-1α. In IL-1α-stimulated fibroblasts, addition of 1 nM 17β-estradiol and 100 nM progesterone to the culture medium essentially suppressed MMP-1 production (Fig. 3, lane 4) when compared with fibroblasts that were maintained in the presence of IL-1α alone (Fig. 3, lane 3). In the presence or absence of IL-1α, the addition of ovarian steroids further inhibited MMP-1 production to levels lower than in nonstimulated fibroblasts (Fig. 3, lanes 2 and 4 versus lane 1; P < 0.05, Student’s t test).
Figure 3.
Ovarian steroids suppress MMP-1 production in rhIL-1α- stimulated fibroblasts. Confluent endometrial fibroblasts were cultured either without (lanes 1 and 2) or with (lanes 3 and 4) 1 ng/ml rhIL-1α, and in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of 1 nM 17β-estradiol and 100 nM progesterone. Collagenase activity was measured in culture supernatants after 24 h. Values are representative of four experiments on cells from different patients and are means ± SEM (n = 3).
IL-1α Release from Endometrial Explants Correlates with MMP-1 Production and Is Inhibited by Ovarian Steroids.
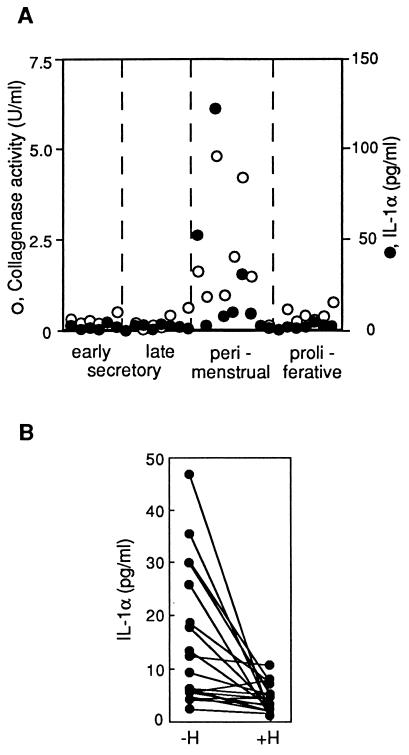
During the first 24 h of culture, endometrial explants closely resemble endometrial tissue in situ, both with respect to preservation of tissue integrity and to MMP-1 expression (11, 12). To analyze IL-1α release along the menstrual cycle, its concentration was measured in conditioned media collected after the first day of culture of endometrial explants from biopsies sampled throughout the cycle. In such media, IL-1α was barely detectable from the early proliferative to the mid-secretory phase. By contrast, samples obtained during the late-secretory and the menstrual phase released large amounts of IL-1α (Fig. 4A), and the resulting IL-1α concentrations correlated with collagenase activity in the corresponding media (r = 0.83, P < 0.005). High amounts of IL-1α were also frequently released when endometrial explants obtained from the proliferative to the mid-secretory phase were cultured for ≥48 h without added ovarian steroids. This enhanced IL-1α release was abolished if ovarian steroids had been added to the culture (Fig. 4B), as was the secretion of MMP-1 (data not shown), thereby being in agreement with previous reports (4, 7, 11).
Figure 4.
IL-1α release from endometrial explants during the menstrual cycle and its repression by ovarian steroids. (A) Biopsies were collected from 30 women at the indicated phases of the menstrual cycle, and an average of 9 (±4) groups of 6 explants from each biopsy were cultured for 24 h in the absence of ovarian steroids. Collagenase activity (○) and IL-1α concentration (•) were measured in pooled conditioned media after 24 h. (B) Explants from nonperimenstrual biopsies were cultured in parallel in the absence (−H) or presence (+H) of 1 nM 17β-estradiol and 100 nM progesterone, and IL-1α concentrations were measured in media conditioned during the second day of culture. Values are means of two measurements per condition with <12% variation.
DISCUSSION
In the cycling human endometrium, synthesis and degradation of extracellular matrix essentially alternate. This is in contrast to other tissues, where both processes take place concomitantly and where matrix steady-state is determined by their balance. The alternance of synthesis and degradation in the endometrium is exemplified by the pattern of MMP-1 expression. This proteinase is absent during the proliferative and most of the secretory phase, when interstitial matrix is synthesized. Just before and during the ensuing menstrual phase, the abrupt and strong expression of MMP-1 is thought to initiate the disruption of fibrillar matrix that leads to menstruation (4). This all-or-none response of the human endometrium provides a particularly convenient model to study the physiological regulation of extracellular matrix breakdown.
In this report, mono- and cocultures of endometrial fibroblasts and epithelial cells were used to analyze how the production of MMP-1 in endometrial fibroblasts is controlled by ovarian steroids. In the absence of 17β-estradiol and progesterone, MMP-1 expression in endometrial fibroblasts depended on paracrine stimulation from epithelial cells, and IL-1α could be identified as the key physiological inducer. Other cytokines, such as tumor necrosis factor α, PDGF-B, EGF, or IL-1β, were not found to participate in the paracrine regulation of MMP-1. Recently, epithelium-derived IL-1α has also been reported to induce MMP-1 production in corneal fibroblasts (26).
Admittedly, a two-cell-type coculture is a simplified model to investigate complex interactions in a tissue. It does not, for example, take into consideration cytokines released from blood-derived inflammatory cells (27) nor the contribution of factors present in the extracellular matrix itself (28, 29). However, both the identical pattern of IL-1α and MMP-1 release after short-term cultures of explants collected throughout the cycle and the strong inhibition by ovarian steroids of IL-1α release in longer cultures of explants point to a crucial involvement of IL-1 α in vivo.
IL-1α is known to be such a potent cytokine, that the occupancy of only a few type I IL-1 receptor molecules per cell can result in considerable responses in vivo and in vitro (reviewed in ref. 30). It is, however, quite possible that autocrine signals modulate MMP-1 expression in addition to the paracrine stimulation by epithelium-derived IL-1α. For example, an extracellular autocrine IL-1α loop is required to render early-passage rabbit corneal fibroblasts competent for collagenase gene expression (31). Even intracellularly active proIL-1α has been found in human epithelial cells (32), where it might act as an intracellular messenger (33). Autocrine stimulation by stromal-cell-derived IL-1α that is exerted through an extracellular or intracellular pathway might explain the low levels of MMP-1 observed in some unstimulated fibroblast monocultures. However, the mechanism of extracellular IL-1α release as well as its putative intracellular action remain obscure (30). Alternatively, transforming growth factor type β, a growth factor released by endometrial fibroblasts in response to progesterone, can suppress the expression of matrilysin in neighboring epithelial cells (34). It is thus conceivable that fibroblast-derived transforming growth factor type β also inhibits MMP-1 secretion via an autocrine mechanism.
Ultimately, MMP-1 expression in endometrial fibroblasts is silenced by ovarian steroids, and the presence of estrogen and progesterone receptors in the endometrium is therefore a key element in this response. The expression of both receptors is up-regulated by estrogen priming during the proliferative phase and peaks around the middle of the cycle. The total concentration of both receptors then decreases during the secretory phase to reach a minimum around menstruation (35). Immunohistochemical studies have further revealed that the pattern of estrogen- and progesterone-receptor distribution among different cell types varies throughout the cycle. In particular, during the late secretory phase, progesterone receptors disappear from glandular epithelial cells but not from stromal cells (36, 37).
We have recently found an inverse relation between areas of ovarian-steroid-receptor detection and foci of MMP-1 expression in biopsies obtained during the menstrual phase. In these foci, the amount of estrogen receptors in fibroblasts and epithelial cells was low, but still detectable. Progesterone receptors were similarly decreased in stromal cells that produced MMP-1 but remarkably were not detectable in neighboring epithelial cells (8). During the perimenstrual phase, when both the plasma concentrations of ovarian steroids and the expression of their receptors in fibroblasts are minimal, the level of occupied receptors should thus fall below a threshold at which it is no longer sufficient to suppress MMP-1 production in these cells. At the same time, the disappearance of progesterone receptors and the minimal residual content of estrogen receptors in glandular epithelium should render these cells unresponsive to the low concentrations of 17β-estradiol and progesterone, thus allowing for the release of the potent MMP-1 inducer IL-1α.
Taken together, these observations suggest a powerful mechanism through which ovarian steroids silence MMP-1 expression. During the proliferative and most of the secretory phase, these steroids provide a dual block, acting both upstream by inhibiting IL-1α release from epithelial cells and downstream by directly suppressing MMP-1 production in fibroblasts. Following the decline of ovarian steroids and their receptors during the late secretory phase, IL-1α is released and quickly induces MMP-1 production via paracrine stimulation. Upon activation, this enzyme cleaves the fibrillar collagens that make up the framework of interstitial matrix, thus initiating the shedding of endometrial tissue.
Acknowledgments
We thank H. Nagase and K. J. Cullen for the gift of materials, B. Casslén for helpful advice, and N. Delflasse for technical assistance. C.F.S. is a recipient of the Haas-Teichen Postdoctoral Fellowship of the International Institute of Cellular and Molecular Pathology, Brussels, Belgium. This work was supported by the Belgian Fonds de la Recherche Scientifique Médicale, by the Fonds de Développement Scientifique of the University of Louvain, and by a grant from Ipsen-Biotech, France. This paper represents research results of the Belgian Programme on Interuniversity Poles of Attraction and of Concerted Research Actions of the “Communauté Française de Belgique.”
ABBREVIATIONS
- MMP
matrix metalloproteinase
- CM
conditioned medium
- rh IL-1α
recombinant human interleukin 1α
- EGF
epidermal growth factor
References
- 1.Johannisson E. In: Contraception and Mechanisms of Endometrial Bleeding. D’Arcangues C, Fraser I S, Newton J R, Odlind V, editors. Cambridge, U.K.: Cambridge Univ. Press; 1990. pp. 53–72. [Google Scholar]
- 2.Woessner J F., Jr FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- 3.Murphy G, Reynolds J J. In: Connective Tissue and Its Heritable Disorders. Royce P M, Steinmann B, editors. New York: Wiley–Liss; 1993. pp. 287–316. [Google Scholar]
- 4.Marbaix, E., Kokorine, I., Donnez, J., Eeckhout, Y. & Courtoy, P. J. (1996) Hum. Reprod. 11, Suppl. 2, 134–143. [DOI] [PubMed]
- 5.Hampton A L, Salamonsen L M. J Endocrinol. 1994;141:R1–R3. doi: 10.1677/joe.0.141r001. [DOI] [PubMed] [Google Scholar]
- 6.Hulboy D L, Rudolph L A, Matrisian L M. Mol Hum Reprod. 1997;3:27–45. doi: 10.1093/molehr/3.1.27. [DOI] [PubMed] [Google Scholar]
- 7.Marbaix E, Donnez J, Courtoy P J, Eeckhout Y. Proc Natl Acad Sci USA. 1992;89:11789–11793. doi: 10.1073/pnas.89.24.11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kokorine I, Marbaix E, Henriet P, Okada Y, Donnez J, Eeckhout Y, Courtoy P J. J Cell Sci. 1996;109:2151–2160. doi: 10.1242/jcs.109.8.2151. [DOI] [PubMed] [Google Scholar]
- 9.Osteen K G, Rodgers W H, Gaire M, Hargrove J T, Gorstein F, Matrisian L M. Proc Natl Acad Sci USA. 1994;91:10129–10133. doi: 10.1073/pnas.91.21.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodgers W H, Matrisian L M, Giudice L C, Dsupin B, Cannon P, Svitek C, Gorstein F, Osteen K G. J Clin Invest. 1994;94:946–953. doi: 10.1172/JCI117461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marbaix E, Kokorine I, Henriet P, Donnez J, Courtoy P J, Eeckhout Y. Biochem J. 1995;305:1027–1030. doi: 10.1042/bj3051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marbaix E, Kokorine I, Moulin P, Donnez J, Eeckhout Y, Courtoy P J. Proc Natl Acad Sci USA. 1996;93:9120–9125. doi: 10.1073/pnas.93.17.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokorine I, Nisolle M, Donnez J, Eeckhout Y, Courtoy P J, Marbaix E. Fertil Steril. 1997;68:246–251. doi: 10.1016/s0015-0282(97)81510-5. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Rees M C P, Bicknell R. J Cell Sci. 1995;108:323–331. doi: 10.1242/jcs.108.1.323. [DOI] [PubMed] [Google Scholar]
- 15.Cherny R A, Findlay J K. Biol Reprod. 1990;43:241–250. doi: 10.1095/biolreprod43.2.241. [DOI] [PubMed] [Google Scholar]
- 16.Eeckhout Y, Delaissé J M, Vaes G. Biochem J. 1986;239:793–796. doi: 10.1042/bj2390793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald R J, Swift G H, Przybyla A E, Chirgwin J M. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- 18.Tabibzadeh S, Sun X Z. Hum Reprod. 1992;7:1214–1221. doi: 10.1093/oxfordjournals.humrep.a137829. [DOI] [PubMed] [Google Scholar]
- 19.Rawdanowicz T J, Hampton A L, Nagase H, Woolley D E, Salamonsen L A. J Clin Endocrinol Metab. 1994;79:530–536. doi: 10.1210/jcem.79.2.8045973. [DOI] [PubMed] [Google Scholar]
- 20.Hanemaaijer R, Koolwijk P, le Clercq L, de Vree W J A, van Hinsbergh V W M. Biochem J. 1993;296:803–809. doi: 10.1042/bj2960803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan E M, Qin H, Kennedy S H, Rouda S, Fox J W, IV, Moore J H. Biochem J. 1995;310:585–588. doi: 10.1042/bj3100585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiraoka K, Sasaguri Y, Komiya S, Inoue A, Morimatsu M. Biochem Int. 1992;27:1083–1091. [PubMed] [Google Scholar]
- 23.Tewari M, Tuncay O C, Milchman A, Reddy P J, Reddy C D, Cressman D E, Taub R, Newton R C, Tewari D S. Arch Oral Biol. 1996;41:461–468. doi: 10.1016/0003-9969(96)00148-3. [DOI] [PubMed] [Google Scholar]
- 24.Ohshima M, Otsuka K, Suzuki K. J Periodontal Res. 1994;29:421–429. doi: 10.1111/j.1600-0765.1994.tb01244.x. [DOI] [PubMed] [Google Scholar]
- 25.Burger D, Chicheportiche R, Giri J G, Dayer J-M. J Clin Invest. 1995;96:38–41. doi: 10.1172/JCI118045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strissel K J, Rinehart W B, Fini E M. Invest Opthalmol Vis Sci. 1997;38:546–552. [PubMed] [Google Scholar]
- 27.Richards C D, Shoyab M, Brown T J, Gauldie J. J Immunol. 1993;150:5596–5603. [PubMed] [Google Scholar]
- 28.Tremble P M, Lane T F, Sage H E, Werb Z. J Cell Biol. 1993;121:1433–1444. doi: 10.1083/jcb.121.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huttenlocher A, Werb Z, Tremble P, Huhtala P, Rosenberg L, Damsky C H. Matrix Biol. 1996;15:239–250. doi: 10.1016/s0945-053x(96)90115-8. [DOI] [PubMed] [Google Scholar]
- 30.Dinarello C A. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 31.West-Mays J A, Strissel K J, Sadow P M, Fini M E. Proc Natl Acad Sci USA. 1995;92:6768–6772. doi: 10.1073/pnas.92.15.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hauser C, Saurat J-H, Schmitt A, Jaunin F, Dayer J-M. J Immunol. 1986;136:3317–3323. [PubMed] [Google Scholar]
- 33.Maier J A M, Voulalas P, Roeder D, Maciag T. Science. 1990;249:1570–1574. doi: 10.1126/science.2218499. [DOI] [PubMed] [Google Scholar]
- 34.Bruner K L, Rodgers W H, Gold L I, Korc M, Hargrove J T, Matrisian L M, Osteen K G. Proc Natl Acad Sci USA. 1995;92:7362–7366. doi: 10.1073/pnas.92.16.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coppens M T, Dhont M A, De Boever J G, Serreyn R F, Vandekerckhove D A, Roels H J. Histochemistry. 1993;99:121–126. doi: 10.1007/BF00571872. [DOI] [PubMed] [Google Scholar]
- 36.Nisolle M, Casanas-Roux F, Wyns C, de Menten Y, Mathieu P E, Donnez J. Fertil Steril. 1994;62:751–759. doi: 10.1016/s0015-0282(16)57000-9. [DOI] [PubMed] [Google Scholar]
- 37.Critchley H O D, Abberton K M, Taylor N H, Healy D, Rodgers P A W. Br J Obstet Gynaecol. 1994;101:428–434. doi: 10.1111/j.1471-0528.1994.tb11917.x. [DOI] [PubMed] [Google Scholar]