Abstract
In a Hungarian family with triosephosphate isomerase (TPI) deficiency, two compound heterozygote brothers were found with the same severe decrease in TPI activity, but only one of them had the classical symptoms. In search for the pathogenesis of the differing phenotype of the same genotypic TPI deficiency, an increase in red cell membrane fluidity was found. There were roughly 100% and 30% more 16:0/20:4 and 18:0/20:4 diacyl-phosphatidylcholine species in erythrocytes from the two TPI-deficient brothers than in the probes from healthy controls. The activities of acethylcholinesterase and calmodulin induced Ca2+ ATPase were significantly enhanced in erythrocytes from the propositus as compared with those of the neurologically symptom-free brother and other members of the TPI-deficient family as well as to those from healthy controls. Both enzymes are crucially involved in the function of nerve cells. The observed differences in membrane fluidity and enzyme activities between the erythrocytes from the phenotypically differing TPI-deficient brothers underline the importance of investigations into the effect of biophysical changes in the lipid environment of the membrane proteins on the development of disseminated focal neurological disorders of unknown pathogenic origin.
A Hungarian family with triosephosphate isomerase (TPI) deficiency has been previously reported with two compound heterozygote brothers, both having <10% TPI activity in their erythrocytes in vitro. Both have congenital hemolytic anemia, but while the propositus has severe extrapyramidal signs and symptoms, his compound heterozygote brother was unique in the world literature from the point of view of the complete lack of any neurological disorder (1). Both brothers inherited a missense mutation 240(TTC[Phe] → CTC[Leu]) from their mother (2). In cooperation with M. Cohen-Solal, a second mutation 145(GAG[Glu] → TAG[stop codon]), inherited from the father, had been revealed. This resulted in a 10- to 20-fold decrease in TPI in both compound heterozygotes (3). Sequencing the whole genomic TPI (all exons and exon–intron border lines) resulted in a completely identical DNA sequence in the two compound heterozygote brothers (M. Cohen-Solal, personal communication). Differences in the noncoding regions (promoter zone and introns) as well as differences in mRNA splicing have still to be excluded. The present results of this “experiment of nature” raise the devilling question of what sort of pathogenesis underlies the differing phenotypes in two genetically identical enzyme deficiencies? Because TPI is not only an enzyme of the glycolytic pathway but also participates in fatty acid synthesis, we have studied the erythrocyte lipids in details. The only significant difference revealed was the increased fatty acid chain mobility in the external lipid layer in the red cells, ghosts, and inside-out vesicles (IOVs) from the propositus as compared with that from his neurologically symptom-free compound heterozygote brother and to those from normal controls. This difference disappeared in the extracted membrane phospholipids pointing to protein–lipid interactions underlying the change in membrane fluidity (4).
Changes in membrane fluidity are known to influence the activity of enzymes (5). The results of our present experiments have shown that the activity of calmodulin stimulated Ca2+ ATPase and of acethylcholinesterase (AChE), both crucially involved in brain cell activity, are increased significantly in the propositus as compared with his neurologically symptom-free compound heterozygote brother.
MATERIALS AND METHODS
Patients.
The main characteristics of the TPI-deficient Hungarian family are summarized in Table 1. More detailed clinical and biochemical findings have been published earlier (1). In each experimental procedure, age-matched controls have been investigated.
Table 1.
Main characteristics of the TPI-deficient family
| Mother heterozygote | Father heterozygote | Propositus compound heterozygote | Brother compound heterozygote | Brother heterozygote | Normal range | |
|---|---|---|---|---|---|---|
| TPI activity, units/g of Hb | 436 | 644 | 9.4 | 6.1 | 745 | 1364-1793 |
| DHAP, nmol/ml of RBC | 19.8 | 23.2 | 904.9 | 580.9 | NT | 5.08-13.0 |
| TPI substitution | 240Phe → Leu | 145 stop | 240Phe → Leu; | 240Phe → Leu; | 240Phe → Leu | None |
| 145 → stop | 145 → stop | |||||
| TPI heat instability | Partial | None | Total | Total | Partial | None |
| PCV, % | 41.4 | 41.4 | 33.3 | 31.2 | 40.0 | 37-55 |
| Hb, g/dl | 12.4 | 15.5 | 11.3 | 11.2 | 13.4 | 12-16 |
| RBC × 103/μl | 3.7 | 4.97 | 3.4 | 3.4 | 4.2 | 4.50-5.30 |
| MCV, fl | 87.0 | 83.3 | 96.0 | 96.6 | 86.4 | 77.0-93.0 |
| MCH, pg | 31.6 | 31.2 | 34.2 | 35.0 | 32.0 | 27.0-32.0 |
| MCHC, g/dl | 33.0 | 37.4 | 34.3 | 34.8 | 32.3 | 31.0-35.0 |
| Reticulocytes, % | 0.8 | 0.8 | 3.0 | 2.0 | 0.8 | 0.4-1.0 |
| Indirect sebilirubin, mg/dl Hb | 0.8 | 0.9 | 7.8 | 3.2 | 0.5 | 0.1-1.0 |
| Neurological disorder | None | None | Severe extrapyramidal | None | None | None |
NT, not tested; DHAP, dihydroxyacetone phosphate; RBC, erythrocytes; PCV, packed cell volume; Hb, hemoglobin; MCV, mean cell volume; MCH, mean cell hemoglobin; MCHC, mean cell hemoglobin concentration.
Analytical Methods.
Preparation of hemoglobin-free erythrocyte ghosts—i.e., right side-out vesicles (ROVs) and inside-out vesicles (IOVs)—were carried out as described by Sarkadi et al. (6).
Ca2+ uptake by IOVs at different Ca2+ concentrations was measured by the membrane filtration technique described by Sarkadi et al. (6). The incubation is carried out at 37°C in small plastic tubes containing 100 μg of membrane protein. The free Ca2+ concentrations are adjusted with varied amount of added EGTA based on calculation by a computer program. The stimulatory effect of calmodulin on the Ca2+ transport of the vesicles was measured in the presence of 10 μg/ml calmodulin.
Measurements of AChE activity and the determination of the ROV/IOV ratios of sealed IOVs in the prepataions were carried out via the AChE assay as described by Steck (7). The applied substrate (acethylthiocholine) cannot reach AChE in sealed IOVs because it is on the originally external surface. In contrast to this, in membranes treated with 0.016% Triton X-100, the full AChE activity is measured. The formation of thiocholin is followed spectrophotometrically with DTNB (5,5 dithiobisnitrobenzoic acid) at 412 nm.
Quantitative determination of the plasma membrane Ca2+ pump protein was carried out by Western blot technique as described by Magócsi and Penniston (8) with the monoclonal antibody 5F10 raised against human plasma membrane calcium pump protein and stained with horseradish peroxidase conjugate (Amersham ECL detection reagents).
Investigation of the structural order of the fatty acid chains in the erythrocyte membranes were carried out by fluorescence anisotropy measurement using a computer-controlled and thermostated Hitachi MPF-2A spectrophotofluorimeter as described in details by Dey and Farkas (9). The lipid soluble fluorophores, 3-[p-(6-phenyl-1,3,5-hexatrienyl)phenyl]propionic acid (DPH-PA) and 1-(4-trimethylammoniumphenyl)-6-phenyl,1,3,5-haxatriene (TMA-DPH) were from Molecular Probes.
Determination of Phospholipid Molecular Species Composition.
Choline and ethanolamine phosphoglycerides were purified by thin layer chromatography according to Fine and Sprecher (10) and were subjected to phospholipase C (Bacillus cereus; Sigma) digestion to obtain diradylglycerols. The diradylglycerols were then converted into anthroyl derivatives according to Takamura and Kito (11). The anthroyl derivatives were segregated into diacyl, alkylacyl, and alkenylacyl subclasses by TLC on fluorescent plates (Merck) using hexane/toluene/diethyl ether (100:80:3). Derivatized 12:0/12:0 diacylglycerol served as internal standard. Separation of molecular species took place on an ODS column (250 × 4.6 mm; Supelco) using acetonitrile/2-propanol (70:30, vol/vol) as solvent. Peaks were identified with the aid of synthetic secondary standards and on the basis of relative elution times given by Bell and Dick (12).
RESULTS
The previously published fluorescence anisotropy measurements with DPH-PA (4) were repeated and, in addition, measurements with TMA-DPH were carried out in each intact fresh red cell samples from which preparations for the investigation of the activity of the Ca2+ pump and AChE were performed. Table 2 depicts the anisotropy values of the erythrocytes from members of the TPI-deficient family.
Table 2.
Anisotropy parameters of DPH-PA and TMA-DPH embedded in erythrocytes from members of the TPI-deficient family
| DPH-PA | TMA-DPH | |
|---|---|---|
| Control | 0.309 ± 0.009 | 0.354 ± 0.034 |
| Propositus | 0.274 ± 0.014 | 0.316 ± 0.042 |
| Compound heterozygote brother* | 0.236 ± 0.013 | 0.337 ± 0.055 |
| Father | 0.275 ± 0.019 | 0.350† |
| Mother | 0.272 ± 0.025 | 0.348 ± 0.019 |
Values are the means (±SD) of five separate experiments.
Without neurological disorder.
Anisotropy measurement was done only in one experiment.
The present repeated measurements with DPH-PA showed, just like in our former studies (4), decreased fluorescence (increased fluidity) in the erythrocyte membranes from all family members, with the most marked decrease in anisotropy values in erythrocytes from the neurologically symptom-free compound heterozygote brother as compared with age-matched controls. DPH-PA due to its anionic nature does not penetrate into the inner leaflet of the membrane and reports membrane regions rich in positively charged phospholipids (sphingomyelin and phosphatidylcholine). In contrast to this, TMA-DPH, with its cationic nature, penetrates into the inner membrane leaflet (13), suggesting increased fluidity in the entire erythrocyte membrane of the propositus.
The composition of the molecular species of phospholipids may change markedly membrane fluidity. Thirteen molecular species of diacyl-phosphatidylcholines, 11 molecular species of diacyl-phosphatidylethanolamines, and 15 molecular species of alkenylacyl-phosphatidylethanolamines were detected in the erythrocytes from normal controls and the two compound heterozygotes for TPI deficiency (Tables 3, 4, 5). The main difference found was that the level of molecular species containing arachidonic acid in the position sn-2 (16:0/20:4, 18:0/20:4) in the diacyl-phosphatidylcholines from the TPI-deficient brothers was almost twice as high as in the probes from the controls. In addition, the level of 18:0/18:1 phosphatidylcholine was found almost 10 times higher than in the control probes. Moreover, 18:0/22:4 and 16:0/18:0 species were represented in a very low amount in the TPI-deficient brothers. The same but less marked changes could be revealed in the phosphatidylethanolamines. Molecular species composition of alkenylacyl-phosphatidylethanolamines was almost identical in healthy controls and the TPI-deficient brothers. The only exception was the difference in the amount of 18:1a/22:4 between the propositus and his neurologically symptom-free brother. Considering the data of Salem and Niebilski (14) and Garwish and Holte (21), the observed pattern of changes in the composition of the phospholipid molecular species of the phosphatidylcholines may explain the increased membrane fluidity.
Table 3.
Molecular species composition of diacyl-phosphatidylcholines of erythrocytes from healthy controls and the TPI-deficient brothers
| Species | Control | Propositus | Compound heterozygote brother* |
|---|---|---|---|
| 16:0/22:6 | 4.0 | 5.10 | 5.60 |
| 18:1/20:4 | 1.13 | 1.96 | 2.44 |
| 16:0/20:4 | 8.20 | 14.0 | 17.57 |
| 18:0/22:6 | 3.82 | 4.33 | 4.45 |
| 16:0/18:2 | 31.70 | 30.93 | 26.36 |
| 18:0/20:4 | 4.88 | 8.25 | 9.72 |
| 18:1/18:1 | 1.13 | 1.89 | 1.44 |
| 16:0/18:1 | 29.77 | 27.0 | 25.26 |
| 16:0/16:0 | 3.14 | 3.08 | 1.01 |
| 18:0/22:4 | 3.15 | 0.13 | 0.10 |
| 18:0/18:1 | 0.21 | 2.50 | 2.17 |
| 16:0/18:0 | 4.65 | 0.69 | 0.40 |
The values are the means from two independent probes. Results are expressed as the percentage of total.
*Without neurologic disorder.
Table 4.
Molecular species composition of diacyl-phosphatidylethanolamines of erythrocytes from healthy controls and the TPI-deficient brothers
| Species | Control | Propositus | Control heterozygote brother* |
|---|---|---|---|
| 18:1/22:6 | 1.4 | 1.5 | 1.5 |
| 16:0/22:6 | 4.9 | 5.1 | 5.1 |
| 18:1/20:4 | 10.4 | 13.0 | 14.0 |
| 16:0/20:4 | 15.1 | 21.2 | 21.4 |
| 18:0/22:6 | 9.0 | 6.0 | 6.1 |
| 16:0/18:2 | 13.0 | 11.8 | 11.0 |
| 18:0/20:4 | 13.8 | 18.3 | 19.1 |
| 18:1/18:1 | 5.6 | 2.2 | 2.9 |
| 16:0/18:1 | 20.1 | 13.6 | 13.7 |
| 16:0/16:0 | 0.2 | 0.2 | 0.1 |
| 18:0/18:1 | 5.2 | 2.7 | 2.8 |
For legend see Table 3.
*Without neurological disorder.
Table 5.
Molecular species composition of alkenylacyl-phosphatidylethanolamines in erythrocytes from TPI-deficient brothers and healthy controls
| Species | Control | Propositus | Compound heterozygote brother* |
|---|---|---|---|
| 18:1a/22:6 | 1.6 | 2.1 | 1.8 |
| 16:0a/22:6 | 3.4 | 2.9 | 3.1 |
| 18:1a/22:5 | 0.6 | 0.6 | 0.6 |
| 18:1a/20:4 | 10.0 | 9.9 | 10.4 |
| 16:0a/20:4 | 14.0 | 12.3 | 14.0 |
| 16:0a/22:5 | 0.3 | 0.2 | 0.3 |
| 18:0a/22:6 | 8.4 | 7.1 | 8.4 |
| 16:0a/18:2 | 10.0 | 12.4 | 10.2 |
| 18:1a/22:4 | trace | 4.0 | 0.4 |
| 18:0a/20:4 | 26.7 | 24.3 | 27.5 |
| 18:0a/22:5 | 0.6 | 0.7 | 0.6 |
| 18:1a/18:1 | 1.2 | 1.4 | 1.2 |
| 16:0a/18:1 | 14.9 | 16.2 | 14.5 |
| 16:0a/16:0 | 0.4 | 0.4 | 0.4 |
| 18:0a/18:1 | 3.4 | 2.6 | 2.2 |
For legend see Table 3.
*Without neurological disorder.
Because changes in membrane fluidity were shown to influence the activity of some enzymes (5), we have investigated in the present studies the effect of changes in membrane fluidity on the activity of Ca2+ ATPase and on that of AChE.
The Ca2+ transport activity was measured in the absence and presence of calmodulin in IOVs. Preparation of IOVs provide an excellent approach for the investigation of the red cell Ca2+ pump in situ. The active center and the regulatory binding sites of the enzyme are exposed on the original cytoplasmic membrane surface to changes in the incubation media. This allows the measurements of uphill Ca2+ transport, its regulation by specific modifications, and the formation of the phosphorylated intermediate of the enzyme. Binding of calmodulin shifts the enzyme from a low Ca2+ activity and affinity to a high affinity and activity state (15).
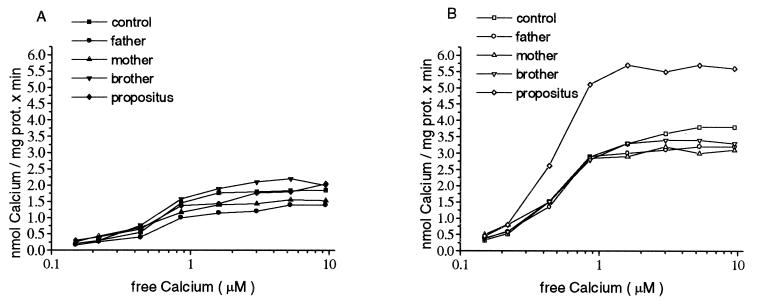
No measurable differences were found in the maximal pump activity in the absence of calmodulin (Fig. 1A). The average value of Vmax measured was 1.64 ± 0.3 nmol of Ca2+ per mg of protein/min and Km was 0.85 ± 0.17 μM Ca2+. In contrast to these results the PMCA from the propositus was enhanced to significantly higher level in the presence of 10 μg/ml calmodulin, as compared with those from his neurologically symptom-free compound heterozygote brother, to other members of the TPI-deficient family, and to those of healthy controls (Fig. 1B). This enhanced pump activity resulted in a higher maximal velocity in the propositus (Vmax = 5.4 ± 0.23) whereas the Vmax average of the other family members and controls were found to be 3.2 ± 0.25 nmol of Ca2+ per mg of protein/min. The increase in Ca2+ pump activity was not accompanied by higher Ca2+ affinity with no significant shift in Km value.
Figure 1.
Calcium transport in IOVs made from erythrocytes. The ATP-dependent Ca2+ uptake is demonstrated as a function of free-Ca2+ concentration. One hundred micrograms of membrane protein was preincubated in the transport medium containing the desired free-Ca2+ concentration at 37°C with (B) or without 10 μg/ml of calmodulin (A) for 5 min. The reaction was started with 0.5 mM ATP and incubated for a further 10 min. The experimental groups are indicated in the figure. Brother designates the neurologically intact compound heterozygote brother. Four independent IOV preparations and Ca2+ transport measurements gave similar results. The Vmax of the calmodulin dependent Ca2+ pump was significantly higher (P < 0.05) in the IOVs prepared from the erythrocytes of the propositus than the Vmax measured in IOVs from the erythrocytes from the control or other members of the family. Data represent the results of one typical experiment (n = 4).
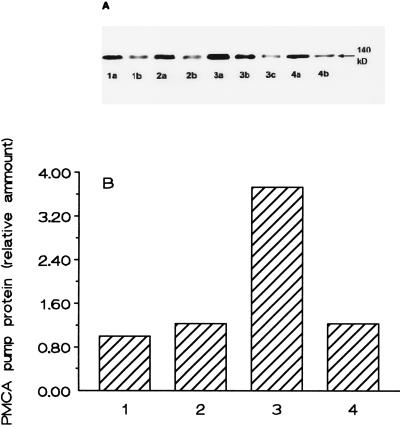
Based on the Western blot analysis of the IOV proteins with 5F10 monoclonal antibody raised against human PMCA the amount of the Ca2+ pump protein was found to be increased by 2.5- to 3.5-fold in the preparations from the propositus as compared with the same membrane preparations from the other family members and healthy age-matched controls (Fig. 2). The immunoblot with mAb 5F10 gave only one sharp band, and no bands pointing to isoforms with differing molecular weight could be revealed. The raised level of the Ca2+ pump protein in the erythrocytes of the propositus cannot simply originate from higher reticulocyte counts because there is no significant difference between the levels of reticulocytosis in the two compound heterozygote brothers. It may be surmised, however, that higher amounts of Ca2+ pump protein is needed in the erythroid precursors of the propositus to prevent a deleterious increase in intracellular Ca2+.
Figure 2.
Western blot analysis of the PMCA pump protein. Erythrocyte IOV proteins were prepared and analyzed by immunoblot from healthy volunteers (lane 1), from the father (lane 2), from the propositus (lane 3), and from the neurologically symptom-free brother (lane 4). In lanes marked with (a) 15 μg protein and in those marked with (b) 7.5 μg protein was analyzed. In the case of the propositus, an extra lane with 3.75 μg protein (c) was also prepared. The quantitative evaluation of the Western blot is presented in B. Data represent the results of one experiment from four independent IOV preparations and Western blot analysis. The average enhancement of PMCA pump protein of the erythrocytes from the propositus was 2.9 ± 0.6 as compared with the control.
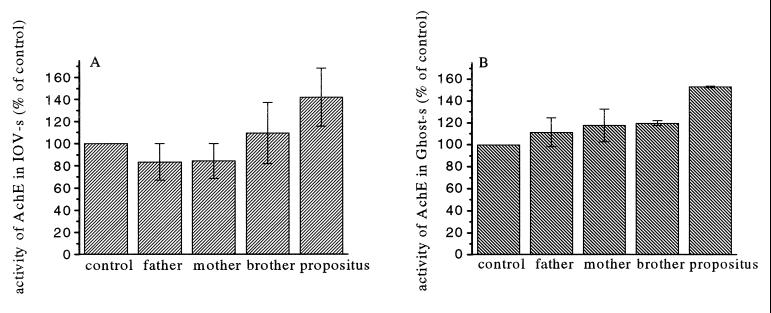
Change in IOV/ROV ratios of the IOV preparations could interfere with the results of the PMCA pump activity. This possibility could be ruled out by measuring the AChE activity, because AChE is an enzyme that specifically binds by a glycosylphosphatidylinositol link to the outer surface of the phospholipid bilayer. The measurement of AChE activity has shown that the ratio of the IOVs did not change. The average ratio of the IOVs in the preparations varied between 62 to 67%. In spite of this, the full AChE enzyme activity was found to be 30–50% higher in the unsealed IOV preparations of the propositus as compared with those from other members of the TPI-deficient family and to those from normal age-matched controls (Fig. 3). The increased AChE activity, measured in nonsealed erythrocyte ghost preparations from the propositus, confirms our observation that membrane enzyme activities are changed parallel with increased membrane fluidity in this TPI-deficient patient with neurological symptoms.
Figure 3.
AChE activity measured in IOVs and ghosts made of erythrocytes. AChE activity of 100 μg IOV membrane protein (A) or 100 μg ghost protein (B) was measured in the presence of 0.016% Triton X-100 as described. Each measurement of the different preparations was carried out in duplicate, and the average of AChE activity was normalized to its own control value (100%). The values with error bars represent the means and standard deviation of the normalized results of five independent experiments. The AChE activity measured in the IOVs or ghosts prepared from the erythrocytes of the propositus was significantly higher (P < 0.05) than the values measured in samples from the control or other members of the family.
DISCUSSION
Membrane fluidity, rotational and translational diffusion of lipids, and proteins are essential for all membrane-bound biological processes. Membrane biophysical properties were shown to have a decisive role in the efficiency of ligand binding, in the outcome of direct cell to cell contacts, and in modulation of the activity of membrane enzymes.
In the search to find an explanation for the differing clinical phenotypes between the two genetically identical compound heterozygote brothers for severe TPI deficiency, significant increase in the activity of calmodulin-induced Ca2+ ATPase and in the activity of AChE were found in the red cell membrane preparations of the propositus with neurological symptoms as compared with those from his neurologically symptom-free brother. We have reported earlier (4) significant increases in fatty acid chain mobility with 2-anthroyloxy-stearic acid fluorophore in the red cell membrane phospholipids of the propositus as compared with those from his neurologically symptom-free brother. Fluorescence anisotropy measurements in the present studies with TMA-DPH in parallel with the estimations of enzyme activities confirmed our former findings of the increased fatty acid chain mobility in the red cell membranes of the propositus as compared with those from his neurologically symptom-free brother. Because this difference in membrane fluidity was shown to disappear in the extract of membrane phospholipids, the difference in TPI function between the two genetically identical brothers may originate from in vivo differences of TPI binding to ultrastructural components of cells, to band 3 in erythrocytes and presumably to tubulin in brain cells. TPI was shown to be a member of the in vivo formed triosephosphate complexes (aldolase + TPI + GDP) that bind in red cells to the negatively charged extreme N-terminal cytoplasmic end of the anion exchanger 1 (band 3) protein (16–19), whereas in the nervous system to tubulin [reviewed by Ovádi (20)]. This binding is markedly influenced by changes in the lipid environment of the binding sites, that significantly differs in various tissues and even among various foci of the same kind of tissue. These differences may result in a variability in focal neurological symptoms in TPI-deficient patients.
Our findings of increased membrane fluidity in the two severely TPI-deficient brothers is strongly supported by the results of the present investigations of the molecular species composition of their erythrocyte phospholipids revealing significant increase in the 16:0/20:4 and 18:0/20:4 molecular species in diacyl-phosphatidylcholines and to a lesser degree in diacyl-phosphatidylethanolamines. Phosphatidylcholine vesicles made of 18:0/20:4 species were shown to exhibit lower DPH fluorescence anisotropy parameter than those made of 18:0/18:1 species (0.167 vs. 0.24) (14) and larger cross-sectional area (70.6 Å2 vs. 66.6 Å2) (21). We found lower steady-state fluorescence anisotropy values for erythrocytes from both compound heterozygotes. The molecular species composition of alkenylacyl-phosphatidylethanolamines in the erythrocytes of the TPI-deficient brothers is identical with those of the controls with the exception of the marked increase in 18:1a/22:4 in the propositus. Whether this results in the observed increase of membrane fluidity should be a subject of further investigations.
In contrast to TPI, Ca2+ ATPase and AChE are typical membrane enzymes crucially involved in specific nerve cell functions. The small acidic protein, calmodulin, is involved in the regulation of many acidic processes controlled by Ca2+-dependent signaling pathways and is suggested to be the primary “decoder” of the rise of the intracellular Ca2+ level. The calmodulin-binding site interacts with the active site of the plasma membrane Ca2+ATPase, repressing it until removed by binding of calmodulin or proteolysis (22). Phosphorylation of the calmodulin-binding domain of the plasma membrane Ca2+-pump by protein kinase C (PKC) causes its dissociation from the active site, inducing thus the activation of the enzyme. The lipid environment of the Ca2+ ATPase was suggested to be critical for its activation by PKC (23).
AChE, through hydrolysis of acetylcholine at the central and peripheral cholinergic synapses, plays an essential role in cholinergic neurotransmission. But in the brain, AChE distribution is not restricted to cholinergic systems nor is the enzyme always membrane bound (24). It has been surmised that AChE may act as a modulator by changing the sensitivity of the nigrostriated cell body to signals received at its distal dendrites (25). This function may play a role in inducing focal neurological symptoms, like the extrapyramidal disorder in our patient.
The increase in calmodulin induced Ca2+ ATPase and AChE activities observed parallel with the increase of membrane fluidity are by no means direct proofs of cause and effect relationship for the explanation of the phenotypical difference between the presence and absence of neurological symptoms in the two genetically identical brothers for severe TPI deficiency. They point, however, to functional differences in the lipid environment influencing the enzyme activities involved in the phenotypic differences. These findings underline the importance of investigating the molecular biophysical changes of lipids that may be involved in the development of disseminated focal neurological disorders of unknown pathogenic origin.
Acknowledgments
We thank Ferenc Antal and Ida Platschek for their skillful technical assistance. These studies were sponsored by the Dave Stewardson Foundation and by grants from the Hungarian National Science Fund (OTKA T 019638 and OTKA T 013177).
ABBREVIATIONS
- TPI
triosephosphate isomerase
- IOV
inside-out vesicle
- ROV
right side-out vesicle
- AChE
acetylcholinesterase
- PMCA
plasma membrane calcium pump activity
- DPH-PA
3-[p-(6-phenyl-1,3,5-hexatrienyl)phenyl]propionic acid
- TMA-DPH
1-(4-trimethylammoniumphenyl)-6-phenyl,1,3,5-haxatriene
References
- 1.Hollán S, Fujii H, Hirono A, Hirono K, Miwa S, Harsányi V, Gyódi É, Inselt-Kovács M. Hum Genet. 1993;92:486–490. doi: 10.1007/BF00216456. [DOI] [PubMed] [Google Scholar]
- 2.Chang M L, Artymiuk P J, Wu X, Hollán S, Lammi A, Maquat L E. Am J Hum Genet. 1993;52:1260–1269. [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider A, Cohen-Solal M. Blood Cells Mol Dis. 1986;22:82–84. doi: 10.1006/bcmd.1996.0011. [DOI] [PubMed] [Google Scholar]
- 4.Hollán S, Dey I, Szollár L, Horányi M, Magócsi M, Harsányi V, Farkas T. Proc Natl Acad Sci USA. 1995;92:268–271. doi: 10.1073/pnas.92.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner R, R. Prog Lipid Res. 1984;23:69–96. doi: 10.1016/0163-7827(84)90008-0. [DOI] [PubMed] [Google Scholar]
- 6.Sarkadi B, Szász I, Gárdos G. Biochim Biophys Acta. 1980;598:326–338. doi: 10.1016/0005-2736(80)90010-3. [DOI] [PubMed] [Google Scholar]
- 7.Steck T L. In: Methods in Membrane Biology. Korn D, editor. Vol. 2. New York: Plenum; 1974. pp. 245–281. [Google Scholar]
- 8.Magócsi M, Penniston J T. Biochim Biophys Acta. 1981;1063:7–14. doi: 10.1016/0005-2736(91)90346-a. [DOI] [PubMed] [Google Scholar]
- 9.Dey I, Farkas T. J Fish Physiol Biochem. 1992;10:347–355. doi: 10.1007/BF00004484. [DOI] [PubMed] [Google Scholar]
- 10.Fine J B, Sprecher H. J Lipid Res. 1982;13:660–663. [PubMed] [Google Scholar]
- 11.Takamura H, Kito M. J Biochem (Tokyo) 1990;109:436–439. doi: 10.1093/oxfordjournals.jbchem.a123399. [DOI] [PubMed] [Google Scholar]
- 12.Bell M V, Dick J R. Lipids. 1991;26:565–573. [Google Scholar]
- 13.Kitagawa S, Matsubayashi M, Kotani K, Usui K, Jametami F. J Membr Biol. 1991;119:221–227. doi: 10.1007/BF01868727. [DOI] [PubMed] [Google Scholar]
- 14.Salem N, Niebilski Ch D. Mol Membr Biol. 1995;12:131–134. doi: 10.3109/09687689509038508. [DOI] [PubMed] [Google Scholar]
- 15.Carafoli E. Physiol Rev. 1991;71:129–153. doi: 10.1152/physrev.1991.71.1.129. [DOI] [PubMed] [Google Scholar]
- 16.Fossel E T, Solomon A K. Biochim Biophys Acta. 1978;510:99–119. doi: 10.1016/0005-2736(78)90133-5. [DOI] [PubMed] [Google Scholar]
- 17.Higoshi T, Richards C S, Uyeda K. J Biol Chem. 1979;254:9542–9550. [PubMed] [Google Scholar]
- 18.Murthy S N P, Liu T H, Kaul R K, Köhler H, Steck T L. J Biol Chem. 1981;256:11203–11208. [PubMed] [Google Scholar]
- 19.Low P S, Rathinavelu P, Harrison M L. J Biol Chem. 1993;268:14627–14631. [PubMed] [Google Scholar]
- 20.Ovádi J. Cell Architecture and Metabolic Channeling. Heidelberg: Springer; 1995. [Google Scholar]
- 21.Garwish K, Holte L L. Chem Phys Lipids. 1996;81:105–116. [Google Scholar]
- 22.James P, Vorherr T, Carafoli E. Trends Biochem Sci. 1995;20:38–42. doi: 10.1016/s0968-0004(00)88949-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang K K W, Wright L C, Machen Ch L, Allen B G, Conigrave A D, Roufogalias B D. J Biol Chem. 1988;266:9078–9085. [PubMed] [Google Scholar]
- 24.Greenfield S A, Chubb I W, Grunwald R A, Henderson A, May J, Portonoy S, Weston J, Wright M C. Exp Brain Res. 1984;54:513–520. doi: 10.1007/BF00235476. [DOI] [PubMed] [Google Scholar]
- 25.Llinas R, Greenfield S A, Jahnsen H. Brain Res. 1984;294:127–132. doi: 10.1016/0006-8993(84)91316-7. [DOI] [PubMed] [Google Scholar]