Abstract
Circulating autoantibodies to phospholipids (aPLs), such as cardiolipin (CL), are found in patients with antiphospholipid antibody syndrome (APS). We recently demonstrated that many aPLs bound to CL only after it had been oxidized (OxCL), but not to a reduced CL analogue that could not undergo oxidation. We now show that the neoepitopes recognized by some aPLs consist of adducts formed between breakdown products of oxidized phospholipid and associated proteins, such as β2 glycoprotein 1 (β2GP1). Addition of human β2GP1, polylysine, native low-density lipoprotein, or apolipoprotein AI to OxCL-coated wells increased the anticardiolipin antibody (aCL) binding from APS sera that first had been diluted so that no aCL binding to OxCL could be detected. No increase in aCL binding was observed when these proteins were added to wells coated with reduced CL. The ability of β2GP1, polylysine, or low-density lipoprotein to be a “cofactor” for aCL binding to OxCL was greatly reduced when the proteins were methylated. Incubation of β2GP1 with oxidized 1-palmitoyl-2-linoleyl-[1-14C]-phosphatidylcholine (PC), but not with dipalmitoyl-[1-14C]-PC, led to formation of covalent adducts with β2GP1 recognized by APS sera. These data suggest that the reactive groups of OxCL, such as aldehydes generated during the decomposition of oxidized polyunsaturated fatty acids, form covalent adducts with β2GP1 (and other proteins) and that these are epitopes for aCLs. Knowledge that the epitopes recognized by many aPLs are adducts of oxidized phospholipid and associated proteins, including β2GP1, may give new insights into the pathogenic events underlying the clinical manifestations of APS.
The antiphospholipid antibody syndrome (APS) is characterized by the presence of clinical features such as venous or arterial thrombosis, fetal loss, autoimmune thrombocytopenia, and circulating antiphospholipid antibodies (aPLs) (1–5). aPLs are a heterogeneous group of autoantibodies so named because they bind in vitro to phospholipid (PL) or PL-containing moieties, but the exact nature of the epitope(s) recognized by aPLs remains uncertain.
Studies by McNeil et al. (6) and Galli et al. (7) first demonstrated the apparent requirement of a plasma “cofactor” for the binding of some anticardiolipin antibodies (aCLs) in solid-phase immunoassays. This cofactor, present on circulating lipoproteins, was identified as apolipoprotein (apo) H or β2 glycoprotein 1 (β2GP1) (8), which binds avidly to anionic PLs, such as phosphatidylserine and phosphatidylinositol, but less well to phosphatidylcholine (PC) or phosphatidylethanolamine (9). A highly charged sequence, KNKEKK, present in the fifth complement control protein domain of β2GP1 (9), as well as two other lysine-rich sites in this domain (10), have been suggested to be responsible for the binding to anionic PL. Rauch, Janoff, and colleagues proposed that β2GP1-induced changes in the three-dimensional structure of cardiolipin (CL) were key to the PL functioning as an antigen in conventional aCL immunoassays and to the immunogenicity of the PL in vivo (11–13). Others also have shown that binding of some aCLs is dependent on interactions between PL and PL-binding proteins (14–17). These data have been interpreted to indicate that as a result of noncovalent protein-lipid interactions, novel, conformational epitopes are created on CL, on β2GP1, or on an admixture of these two, and that aCLs are directed against one or more of these epitopes. In addition, some investigators have suggested that β2GP1 alone is the target antigen for aCLs (7, 15–19).
Recently, we demonstrated that CL was rapidly oxidized when plated on microtiter wells and exposed to air (the standard conditions for solid-phase aCL immunoassays) (20). We also demonstrated that both intact reference APS sera, as well as affinity-purified aCL-IgG from APS patients, bound to OxCL, but did not bind at all to a “reduced” CL analogue that was unable to undergo lipid peroxidation (all unsaturated fatty acids in reduced CL had been hydrogenated to saturated fatty acids) (20). We concluded that many aCLs bind to neoepitopes generated when phospholipids undergo oxidation. Our data also showed increased binding of a mAb against malondialdehyde-lysine to OxCL, and therefore strongly suggested that at least some of the epitopes generated on the microtiter wells were, in fact, adducts formed between degradation products of polyunsaturated fatty acid oxidation (e.g., malondialdehyde) and associated protein (BSA in our assays).
In this paper we have expanded our investigation of the nature of the neoepitopes recognized by aPLs by focusing on the possible role of cofactor proteins. Because we already knew that aPL binding was dependent on oxidation of the PL, we wanted to examine if the epitopes for aPLs were generated when breakdown products of OxPL covalently modified lysine residues of associated proteins, such as β2GP1. This kind of protein modification would be very similar to that which occurs during the oxidative modification of low-density lipoprotein (LDL), in which a variety of highly reactive breakdown products of polyunsaturated fatty acid are formed, such as malondialdehyde and 4-hydroxynonenal, which then can form Schiff bases and Michael adducts with lysine residues of LDL apoB (21–23), as well as crosslinks with other proteins and lipids. A large number of different lipid-protein and lipid-lipid adducts could form. We (24, 25) and others (26–29) have shown earlier that such epitopes are generated when LDL is oxidatively modified and exist in vivo in atherosclerotic lesions. Also, these epitopes are highly immunogenic (20, 23–25, 30–32). Because β2GP1 binds avidly to phospholipids, the ɛ-amino groups of its lysine residues also could be covalently modified when the associated lipid is oxidized. These covalent adducts could be immunogens and the antibodies generated would recognize OxCL-β2GP1 adducts but not “native” CL or β2GP1. The results presented in this paper demonstrate that adduct formation between lysine residues of β2GP1 (and other proteins) and OxCL is involved in the epitope formation of aPLs found in reference APS sera.
METHODS
Materials.
Cardiolipin (bovine heart), hydrogenated (“reduced”) cardiolipin, 1,2-dipalmitoyl-PC, 1,2-dioleoyl-PC, 1,2-dilinoleyl-PC, and 1,2-diarachidonoyl-PC were obtained from Avanti Polar Lipids. Fatty acid analysis confirmed that linoleic acid accounted for 91–92% of the fatty acids of CL, whereas all fatty acids in reduced CL were saturated (20). l-α-dipalmitoyl-[1-14C]-PC and l-α-1-palmitoyl-2-linoleyl-[1-14C]-PC were obtained from Andotek Life Sciences (Irvine, CA). Human purified β2GP1 was obtained from Crystal Chem (Chicago, IL) and polylysine from Sigma. Purified apoAI was a gift from C. Banka (Scripps Research Institute). Goat anti-human-β2GP1 antiserum was from Enzyme Research Laboratories (South Bend, IN), and LumiPhos 530 was from Lumigen.
Human Subjects.
Reference sera from women with APS were collected at the Department of Obstetrics and Gynecology of the University of Utah Hospital as described (20). Patients with APS had one or more of the following clinical features: (i) a history of either one or more fetal deaths or ≥ three consecutive pregnancy losses, (ii) venous or arterial thrombosis, or (iii) autoimmune thrombocytopenia (1, 2, 4). In addition, all patients had both lupus anticoagulant and medium to high positive levels of IgG aCLs (20).
Chemiluminescent Immunoassay for Antibody Binding to CL.
The chemiluminescent assay was performed as previously described in detail (20). In brief, CL or reduced CL (40 μg/ml in 100% ethanol) was added at 25 μl per well to 96-well white round-bottomed MicroFluor (Dynatec) microtitration plates, dried under nitrogen, and exposed to air for the indicated time at room temperature. Absolute ethanol was added to blank wells. Human sera were diluted with Tris-buffered saline (TBS) buffer containing 3% BSA, 0.27 mM EDTA, 0.02% NaN3, and 20 μM butylated hydroxytoluene, and incubated in the wells for 1 hr. β2GP1 or methylated β2GP1 were added directly into the serum samples or incubated in the PL-coated wells for 1 hr before addition of the sera. Polylysine and LDL (in TBS buffer) were added to PL-coated wells for 10 min before addition of the sera. A 1:500 dilution of human sera was selected because it was shown in preliminary experiments to have minimal binding to OxCL. The amount of antibody bound was measured with alkaline phosphatase-labeled goat anti-human-IgG (Sigma). Protein A purified goat anti-human-β2GP1 antiserum was labeled with alkaline-phosphatase and used to measure the amount of β2GP1 or methylated β2GP1 bound. The goat anti-human-β2GP1 antibody did not react with CL or reduced CL (not shown). The amount of antibody bound was quantified using a chemiluminescent technique (20).
Methylation of β2GP1, Polylysine, and LDL.
β2GP1, polylysine, and LDL were methylated as described (33). The extent of methylation, determined by the trinitrobenzenesulfonic acid method (34), showed that for β2GP1, polylysine, and LDL nearly 100%, 88.5%, and 73% of available lysines were methylated, respectively.
Binding of β2GP1 and LDL to 14C-Labeled PL.
l-α-dipalmitoyl-[1-14C]-PC and l-α-1-palmitoyl-2-linoleyl-[1-14C]-PC were dried under nitrogen and exposed to air at room temperature for 0 or 22 hr. Ten micrograms of β2GP1 in TBS buffer with 10 mM NaCNBH3 was added to the PL-containing tubes and incubated for 5 hr at 37°C. The mixture was exposed to 500 μl of 40 mM n-octyl glucoside as detergent for 15 min, and 30 μg of BSA was added as a carrier protein immediately before adding trichloroacetic acid (TCA; final concentration 10%) to precipitate the protein. After vortexing, the tubes were incubated at 4°C for 20 min, and the protein was pelleted by centrifugation. The pellet was rinsed with 10% TCA in 40 mM n-octyl glucoside and centrifuged, and radioactivity in the pellet was counted with a LS 6800 Liquid Scintillation System (Beckman). When using LDL, the unbound PC was removed with single-phase methanol-chloroform extraction overnight at −20°C. Control incubations with labeled PL were carried out under identical conditions without β2GP1 (only TBS buffer and 10 mM NaCNBH3) and showed a background of only 34 cpm for l-α-dipalmitoyl-[1-14C]-PC and 44 cpm for l-α-1-palmitoyl-2-linoleyl-[1-14C]-PC.
Absorption Experiments.
Increasing amounts of various PL were dried onto the surface of glass tubes, and then a serum pool from five APS patients (1:100 dilution in 2% BSA-TBS) was added. After vortexing, the tubes were incubated at 37°C for 45 min and centrifuged at 13,000 rpm for 30 min (4°C). The supernatants then were tested for antibody binding to OxCL by immunoassay.
RESULTS
Binding of aCL IgG from APS Sera to OxCL and Reduced CL in the Presence of β2GP1.
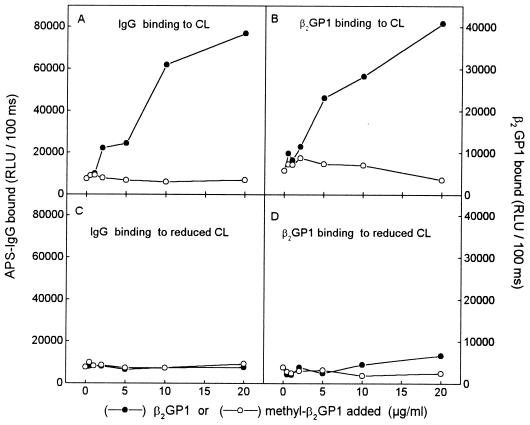
To examine the role of β2GP1 on APS antibody binding to OxCL and reduced CL, a serum pool from 10 well characterized APS patients (20) first was diluted with 3% BSA-TBS so that no antibody binding to OxCL was detected (1:500) (0 point in Fig. 1A). Subsequently, the IgG binding from the same 1:500 dilution of pooled sera to CL and reduced CL was retested after adding increasing amounts of purified human β2GP1 directly into the pooled sera. IgG binding to OxCL (Fig. 1A), but not to reduced CL (Fig. 1C), increased substantially when increasing amounts of β2GP1 were added. In parallel wells, β2GP1 showed significant binding to OxCL (Fig. 1B), but not to reduced CL (Fig. 1D).
Figure 1.
(A and C) Chemiluminescent immunoassay for IgG binding from a serum pool of 10 APS patients to OxCL and reduced CL in the absence or presence of increasing amounts of native (•) or methylated (○) human β2GP1. The serum pool was first diluted (1:500) so that no antibody binding to OxCL was detected (0 point). Increasing amounts of native or methylated human β2GP1 were added to the sera before addition to the PL-coated wells. Binding of IgG to OxCL and reduced CL was measured with alkaline phosphatase-labeled goat anti-human-IgG antibody. (B and D) In parallel wells the binding of β2GP1 and methylated β2GP1 was detected with alkaline phosphatase-labeled goat anti-human-β2GP1 antibody. Preliminary data indicated that this antibody bound equally well to native and methylated β2GP1. RLU, relative light units. Each point is the mean of triplicate determinations.
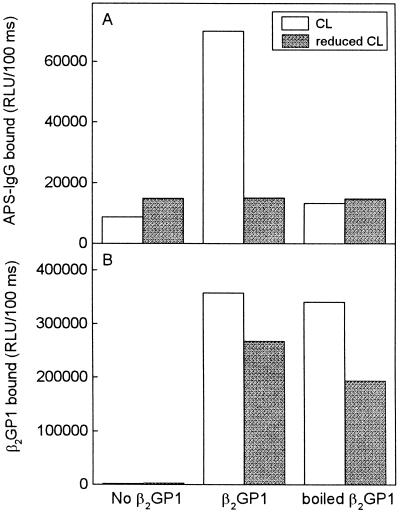
In the experiment shown in Fig. 1 we had added β2GP1 to APS sera and incubated these together in PL-containing wells. In further experiments, we added 5 μg/ml of β2GP1 in the absence of serum (which potentially could bind β2GP1 via its lipoproteins) directly to wells coated with OxCL or reduced CL, and allowed it to bind to the plated PL 1 hr before washing the wells and adding the APS sera. Under these conditions the amount of β2GP1 binding to the reduced CL approached that to OxCL (Fig. 2B); yet aCL binding still occurred only to OxCL-containing wells (Fig. 2A). Furthermore, if the β2GP1 was first denatured by boiling, binding to OxCL was not inhibited, but the ability to generate aCL epitopes was abolished. This strongly suggests that simple binding to CL (or even to OxCL) is not sufficient to induce aCL epitope formation.
Figure 2.
(A) Chemiluminescent immunoassay for IgG binding from a serum pool of five APS patients to OxCL and reduced CL. Native β2GP1 or β2GP1 boiled for 30 min (5 μg/ml in 3% BSA-TBS) was incubated in the PL-coated wells for 1 hr. The serum pool was first diluted 1:750 so that minimal antibody binding to OxCL was detected. The IgG bound was measured using alkaline phosphatase-labeled goat anti-human-IgG antibody. (B) In parallel wells the binding of β2GP1 to OxCL and reduced CL was detected with goat anti-human-β2GP1 antibody, which then was detected with alkaline phosphatase-labeled rabbit anti-goat-IgG secondary antibody. Each point is the mean of triplicate determinations.
Binding of Methylated β2GP1 to OxCL and Reduced CL.
If the antibodies binding to OxCL in the presence of β2GP1 shown in Fig. 1 are directed against oxidized lipid-protein adducts, such as those generated by Schiff base formation, then blocking of free lysine groups of the protein should prevent adduct formation, and therefore, also aCL binding. The amino groups were blocked by reductive methylation, which does not change the charge of the protein (35). Fig. 1A shows that in contrast to native β2GP1, the addition of increasing amounts of methylated β2GP1 failed to increase binding of IgG antibodies from APS sera to OxCL. In addition, methylated β2GP1 did not bind to OxCL or to reduced CL (Fig. 1 B and D). In control studies, we demonstrated that the binding of the anti-human-β2GP1 antibody was similar to native and methylated β2GP1 (not shown). These data support the hypothesis that lysine groups of β2GP1 are forming adducts with oxidation products of OxCL and that these adducts form the epitopes recognized by aCLs in sera of patients with APS.
Binding of β2GP1 to 14C-Labeled PL.
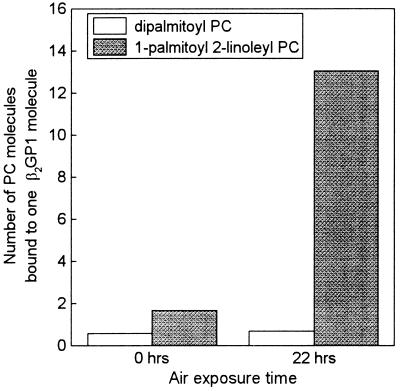
To further test the hypothesis that OxPL forms covalent adducts with β2GP1, we incubated purified human β2GP1 with PL containing either saturated or unsaturated fatty acids. Dipalmitoyl-[1-14C]-PC and 1-palmitoyl-2-linoleyl-[1-14C]-PC were dried under nitrogen, oxidized by exposure to air, and incubated with β2GP1. To stabilize the Schiff bases formed, we included NaCNBH3 in the incubations. Fig. 3 shows that only after the 1-palmitoyl-2-linoleyl-PC had been oxidized was it able to bind to β2GP1, whereas dipalmitoyl-PC (which could not undergo oxidation) was unable to bind at all to β2GP1, even after 22 hr of air exposure. Because the label was in the 1-C position, these data strongly suggest that after oxidation of the sn-2 linoleic acid, the aldehyde or other reactive groups generated on the residual fatty acyl chain still attached to the glycerol backbone (e.g., as a result of β-scission) reacted covalently with a lysine group in β2GP1.
Figure 3.
Binding of dipalmitoyl-[1-14C]-PC and 1-palmitoyl-2-linoleyl-[1-14C]-PC to β2GP1. PCs were dried under nitrogen, exposed to air for 0 or 22 hr and then incubated with β2GP1 for 6 hr in the presence of 10 mM NaCNBH3. The amount of PC bound to β2GP1 was determined after solubilizing the mixture in n-octyl glucoside and precipitating protein with 10% trichloroacetic acid.
In a parallel set of experiments, similar results were obtained when LDL was incubated with the 1-14C-labeled PC compounds. Incorporation of 1-14C into LDL occurred only from the oxidized linoleyl-PC, but not from the dipalmitoyl-PC, and methylation of LDL decreased the binding of oxidized 1-palmitoyl-2-linoleyl-PC to LDL (not shown).
Binding of aCL IgG from APS Sera to PC.
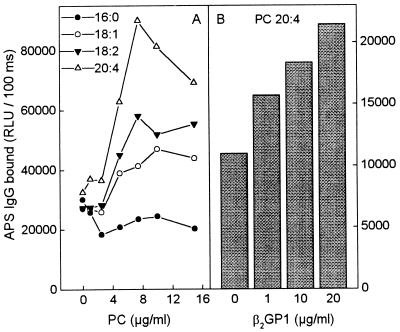
To demonstrate that APS sera would have aPL binding activity against PC containing oxidizable fatty acids, we plated PC in which both fatty acids were saturated (1,2-dipalmitoyl-[16:0]-PC), monounsaturated (1,2-dioleoyl-[18:1]-PC), or polyunsaturated (1,2-dilinoleyl-[18:2]-PC and 1,2-diarachidonoyl-[20:4]). Fig. 4A shows that the PC that could not undergo oxidation (1,2-dipalmitoyl-PC) yielded no aPL activity, whereas all the PCs with oxidizable fatty acids displayed increased IgG binding activity: 1,2-dioleoyl-PC being the lowest and 1,2-diarachidonyl-PC being the highest. Furthermore, addition of purified human β2GP1 to oxidized 1,2-diarachidonyl-PC-coated wells increased the IgG binding from APS sera (Fig. 4B), but had no effect when added to wells coated with dipalmitoyl-PC (not shown).
Figure 4.
(A) Chemiluminescent immunoassay for IgG binding from a serum pool of five APS patients (1:100 dilution) to different PC: 1,2-dipalmitoyl-PC (16:0), 1,2-dioleoyl-PC (18:1), 1,2-dilinoleyl-PC (18:2), and 1,2-diarachidonyl-PC (20:4). (B) IgG binding from the same serum pool to 1,2-diarachidonyl-PC (20:4) in the presence of β2GP1. The serum pool was first diluted 1:750 so that minimal antibody binding to OxCL was detected. The IgG bound was measured using alkaline phosphatase-labeled goat anti-human-IgG antibody. Each point is the mean of triplicate determinations.
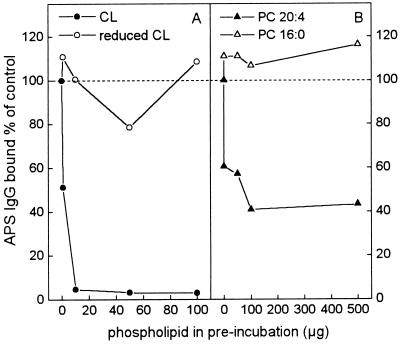
Absorption of aCL IgG from APS Sera by Preincubation with OxPL.
To demonstrate the specificity of binding of aCL IgG in APS sera to OxCL, the pool of APS sera was preincubated with increasing amounts of OxCL, reduced CL, 1,2-diarachidonyl-[20:4]-PC, or 1,2-dipalmitoyl-[16:0]-PC. In these assays, BSA and proteins in the APS sera presumably served as cofactors as previously described (20). Fig. 5A shows that preincubation of APS sera with OxCL removed over 95% of the original aCL binding activity, whereas preincubation with reduced CL did not remove any activity. Similarly, preincubation of APS sera with PC containing oxidizable fatty acids (1,2-diarachidonyl-[20:4]-PC) was able to remove about 60% of the aCL binding activity, whereas PC that could not undergo oxidation (1, 2-dipalmitoyl-[16:0]-PC) did not remove any aCL binding activity (Fig. 5B).
Figure 5.
Competition immunoassay of the binding of IgG from APS sera to OxCL. A serum pool from five APS patients (1:100 dilution) was preincubated with OxCL (•), reduced CL (○), 1,2-diarachidonyl-[20:4]-PC (▴), or 1,2-dipalmitoyl-[16:0]-PC (▵). After preincubation, the immune complexes were pelleted by centrifugation and the supernatants tested for aCL IgG binding to OxCL. Results are expressed as percentage of control (control = APS sera preincubated without any PL). Each point is the mean of four replicate determinations.
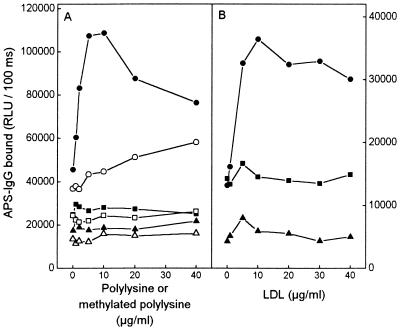
Antibody Binding to CL in the Presence of Polylysine and LDL.
If the antibodies from the APS sera recognize epitopes formed when lysine residues are modified by breakdown products of OxPL, then theoretically, any protein rich in available lysine residues, such as LDL apoB, or even polylysine alone, should be able to form epitopes recognized by some “aCLs.” To test this, increasing concentrations of native and methylated polylysine (molecular weight 3,800) or LDL were incubated in wells coated with OxCL, reduced CL, or ethanol (all dried under nitrogen and exposed to air for 3 hr) before adding the APS sera. Fig. 6 shows a nearly 3-fold increase in IgG binding to OxCL when native polylysine was added (Fig. 6A). The increase was much smaller when methylated polylysine was added. In this experiment, 88.5% of available lysines were methylated. In three different experiments, the minimal increase in IgG binding observed with the methylated polylysine correlated with the amount of free lysine groups still remaining in the various polylysine preparations after methylation, as measured with the trinitrobenzenesulfonic acid reaction (R = 0.996, P = 0.05, n = 3). Note that when polylysine was added to wells coated with reduced CL or ethanol only (Fig. 6) no increase in IgG binding occurred. Binding of pooled sera (at 1:500 dilution) from five healthy control subjects to OxCL in the presence of polylysine was only 25% of the binding of pooled sera from APS patients, and no increase occurred when polylysine was added to wells coated with reduced CL and ethanol only (not shown). Control incubations without the primary antibody (sera) showed only a minimal increase in binding of the secondary antibody to polylysine (not shown), and this was most prominent in ethanol-coated wells. This indicates that the increase seen in the aCL binding to OxCL and polylysine was not caused by nonspecific binding to the polylysine itself.
Figure 6.
Chemiluminescent immunoassay for IgG binding from a serum pool of five APS patients to OxCL (circles), reduced CL (squares), and ethanol-only control wells (triangles). (A) Binding in the presence of polylysine (solid figures) or methylated polylysine (open figures). (B) Binding in the presence of LDL. Each compound (in TBS) was incubated for 10 min in wells coated with OxCL, reduced CL, or ethanol. The serum pool was first diluted 1:500 so that minimal antibody binding to OxCL was detected. The IgG binding was measured with alkaline phosphatase-labeled goat anti-human-IgG antibody. Each point is the mean of four replicate determinations.
The addition of native LDL to wells coated with OxCL also increased the IgG binding from APS sera more than 2-fold, whereas no effect on IgG binding was seen when LDL was added to reduced CL (Fig. 6B). Methylation of 73% of lysine residues of LDL reduced the binding of IgG to OxCL by approximately 63%. In parallel wells the binding of LDL to OxCL, reduced CL, or ethanol-coated wells was verified to be similar using the apoB-specific mAb MB47 (not shown). In a similar set of experiments, we also demonstrated that apoAI, an amphipathic protein with high affinity for phospholipids, also could serve as a cofactor for aCLs from APS sera (not shown).
DISCUSSION
We recently demonstrated that intact reference sera and affinity-purified aCL-IgG from APS patients bound to OxCL, but did not bind at all to a reduced cardiolipin analogue unable to undergo lipid peroxidation (20). These data suggest that many (if not most) aCLs bind to neoepitopes of OxPL or to neoepitopes generated by adduct formation between reactive breakdown products of OxPL and associated proteins. In this paper, we demonstrate that adduct formation between lysine residues of β2GP1 (and other proteins) and OxCL generates neoepitopes recognized by aCLs in APS sera. Thus, we offer the alternative (or additional) explanation that some epitopes for aPLs are formed, not as a result of conformational changes in β2GP1, or CL, but as a consequence of covalent adduct formation between OxPL and β2GP1 (or other associated proteins).
β2GP1 has been reported to be the primary serum cofactor for aCLs (6, 7). Therefore, we first investigated its ability to form neoepitopes with OxCL. β2GP1 binds avidly to PL (6–10, 36), and a lysine-rich domain of β2GP1 has been postulated to be involved in this binding (9, 10). Thus, we postulated that if CL underwent oxidation, the possibility of adduct formation would be greatly enhanced by the close proximity of the lysine-rich domains of β2GP1 and reactive groups, such as aldehydes, in OxCL. Indeed, when the endogenous content of β2GP1 in serum was diluted out, the addition of β2GP1 markedly increased IgG binding from the reference APS sera to OxCL (Fig. 1A). In contrast, when β2GP1 was added to wells coated with reduced CL, no epitopes for aCLs were formed (Fig. 1C), even when large amounts of β2GP1 were added and even when some β2GP1 was bound to the reduced CL (Figs. 1D and 2). These data support the idea that a simple PL-β2GP1 complex is not sufficient to generate aCL epitopes, unless the PL first undergoes oxidation. Our data also demonstrate that denatured β2GP1 was able to bind to OxCL, but failed to yield binding of IgG from the APS sera (Fig. 2), implying that epitope formation occurs only when adducts are formed with native β2GP1, or that denatured β2GP1 bound to OxCL nonspecifically, in a way that precluded adduct formation.
Our studies with radiolabeled PL demonstrate that under the actual conditions used in these experiments covalent adducts between OxPL and β2GP1 occurred, whereas such adducts could not be demonstrated when the PL was not oxidized (Fig. 3). To further support the idea that covalent adduct formation between the OxPL and lysine residues of the β2GP1 generated epitopes recognized by some aCLs, we methylated β2GP1 at pH 7.4, which does not change the net charge of the protein (35). Under these conditions methylated β2GP1 lost its ability to be a cofactor for aCLs (Fig. 1A). It might be argued that the methylation prevented the binding of β2GP1 to OxCL by nonspecific means, and thus prevented a conformationally induced epitope from forming. However, the fact that three very different compounds, such as polylysine, LDL, and apoAI, also could serve as aCL cofactors, albeit weaker, and the fact that again methylation abolished formation of these epitopes makes it highly unlikely that these aCLs are seeing conformational epitopes consisting of noncovalent β2GP1/CL complexes. Furthermore, the observations that APS IgG bound to PC containing oxidizable fatty acids, but not to PC with saturated fatty acids; that β2GP1 could serve as a cofactor for this binding; and that 60% of the IgG in APS sera binding to OxCL could be preabsorbed by an oxidizable PC, all support the idea of common structural neoepitopes. Thus it appears that similar products of OxCL are forming common covalent adducts with lysine residues of different proteins. β2GP1 appears to be highly efficient in forming the adducts (and epitopes), presumably because of its high avidity for binding to CL. In our experiments, lysine-OxPL adducts appear to be prominent epitopes, but it is likely that in vivo other types of adducts also may occur.
The formation of neoepitopes as the result of reactions between breakdown products of OxPL and associated proteins is analogous to what happens when LDL undergoes oxidation, as described above. Not only can breakdown products of polyunsaturated fatty acid form adducts with lysine residues of any closely associated proteins, but aldehydes (and other reactive products) that remain on the residual fatty acid fragment attached to the PL backbone after oxidative decomposition also can form similar adducts with proteins or even with other amino PL. Literally hundreds of different structures could occur (see figure 1 in ref. 20). All of these could be immunogenic (23–32), and autoantibodies to many of these epitopes are present in sera of animals and humans (23–25, 30). For example, considerable data exist that elevated titers of antibodies to oxidized LDL are found in animals and humans with increased atherosclerosis (reviewed in ref. 32). Serum proteins with high affinity binding to phospholipids, such as β2GP1, would be particularly amenable to similar kinds of oxidative modification. If oxidation of phospholipids occurred in vivo, for example, on the surface of a cell undergoing oxidative stress or apoptosis, then such reactive groups could bind β2GP1 in vivo, be immunogenic, and/or bind “aCLs” or “aPLs” as well as antioxidized LDL antibodies (37). Some antibodies might recognize highly restricted epitopes, such as the adduct formed between 1-palmitoyl-2-(9-oxononanoyl)-PC and lysine (29) or malondialdehyde-lysine (23, 24), whereas the epitopes of other antibodies might encompass the PL and broader regions of a protein. Similar epitopes also could be generated on the surface of activated platelets, e.g., between OxPL (such as exposed phosphatidylserine) and associated proteins (either endogenous cellular proteins or β2GP1 acquired from plasma), leading to binding of some aPLs and resulting thrombocytopenia (38).
As noted above, there is likely to be much heterogeneity among aPLs (3, 5, 16, 39). In addition to aPLs directed to OxPL-protein adducts there may be oxidation-independent antibodies, such as those proposed against conformational changes either in CL (11–12) or in β2GP1 (7, 15, 18, 19, 40). In this respect it is interesting to note that many of the reports observing antibody binding directly to β2GP1 have used activated plates that are either chemically treated or irradiated. In the latter case, the gamma irradiation is known to initiate radical formation and in the presence of air to lead to the incorporation of 10–20% of surface oxygen. The oxidized polystyrene surface contains numerous reactive groups, including α, β-unsaturated carbonyl groups (41), which likely form adducts with the β2GP1 (15, 40). In addition, investigators have reported that some aPLs bind to various coagulation factors, such as prothrombin (42), and again it is interesting that in some cases these antibodies may be directed at PL-bound prothrombin (43).
Our studies have not yet addressed the issue of whether the aCLs directed to neoepitopes of OxPL have important biological activities, such as altering coagulation properties. However, the clinical association of elevated aPLs with various disease states has been based on the presence of antibodies to CL determined under assay conditions that would lead inheritantly to oxidation of the PL (e.g., air drying on microtiter wells) and modification of associated proteins in the assay. Olee and colleagues (44) recently have cloned a human monoclonal IgG aCL from a patient with the APS and demonstrated that it was thrombogenic in mice. In preliminary studies we have demonstrated that binding of this antibody to CL is dependent on oxidation of the CL (S.H., T. Olee, E.M., D.W.B., V. L. Woods, W.P., P. C. Chen, and J.L.W., unpublished work), similar to the binding of APS sera shown previously (20). It will be important to determine the epitopes of such mAbs and their biological effects. Knowledge that the epitopes recognized by many aPLs are adducts of OxPL and associated proteins may give new insights into pathogenic events underlying the clinical manifestations of APS.
Acknowledgments
We thank Dr. D. Steinberg for advice and support. These studies were supported by National Heart, Lung, and Blood Institute Grants 14197 (La Jolla Specialized Center of Research) and HL57505 (J.L.W.), the Emil Aaltonen Foundation, the Academy of Finland, and the Finnish Foundation for Cardiovascular Research.
ABBREVIATIONS
- apo
apolipoprotein
- APS
antiphospholipid antibody syndrome
- aPL
antiphospholipid antibody
- aCL
anticardiolipin antibody
- CL
cardiolipin
- OxCL
oxidized cardiolipin
- PL
phospholipid
- LDL
low-density lipoprotein
- OxPL
oxidized phospholipid
- β2GP1
β2 glycoprotein 1
- PC
phosphatidylcholine
- TBS
Tris-buffered saline
References
- 1.Alarcon-Segovia D, Deleze M, Oria C, Sanchez-Guerrero J, Gomez-Pancheco L, Cabiedes J, Fernandez L, Ponce de Leon S. Medicine. 1989;58:353–363. doi: 10.1097/00005792-198911000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Asherson R A, Khamashta M A, Ordi-Ros J, Derksen R H W M, Machin S J, Barquinero J, Out H H, Harris E N, Vilardell-Torres M, Hughes G R V. Medicine. 1989;68:366–374. [PubMed] [Google Scholar]
- 3.Harris E N, Pierangeli S S. Springer Semin Immunopathol. 1994;16:223–245. doi: 10.1007/BF00197519. [DOI] [PubMed] [Google Scholar]
- 4.Silver R M, Draper M L, Scott J R, Lyon J L, Reading J, Branch D W. Obstet Gynecol. 1994;83:372–377. [PubMed] [Google Scholar]
- 5.Lockshin M D. New Engl J Med. 1995;333:667. [PubMed] [Google Scholar]
- 6.McNeil H P, Simpson R J, Chesterman C N, Krilis S A. Proc Natl Acad Sci USA. 1990;87:4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galli M, Comfurius P, Maassen C, Hemker H C, de Baets M H, van Breda-Vriesman P J, Barbui T, Zwaal R F, Bevers E M. Lancet. 1990;335:1544–1547. doi: 10.1016/0140-6736(90)91374-j. [DOI] [PubMed] [Google Scholar]
- 8.Polz E, Kostner G M. FEBS Lett. 1979;102:183–186. doi: 10.1016/0014-5793(79)80955-2. [DOI] [PubMed] [Google Scholar]
- 9.Kertesz Z, Yu B B, Steinkasserer A, Haupt H, Benham A, Sim R B. Biochem J. 1995;310:315–321. doi: 10.1042/bj3100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt K, Krilis S. J Immunol. 1994;152:653–658. [PubMed] [Google Scholar]
- 11.Rauch J, Janoff A S. Proc Natl Acad Sci USA. 1990;87:4112–4114. doi: 10.1073/pnas.87.11.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rauch J, Tannenbaum M, Tannenbaum H, Ramelson H, Cullis P R, Tilcock C P, Hope M J, Janoff A S. J Biol Chem. 1986;261:9672–9677. [PubMed] [Google Scholar]
- 13.Rauch J, Tannenbaum M, Janoff A S. Thromb Haemostasis. 1989;62:892–896. [PubMed] [Google Scholar]
- 14.Bevers E M, Galli M. Lupus. 1992;1:51–53. doi: 10.1177/096120339200100201. [DOI] [PubMed] [Google Scholar]
- 15.Matsuura E, Igarashi Y, Fujimoto M, Ichikawa K, Suzuki T, Sumida T, Yasuda T, Koike T. J Immunol. 1992;148:3885–3891. [PubMed] [Google Scholar]
- 16.Roubey R A. Blood. 1994;84:2854–2867. [PubMed] [Google Scholar]
- 17.Sugi T, McIntyre J A. Blood. 1995;86:3083–3089. [PubMed] [Google Scholar]
- 18.Keeling D M, Wilson A J, Mackie I J, Machin S J, Isenberg D A. Br J Haematol. 1992;82:571–574. doi: 10.1111/j.1365-2141.1992.tb06469.x. [DOI] [PubMed] [Google Scholar]
- 19.Viard J P, Amoura Z, Bach J F. Am J Med. 1992;93:181–186. doi: 10.1016/0002-9343(92)90049-h. [DOI] [PubMed] [Google Scholar]
- 20.Hörkkö S, Miller E, Dudl E, Reaven P, Curtiss L K, Zvaifler N J, Terkeltaub R, Pierangeli S S, Branch D W, Palinski W, Witztum J L. J Clin Invest. 1996;98:815–825. doi: 10.1172/JCI118854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinberg D, Parthasarathy S, Carew T E, Khoo J C, Witztum J L. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 22.Witztum J L, Steinberg D. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palinski W, Ylä-Herttuala S, Rosenfeld M E, Butler S, Socher S A, Parthasarathy S, Curtiss L K, Witztum J L. Arteriosclerosis. 1990;10:325–335. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- 24.Palinski W, Rosenfeld M E, Ylä-Herttuala S, Gurtner G C, Socher S A, Butler S, Parthasarathy S, Carew T E, Steinberg D, Witztum J L. Proc Natl Acad Sci USA. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palinski W, Ord V, Plump A S, Breslow J L, Steinberg D, Witztum J L. Arterioscler Thromb. 1994;14:605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- 26.Haberland M E, Fong D, Cheng L. Science. 1988;241:215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- 27.Boyd H C, Gown A M, Wolfbauer G, Chait A. Am J Pathol. 1989;135:815–825. [PMC free article] [PubMed] [Google Scholar]
- 28.Hammer A, Kager G, Dohr G, Rabl H, Ghassempur I, Jürgens G. Arterioscler Thromb Vasc Biol. 1995;15:704–713. doi: 10.1161/01.atv.15.5.704. [DOI] [PubMed] [Google Scholar]
- 29.Itabe H, Yamamoto H, Suzukis M, Kawai Y, Nakagawa Y, Suzuki A, Imanaka T, Takano T. J Biol Chem. 1996;271:33208–33217. doi: 10.1074/jbc.271.52.33208. [DOI] [PubMed] [Google Scholar]
- 30.Palinski W, Hörkkö S, Miller E, Steinbrecher U P, Powell H C, Curtiss L K, Witztum J L. J Clin Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stemme S, Faber B, Holm J, Wiklund O, Witztum J, Hansson G K. Proc Natl Acad Sci USA. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witztum J L, Palinski W. In: Immune Functions of the Vessel Wall. Hansson G K, Libby P, editors. New York: Harwood; 1996. pp. 159–172. [Google Scholar]
- 33.Weisgraber K H, Innerarity T L, Mahley R W. J Biol Chem. 1978;253:9053–9062. [PubMed] [Google Scholar]
- 34.Habeeb A F S A. Anal Biochem. 1966;14:328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- 35.Means G E, Feeney R E. In: Chemical Modifications of Proteins. Means G E, Feeney R E, editors. San Francisco: Holden-Day; 1971. pp. 130–131. [Google Scholar]
- 36.Wurm H. Int J Biochem. 1984;16:511–515. doi: 10.1016/0020-711x(84)90168-x. [DOI] [PubMed] [Google Scholar]
- 37.Casicola-Rosen L, Rosen A, Petri M, Schlissel M. Proc Natl Acad Sci USA. 1996;93:1624–1629. doi: 10.1073/pnas.93.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi W, Chong B H, Chesterman C N. Blood. 1993;81:1255–1262. [PubMed] [Google Scholar]
- 39.Vaarala O, Puurunen M, Lukka M, Alfthan G, Leirisalo-Repo M, Aho K, Palosuo T. Clin Exp Immunol. 1996;104:269–274. doi: 10.1046/j.1365-2249.1996.21728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roubey R A, Eisenberg R A, Harper M F, Winfield J B. J Immunol. 1995;154:954–960. [PubMed] [Google Scholar]
- 41.Onyiriuka E C, Hersh I S, Herti W. Appl Spectrosc. 1990;44:808–811. [Google Scholar]
- 42.Permpikul P, Rao L V, Rapaport S I. Blood. 1994;83:2878–2892. [PubMed] [Google Scholar]
- 43.Oosting J D, Derksen R H, Bobbink I W, Hackeng T M, Bouma B N, de Groot P G. Blood. 1993;81:2618–2625. [PubMed] [Google Scholar]
- 44.Olee T, Pierangeli S S, Handley H H, Le D T, Wei X, Lai C-J, En J, Novotny W, Harris E N, Woods V L, Chen P P. Proc Natl Acad Sci USA. 1996;93:8606–8611. doi: 10.1073/pnas.93.16.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]