Abstract
Induction of phase 2 detoxication enzymes [e.g., glutathione transferases, epoxide hydrolase, NAD(P)H: quinone reductase, and glucuronosyltransferases] is a powerful strategy for achieving protection against carcinogenesis, mutagenesis, and other forms of toxicity of electrophiles and reactive forms of oxygen. Since consumption of large quantities of fruit and vegetables is associated with a striking reduction in the risk of developing a variety of malignancies, it is of interest that a number of edible plants contain substantial quantities of compounds that regulate mammalian enzymes of xenobiotic metabolism. Thus, edible plants belonging to the family Cruciferae and genus Brassica (e.g., broccoli and cauliflower) contain substantial quantities of isothiocyanates (mostly in the form of their glucosinolate precursors) some of which (e.g., sulforaphane or 4-methylsulfinylbutyl isothiocyanate) are very potent inducers of phase 2 enzymes. Unexpectedly, 3-day-old sprouts of cultivars of certain crucifers including broccoli and cauliflower contain 10–100 times higher levels of glucoraphanin (the glucosinolate of sulforaphane) than do the corresponding mature plants. Glucosinolates and isothiocyanates can be efficiently extracted from plants, without hydrolysis of glucosinolates by myrosinase, by homogenization in a mixture of equal volumes of dimethyl sulfoxide, dimethylformamide, and acetonitrile at −50°C. Extracts of 3-day-old broccoli sprouts (containing either glucoraphanin or sulforaphane as the principal enzyme inducer) were highly effective in reducing the incidence, multiplicity, and rate of development of mammary tumors in dimethylbenz(a)anthracene-treated rats. Notably, sprouts of many broccoli cultivars contain negligible quantities of indole glucosinolates, which predominate in the mature vegetable and may give rise to degradation products (e.g., indole-3-carbinol) that can enhance tumorigenesis. Hence, small quantities of crucifer sprouts may protect against the risk of cancer as effectively as much larger quantities of mature vegetables of the same variety.
Keywords: chemoprotection, glucosinolates, isothiocyanates, sulforaphane, glucoraphanin
Many types of chemoprotectors against cancer evoke large inductions of phase 2 enzymes of xenobiotic metabolism and increase glutathione levels in animal tissues (1). These cellular responses accelerate the detoxication of electrophiles and reactive forms of oxygen, and thereby protect cells against mutagenesis and neoplasia. Substantial evidence suggests that induction of these detoxication enzymes provides a major strategy for achieving protection against malignancy (1). The chemical specificity of the inducers and the molecular mechanisms of regulation of phase 2 enzymes are under active investigation in several laboratories (2–6). Edible plants contain a wide variety of minor metabolites, some of which are phase 2 enzyme inducers. Since extensive epidemiological evidence, backed by animal experiments, shows that diets rich in fruits and vegetables are associated with large and dose-related reductions in the risk of developing cancer (7–10), it is likely that these metabolites are at least partially responsible for protection. Crucifers (e.g., broccoli, cauliflower, kale, and Brussels sprouts), which are rich in phase 2 enzyme inducers (11), may play a special role in affording such protection (12, 13).
A simple cell culture system, developed to detect and quantitate the potency of phase 2 enzyme inducers, measures the elevation of NAD(P)H:quinone reductase (QR; a typical phase 2 enzyme) in murine hepatoma cells grown in 96-well microtiter plates (11, 14). This assay was critical for the isolation of the isothiocyanate sulforaphane as the principal and exceedingly potent monofunctional enzyme inducer in broccoli (15). Sulforaphane induces several phase 2 enzymes in both cultured cells and mouse tissues (15), blocks 7,12-dimethylbenz(a)anthracene (DMBA)-initiated mammary tumor formation in rats (16), and inhibits neoplastic nodule formation in cultured mouse mammary glands (17).
Isothiocyanates, including sulforaphane, are synthesized and stored in plants as relatively stable precursors, known as glucosinolates (β-thioglucoside N-hydroxysulfates), which are hydrolyzed to isothiocyanates by myrosinase (β-thioglucoside glucohydrolase; EC 3.2.3.1). Myrosinase (see ref. 18) is normally segregated from glucosinolates and is released when plant cells are injured. Myrosinase catalyzes the following reaction:
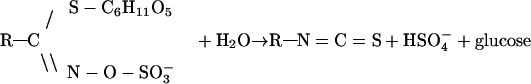 |
Determination of the isothiocyanate and glucosinolate contents of plants has been complicated by the difficulty of quantitatively extracting both types of compounds while avoiding the hydrolysis of glucosinolates by myrosinase. We have overcome this problem by a novel method that provides extracts suitable for nondestructive glucosinolate analysis by paired-ion chromatography (19) and for spectroscopic measurement of isothiocyanates and glucosinolates (after myrosinase treatment) by a cyclocondensation reaction with 1,2-benzenedithiol (20, 21).
As part of our long-term goals of identifying and developing edible plants for chemoprotection, we describe the presence of extremely high concentrations of phase 2 enzyme inducer activity in young sprouts of cruciferous plants (e.g., broccoli), relate this inducer activity to the presence of specific glucosinolates, and demonstrate the high potency of extracts of these plants as chemoprotectors against experimental mammary tumors.
MATERIALS AND METHODS
Plant Source and Cultivation.
Seeds not treated with pesticides were obtained commercially. Sprouts were produced from seeds surface-sterilized by a 1-min rinse in 70% ethanol, a 15-min exposure to 1.3% sodium hypochlorite containing 0.001% Alconox detergent, and exhaustive rinsing with sterile distilled water. The broccoli (Brassica oleracea var. italica) cultivar used was SAGA, unless stated otherwise. Sprouts were grown without added nutrients either aseptically on 0.7% agar or in inclined perforated trays (35 × 40 cm) watered with four 15-s gentle spray cycles per h. All sprouts were grown with a 16-h light and 8-h dark photoperiod and a corresponding 25/20°C cycle for agar-grown sprouts or a constant 25°C for tray-grown sprouts. Sprouts were rapidly and gently collected from the surface of the agar or spray tray immediately before extraction to minimize hydrolysis of glucosinolates by endogenous myrosinase. Mature and frozen vegetables were obtained from local supermarkets. Plants not extracted on the day of collection were stored at −80°C.
Extraction of Phase 2 Enzyme Inducers from Plants.
Vegetables were homogenized with 10 vol of a mixture of equal volumes of dimethyl sulfoxide, dimethylformamide, and acetonitrile (triple solvent) maintained at about −50°C in a dry-ice/ethanol bath. Samples were homogenized, depending on sample size, in a glass homogenizer, a Brinkmann Polytron homogenizer, or a Waring Blendor. Other extractive solvents were boiling methanol, boiling water, ice-cold water, and acetonitrile. In all cases homogenates were centrifuged to remove remaining particulates and stored at −20°C until analyzed.
Myrosinase Purification.
The enzyme was purified from 8-day-old Raphanus sativus (daikon) seedlings by sequential chromatography procedures to be published separately. The dimeric 120-kDa ascorbic acid-requiring enzyme was purified approximately 230-fold to a specific activity of 184 μmol of sinigrin hydrolyzed per min per mg of protein and was used to hydrolyze glucosinolates for chemical and inducer activity assays.
Bioassay of Inducer Potency.
Induction of quinone reductase was measured in Hepa 1c1c7 murine hepatoma cells grown in 96-well microtiter plates (11, 14). Usually, 15 μl of the solution to be assayed (in water, methanol, acetonitrile, or triple solvent) was diluted to 3.0 ml with medium and serial dilutions were used for the microtiter plates. Excess purified myrosinase and 500 μM ascorbate were added to each well to achieve complete glucosinolate hydrolysis. The final concentration of organic solvent was ⋜0.5% by volume. Modifications of the published procedure (11) included (i) use of fetal calf serum treated with charcoal (1 g/100 ml) for 90 min at 55°C and (ii) assessment of cytotoxicity by measurement of protein concentration as follows: a 20-μl aliquot of the digitonin cell lysate was transferred to a replica 96-well microtiter plate and 300 μl of bicinchoninic acid reagent was added (22). One unit of inducer activity is the amount that doubles the QR activity in a microtiter well containing 150 μl of medium. Hence, a compound with a CD value (the concentration of a compound required to double the QR specific activity in Hepa 1c1c7 murine hepatoma cells) of 1.0 μM has 6,667 units of inducer activity per μmol. We express the inducer potency of plant extracts as units/g fresh weight (fr. wt.) or dry weight.
Measurement of Isothiocyanates and Glucosinolates.
Isothiocyanate concentrations of plant extracts were determined spectroscopically by cyclocondensation with 1,2-benzenedithiol to produce 1,3-benzodithiole-2-thione (ɛ of 23,000 M−1⋅cm−1 at 365 nm) (20, 21). Aqueous extracts were used directly; organic solvent extracts were first evaporated to dryness and then redissolved in water. Glucosinolates in these extracts were quantitatively converted to isothiocyanates by treatment with purified myrosinase for 2 h at 37°C and then subjected to cyclocondensation. Indole glucosinolates cannot be measured in this fashion because their isothiocyanates are unstable (see below).
Paired-Ion Chromatography of Glucosinolates.
Plant extracts were chromatographed in acetonitrile/water (1:1, vol/vol) containing 5 mM tetradecylammonium bromide at a flow rate of 3 ml/min, on reverse-phase columns (Whatman Partisil 10 ODS-2; 250 × 4 mm) on a Waters HPLC system equipped with a photodiode array detector (19). Sinigrin (allyl glucosinolate) was used as a standard. The relative integrated absorbance areas at 235 nm for alkylthioglucosinolates such as glucoraphanin, glucobrassicin, and neoglucobrassicin were 1.00, 1.22, and 2.70 times, respectively, those of an equimolar quantity of sinigrin.
Inhibition of Mammary Tumor Development in Rats.
Mammary tumors were produced in female Sprague–Dawley rats by feeding single 10-mg doses of DMBA by gavage at age 50 days (16). Glucosinolate or isothiocyanate preparations obtained from broccoli sprouts and vehicle control (all in 1.0 ml of equal volumes of Emulphor 620P and water) were administered by daily gavage on days 47–51 (2 h before the DMBA dose on day 50).
Plant Preparation.
Three-day-old broccoli sprouts were rapidly plunged into 5 vol of boiling water and boiling was continued for 3 min. The mixture was then cooled, filtered, and lyophilized to provide a dry powder.
To prepare glucosinolates, the dried powder was dissolved in water and analyzed for inducer activity and for glucosinolate and isothiocyanate content. Excess purified myrosinase was added to both analyses. The preparation was then diluted with water and mixed with an equal volume of Emulphor 620P, so that 1.0 ml contained 25 or 100 μmol of glucosinolates (Table 1).
Table 1.
Analyses of 3-day-old broccoli sprout preparations used for rat mammary tumor inhibition studies
| Preparation | Bioassay of inducer activity, units/mg dry wt. | Concentration of inducer, nmol/mg dry wt.
|
|
|---|---|---|---|
| Calculated from bioassay | Measured by cyclocondensation | ||
| Glucosinolate | 10,300 | 309* | 334 |
| Isothiocyanate | 7,860 | 236* | 228 |
These values assume that the inducer activity is entirely derived from myrosinase-hydrolyzed glucoraphanin, i.e., sulforaphane (CD = 0.2 μM, or 33.3 units of inducer activity per nmol). The dry weights of the powder for the 100-μmol dose were 324 and 424 mg for the glucosinolate and isothiocyanate preparations, respectively.
To prepare isothiocyanates, the above-described powder was mixed with 2% (wt/wt) (based on the original fr. wt. of broccoli sprouts) of 9-day-old daikon (Raphanus sativus) sprouts as an abundant source of crude myrosinase. The mixture was homogenized and incubated for 3 h at 37°C, which resulted in quantitative conversion of glucosinolates to isothiocyanates. It was filtered, lyophilized, and assayed for inducer activity and for isothiocyanate content. The added daikon accounted for less than 1% of the isothiocyanate content and of the total inducer activity. The preparation was then diluted with water and mixed with an equal volume of Emulphor 620P so that 1.0 ml contained 25, 50, or 100 μmol of isothiocyanates (Table 1).
Progression of tumor development was monitored by palpation at weekly intervals. Kaplan–Meier analyses followed by log-rank comparisons were used to test for differences in tumor incidence. Animals were necropsied at 167 days of age, and mammary tumors were excised, counted, and weighed. All animal experiments were in compliance with National Institutes of Health Guidelines and were approved by a Johns Hopkins University Animal Care and Use Committee.
RESULTS AND DISCUSSION
Extraction and Quantitation of Glucosinolates and Isothiocyanates from Crucifers.
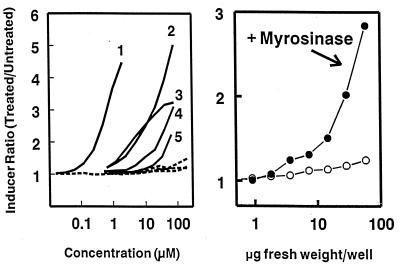
The isothiocyanate sulforaphane was isolated from acetonitrile extracts of lyophilized aqueous homogenates of broccoli and was identified as the principal and very potent phase 2 enzyme inducer of this crucifer (15). This finding, and the subsequent demonstration of the tumor blocking activity of sulforaphane (16), focused our attention on the potential of plant-derived isothiocyanates as chemoprotectors and emphasized the long-recognized chemoprotective properties of this class of compounds (23). However, it is now apparent that the isolated sulforaphane was derived largely from its glucosinolate (glucoraphanin) through the action of endogenous myrosinase during the isolation procedure. Glucosinolates per se are not inducers but are converted quantitatively by treatment with myrosinase to their cognate isothiocyanates, which are inducers of phase 2 enzymes (Fig. 1 Left).
Figure 1.
Induction of quinone reductase in Hepa 1c1c7 murine hepatoma cells as a function of concentration of glucosinolates (Left) and of an extract of mature fresh broccoli (Right). (Left) Five glucosinolates (GS). Curves: 1, glucoraphanin (the glucosinolate of sulforaphane); 2, gluconapin (3-butenyl-GS); 3, sinigrin (allyl-GS); 4, progoitrin (2-hydroxy-3-butenyl-GS); 5, glucobrassicin (indol-3-ylmethyl-GS). Solid lines, myrosinase treated; dashed lines, untreated. (Right) A triple solvent extract of mature broccoli obtained from a local supermarket. Induction potencies were measured, in the absence and presence of purified myrosinase, in 96-well microtiter plates containing serial dilutions of aqueous solutions of the pure glucosinolates (expressed as micromolar concentrations) or of broccoli extracts (expressed as fr. wt. equivalents in μg per well). Note the logarithmic concentration scales. From the CD value of 30 μg fr. wt. equivalent per well, one can calculate that this sample of mature broccoli contained 33,300 units/g fr. wt. of latent inducer activity (compare with Fig. 2).
Our search for edible plants with high levels of inducer activity required development of methods for efficient extraction and quantitation (preferably in the same extracts) of generally hydrophobic isothiocyanates and water-soluble glucosinolates, under conditions that prevented the hydrolysis of glucinolates by myrosinase. It was also desirable to obtain extracts without use of hot solvents to avoid loss of volatile and highly reactive isothiocyanates and to use water-miscible solvents that could be directly assayed for QR inducer potency (11, 14) and for isothiocyanate content by cyclocondensation with 1,2-benzenedithiol (20, 21).
Of a range of solvents and solvent combinations examined, a mixture of equal volumes of dimethyl sulfoxide, dimethylformamide, and acetonitrile (triple solvent) was particularly effective. Rapid immersion and homogenization of fresh or frozen plant samples in 10 vol of triple solvent at −50°C, followed by filtration or centrifugation, provided soluble extracts that contained both glucosinolates and isothiocyanates in high yield. We compared the inducer activities of myrosinase-treated extracts of five freshly harvested adult heads of broccoli. Several extraction procedures were used including ice-cold water, boiling water, boiling aqueous 80% methanol, and triple solvent, as well as acetonitrile extraction of lyophilized aqueous homogenates. The triple solvent and 80% methanol extracts had very high inducer activity (38,000–45,000 units/g fr. wt.), whereas ice-cold and boiling water extracts contained only about 30% and acetonitrile extracts contained about 10% of this activity. Furthermore, triple solvent extracts showed essentially no inducer activity without added myrosinase, which is not itself an inducer (Fig. 1 Right), whereas the smaller amounts of inducer activity extracted with water were not increased by myrosinase treatment. Cold triple solvent is a simple, efficient, and reproducible extractant for both glucosinolates and isothiocyanates and also prevents myrosinase action. By using this extraction procedure, followed by the addition of purified myrosinase, we have shown that nearly all the inducer activity of the cruciferous plants evaluated is derived from glucosinolates.
Comparison of Inducer Activity of Fresh and Frozen Mature Stage Vegetables.
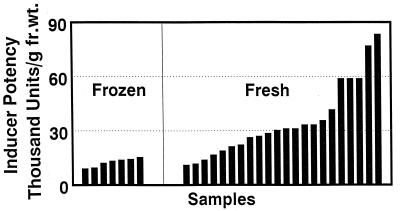
By using triple solvent for extracting glucosinolates and converting them to their bioactive isothiocyanates with myrosinase, we compared phase 2 enzyme inducer activities from a variety of cruciferous plants (arugula, broccoli, Brussels sprouts, cabbage, cauliflower, Chinese cabbage, collards, crambe, daikon, kale, kohlrabi, mustard, red radish, turnip, and watercress). Although our earlier study found less than 3,400 units of inducer activity per g fr. wt of a variety of such vegetables including broccoli (recalculated from ref. 11 to permit dry/fr. wt. comparisons), extraction with triple solvent provided between 10,000 and 100,000 units/g fr. wt. This finding of dramatically higher inducer activities is attributable to much more efficient extraction of the inducers and hydrolysis of glucosinolates by added myrosinase. Because broccoli extracts were typically the most potent, we compared the inducer activities of 7 samples of frozen broccoli (five national brands) with those of 22 randomly collected fresh broccoli samples (cultivars unknown) obtained from local supermarkets. Activities of frozen samples ranged from 9,000 to 15,000 units/g fr. wt., whereas the fresh samples had an almost 8-fold range of potencies from 11,100 to 83,300 units/g fr. wt. (mean = 35,100 units/g fr. wt.; median = 30,800 units/g fr. wt.; Fig. 2). The inducer activities of the fresh broccoli samples were unrelated to their physical appearance or whether grown under conventional or organic conditions. In all 7 samples of frozen broccoli and in 21 of 22 triple solvent extracts of fresh mature broccoli, no inducer activity was detectable before addition of myrosinase. Therefore, we conclude that the contribution of phytochemicals other than glucosinolates to the inducer activity of these extracts is negligible. The generally lower potencies of frozen broccoli samples may have been due to unfavorable storage conditions or to removal of glucosinolates during the blanching process. Both cultivar and many environmental factors have significant effects on glucosinolate content and, consequently, on inducer potency.
Figure 2.
Quinone reductase inducer activities of randomly selected fresh and frozen broccoli samples purchased at Baltimore area supermarkets. Florets of seven frozen broccoli lots representing five national brands (Hanover, America’s Choice, Green Giant, BirdsEye, and BelAir) were extracted with cold triple solvent. Each bar represents the analysis of a portion of a pooled 3- to 5-lb. sample from a single lot (1 lb. = 0.45 kg). Mean inducer potencies of the frozen broccoli samples were 12,000 units/g fr. wt. Twenty-two fresh bunches (one to four heads each) of conventionally and organically grown broccoli were obtained from local supermarkets. The florets were homogenized in cold triple solvent and assayed for inducer activity in the presence of added purified myrosinase. Mean potency is 35,000 units/g fr. wt. Negligible quantities of inducer activity were detected in most samples before addition of myrosinase (see text).
Effects of Plant Age on Inducer Potencies of Cruciferous Vegetables.
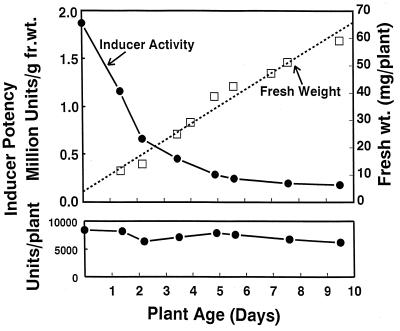
Preliminary experiments indicated that inducer potencies (expressed per g of plant) of extracts of young sprouts of arugula, bok choy, broccoli, Brussels sprouts, cabbage, cauliflower, Chinese cabbage, collards, cress, daikon, kale, kohlrabi, mustard, turnip, and watercress ranged from 10 to 100 times those of mature field-grown plants. Similarly, in sprouts of eight broccoli cultivars, grown without exogenous nutrients, the inducer activity (nearly all of which arose from glucosinolates) per unit plant weight declined initially in an exponential manner from a maximum in the seed (Fig. 3) and continued to decline thereafter, approaching the values in mature broccoli heads after about 15 days (data not shown), whereas the total inducer activity per plant remained constant. The inducer activity fell from 1.8 million units/g of seeds to 180,000 units/g fr. wt. at 9 days, largely due to an increase in plant weight from seeds (3.3 mg) to 9-day-old sprouts (60 mg). Apparently no significant net synthesis of glucosinolates occurred under these conditions.
Figure 3.
Effect of age on the inducer potency of broccoli sprout extracts. Broccoli sprouts were harvested daily for 9 days. Extracts were prepared by homogenization in cold triple solvent and assayed for inducer activity with myrosinase and ascorbate added to the microtiter well plates. Inducer potencies of the sprouts are expressed both “per mg fresh plant weight” (Upper) and “per plant” (Lower). Mean fresh weights were determined on samples of at least 20 sprouts per time point. The initial seed weight is about 3.5 mg, and sprout weight increases by about 6.6 mg/day. Inducer specific activities of myrosinase-treated sprout extracts are compared with those of seeds. Without myrosinase treatment, extracts contained <1,000 units/g fr. wt.
When broccoli sprout extracts were tested with mutant murine hepatoma cells defective in cytochrome P450 activity, they were fully active as inducers (data not shown), demonstrating that they contain inducers that are monofunctional (24). This is to be expected since most of the inducer activity of sprouts arises from sulforaphane, which is a monofunctional inducer (15). In contrast bifunctional inducers raise both phase 2 enzymes and certain phase 1 enzymes (cytochromes P450) via an aryl hydrocarbon receptor-dependent mechanisms.
Whereas inducer activity was high for sprouts of all crucifers examined, it was consistently highest for broccoli and cauliflower cultivars. Three-day-old sprouts of random commercial cultivars of broccoli (n = 26) and cauliflower (n = 28) produced inducer potencies ranging from 92,500 to 769,000 units/g fr. wt. (mean = 293,000 units/g fr. wt.) and from 50,000 to 560,000 units/g fr. wt. (mean = 251,000 units/g fr. wt.), respectively. Repetitive kilogram-scale harvests of 3-day-old broccoli (cultivar SAGA) sprouts yielded an average of 511,000 units/g fr. wt. (n = 14).
Glucosinolate Profiles of Sprouts and Mature Broccoli.
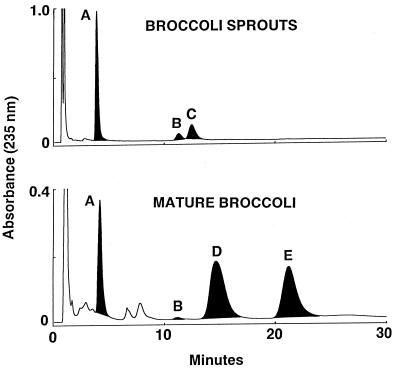
The dramatic quantitative differences between the inducer potencies of young sprouts and mature crucifers grown from the same seed lots are associated with equally striking qualitative and quantitative differences in the glucosinolate profiles of these plants. Paired-ion chromatography confirmed that the major glucosinolates in mature broccoli are typically indoles: glucobrassicin, neoglucobrassicin (18, 25, 26), and smaller quantities of 4-hydroxyglucobrassicin (for example, see Fig. 4). Although the indole glucosinolate composition varied considerably among cultivars and even among different samples of the same cultivar, indole glucosinolates accounted, on average, for 67% of the total glucosinolates in extracts prepared from 7 broccoli cultivars (DeCicco, Emperor, Everest, Excelsior, Green Comet, Green Valiant, and SAGA). In contrast, in most 3-day-old broccoli and cauliflower sprouts and seeds (>70 cultivars examined), less than 10% of total glucosinolates were indoles, and in many cultivars none could be detected (Fig. 4). These sprouts contained primarily alkylthioglucosinolates: typically glucoraphanin with smaller quantities of glucoerucin and glucoiberin (3-methylsulfinylpropyl glucosinolate). The total glucosinolate contents are 22.7 and 3.37 μmol/g fr. wt. for the sprouts and mature broccoli, respectively. Indole glucosinolates, however, accounted for only 3% of the total glucosinolates in these sprouts, compared with 68% of those in the mature plant. There are 20 times more methylsulfinylalkyl glucosinolates (glucoraphanin and glucoerucin) in the sprouts compared with the mature broccoli. A 100-g serving of mature broccoli would, therefore, provide 108 μmol of methylsulfinylalkyl glucosinolates and 229 μmol of indole glucosinolates, whereas consumption of an equivalent quantity of methylsulfinylalkyl glucosinolates via a much smaller serving of sprouts (5 g) would result in the consumption of only 11.2 μmol of indole glucosinolates.
Figure 4.
Comparison of glucosinolate HPLC profiles of 2-day-old broccoli sprouts with those of florets from heads of mature broccoli grown from the same seeds of cultivar SAGA. All samples were extracted with triple solvent and the component glucosinolates were resolved by paired-ion chromatography (18). Extracts equivalent to 62.4 mg fr. wt. of mature broccoli and 9.45 mg fr. wt of sprouts were analyzed. Glucosinolates were identified by reanalysis after treatment with myrosinase; the shaded black peaks disappeared upon such treatment. The glucosinolates are as follows. Peaks: A, glucoraphanin; B, 4-hydroxyglucobrassicin; C, glucoerucin; D, glucobrassicin; E, neoglucobrassicin. The chemical identities of peaks A, C, D, and E were confirmed by mass spectrometry and NMR (18). In the sprout extract, the major peaks are glucoraphanin (16.6 μmol/g fr. wt.) and glucoerucin (5.41 μmol/g fr. wt.), and only 0.71 μmol/g fr. wt. of 4-hydroxyglucobrassicin. The mature broccoli extract contained glucoraphanin (1.08 μmol/g fr. wt.) and the indoles glucobrassicin (1.67 μmol/g fr. wt) and neoglucobrassicin (0.62 μmol/g fr. wt.).
These differences in glucosinolate profiles between young sprouts and mature broccoli are of considerable interest and potential importance in devising chemoprotective strategies in humans. The methylsulfinylalkyl glucosinolates contained in high concentrations in sprouts are monofunctional inducers (see above, and ref. 15). Moreover, sulforaphane does not appear to be significantly genotoxic in that it stimulates neither unscheduled DNA synthesis in hepatocytes nor the formation of histidine revertants in the Salmonella typhimurium test (27). In contrast, hydrolysis of indole glucosinolates by myrosinase gives rise to bifunctional inducers, such as indole-3-carbinol and indole-3-nitrile, and to condensation products, such as 3,3′-diindolylmethane and indole-3-carbazole, which bind to the aryl hydrocarbon receptor (28). Indole-3-carbinol is both an inhibitor and an enhancer of tumor formation in animals, depending upon the experimental system and the timing of administration in relation to exposure to carcinogen (see references in ref. 29). Consequently, there are potential limitations to the use of indole glucosinolates as chemoprotectors in humans because they (i) are weak inducers of phase 2 enzymes, (ii) are bifunctional inducers that activate phase 1 enzymes, (iii) may have estrogen receptor binding activity (30), and (iv) are potential tumor promoters.
Inhibition of DMBA-Elicited Mammary Tumor Development in Rats by Broccoli Sprout Extracts.
Sulforaphane and several synthetic acetylnorbornyl isothiocyanate analogues, which are potent phase 2 enzyme inducers, reduced the incidence, multiplicity, and weight of mammary tumors and retarded tumor development in female Sprague–Dawley rats treated with a single dose of DMBA (16). We therefore reasoned that extracts of cruciferous sprouts containing high levels of glucoraphanin and associated high inducer activity should likewise have antitumor activity. Accordingly, boiling water extracts of 3-day-old broccoli sprouts rich in glucoraphanin were evaluated as inhibitors in the DMBA rat mammary tumor system. Although triple solvent is a more efficient solvent, it was not used to make these preparations because solvent residues could confound interpretation of feeding experiments. Since the inducer activity of 3-day-old broccoli sprout extracts is derived nearly exclusively from two glucosinolates (usually about 75% glucoraphanin and 25% glucoerucin), we examined the extracts before and after myrosinase treatment to determine the effects of glucosinolates and isothiocyanates, respectively, on tumor inhibition. These sprout preparations were standardized according to their inducer activities in the murine hepatoma cell assay, by assuming as an approximation that the entire inducer activity was derived from glucoraphanin or sulforaphane. Daily doses of glucosinolates (25 and 100 μmol) and isothiocyanates (25, 50, and 100 μmol) were administered on days 47–51 to groups of 20 rats that also received 10 mg of DMBA at 50 days of age, 2 h after administration of the sprout extract, according to a standard protocol (16).
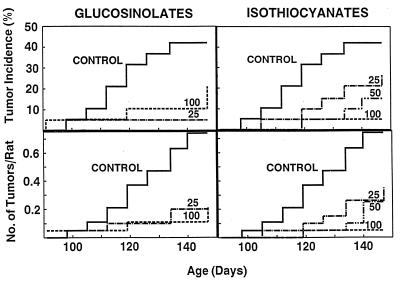
Mammary tumor development, monitored by palpation at weekly intervals, was significantly retarded compared with controls, with respect to tumor multiplicity (tumors per animal), and incidence (fraction of tumor-bearing animals) (Fig. 5). The tumor incidence at all doses of glucosinolate and isothiocyanate treatment was lower than in controls (P = 0.0197 and 0.0190, respectively). Analysis of tumor progression by palpation was terminated at 147 days of age. Retardation of tumor development was clearly dose-dependent for the isothiocyanates, but the potency of the 25 μmol dose of glucosinolates appeared to be similar to that of the 100-μmol dose, suggesting that the lower dose may have produced a nearly maximal effect. All treatments markedly reduced the total number of tumors found at necropsy. There were 34 tumors in the control group (20 surviving animals) and 11–19 tumors in the treated groups (19–20 surviving animals).
Figure 5.
Effects of hot-water extracts of 3-day-old broccoli sprouts on mammary tumor development in DMBA-treated female Sprague–Dawley rats. The analytical composition of the glucosinolate and isothiocyanate extracts is given in Table 1. The animals were examined for tumors at weekly intervals. (Upper) Tumor incidence (percent of rats with tumors). (Lower) Tumor multiplicity (no. of tumors/no. of rats at risk). Progression of tumor development in the single control group is shown in all panels. The animals received daily intragastric doses of 25 or 100 μmol of glucosinolates (Left) and 25, 50, or 100 μmol of isothiocyanates (Right) at age 47–51 days. The 25-μmol doses corresponded to 81 and 106 mg of lyophilized extract for the glucosinolate and isothiocyanate preparations, respectively. Statistical significance of the retardation of tumor development (incidence) compared with controls was as follows. For 25 and 100 μmol doses of glucosinolates, P = 0.0079 and 0.138, respectively. For 25, 50, and 100 μmol doses of isothiocyanates, P = 0.214, 0.0422, and 0.0058, respectively.
The tumor inhibitory effects of sprout extracts in these experiments are comparable to those observed with 75 μmol of synthetic sulforaphane in a similar protocol (16). It therefore seems unlikely that broccoli sprouts contain significantly potent chemoprotectors other than the glucosinolates/isothiocyanates identified in these studies. Although the chemoprotective effects of isothiocyanates are well recognized (23), to our knowledge the only antitumor experiments with glucosinolates are those of Wattenberg et al. (31) who reported that relatively large single doses of benzyl glucosinolate (147 μmol) and glucobrassicin (134 μmol) significantly decreased mammary tumor formation in DMBA-treated rats. The possibility that the chemoprotective effect of glucosinolates may not depend on hydrolysis to isothiocyanates needs consideration.
The high potency of lyophilized hot-water extracts of broccoli sprouts in suppressing mammary tumor development is in sharp contrast to the few previous studies on the tumor suppressive effects of feeding dried mature crucifers to rodents. In the present study (Fig. 5) daily administration of dried sprout extracts ranging from 81 to 424 mg (i.e., 0.54 to 2.83% of an estimated 15-g daily food intake) provided a highly significant tumor suppressor effect. In contrast, 5–40% dried mature crucifers in the diet were required to achieve protection (see ref. 10 for references).
CONCLUSIONS
Large quantities of inducers of enzymes that protect against carcinogens can be delivered in the diet by small quantities of young crucifer sprouts (e.g., 3-day-old broccoli sprouts) that contain as much inducer activity as 10–100 times larger quantities of mature vegetables. Moreover, the inducer activity arises primarily from glucoraphanin (the glucosinolate of sulforaphane) and such sprouts contain relatively low quantities of indole glucosinolates, which are potential tumor promoters. Because little is known of the metabolism of glucosinolates in humans, we have undertaken studies (to be published separately) that demonstrate efficient conversion of glucosinolates to isothiocyanates in humans in the absence of plant myrosinase.
Acknowledgments
Observations reported in this paper are the subject of issued and pending patents. We thank Katherine K. Stephenson, Kristina L. Wade, and Mark Wrona for expert technical assistance; W. David Holtzclaw and Tory Prestera for help in isolation and identification of the glucosinolates; Mikio Shikita for purification of myrosinase; Patrick M. Dolan for assistance with the rat mammary tumor experiments; Stephen J. Gange for statistical analysis; and Thomas W. Kensler and Theresa A. Shapiro for constructive review of the manuscript. We are grateful to the following growers for providing mature cruciferous vegetables grown to our specifications: G. & Y. Johnson, M. Rice, M. Voelkel, M. Heller, J. Martin, W. & S. Hastings, J. & L. Carty, P. Holloway, and T. & L. Harding. These studies were supported by generous gifts from Lewis B. Cullman, Charles B. Benenson, and other Friends of the Brassica Chemoprotection Laboratory; by a Program-Project Grant (P01 CA 44530) from the National Cancer Institute, Department of Health and Human Services; and by grants from the American Institute for Cancer Research and the Cancer Research Foundation of America.
ABBREVIATIONS
- QR
quinone reductase [NAD(P)H:(quinone-acceptor) oxidoreductase, EC 1.6.99.2]
- DMBA
7,12-dimethylbenz(a)anthracene
- glucoraphanin
the glucosinolate of sulforaphane or 4-methylsulfinylbutyl isothiocyanate [CH3S(O)(CH2)4NCS]
- glucoerucin
the glucosinolate of erucin or 4-methylthiobutyl isothiocyanate [CH3S(CH2)4NCS]
- glucobrassicin
indol-3-ylmethyl glucosinolate
- neoglucobrassicin
1-methoxyindol-3-ylmethyl glucosinolate
- 4-hydroxyglucobrassicin
4-hydroxyindol-3-ylmethyl glucosinolate
- fr. wt.
fresh weight
References
- 1.Talalay P, Fahey J W, Holtzclaw W D, Prestera T, Zhang Y. Toxicol Lett. 1995;82/83:173–179. doi: 10.1016/0378-4274(95)03553-2. [DOI] [PubMed] [Google Scholar]
- 2.Talalay P, De Long M J, Prochaska H J. Proc Natl Acad Sci USA. 1988;85:8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prestera T, Holtzclaw W D, Zhang Y, Talalay P. Proc Natl Acad Sci USA. 1993;90:2965–2969. doi: 10.1073/pnas.90.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friling R S, Bergelson S, Daniel V. Proc Natl Acad Sci USA. 1992;89:668–672. doi: 10.1073/pnas.89.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen T, Rushmore T H, Pickett C B. J Biol Chem. 1994;269:13656–13662. [PubMed] [Google Scholar]
- 6.Wasserman W W, Fahl W E. Proc Natl Acad Sci USA. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Block G, Patterson B, Subar A. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 8.Doll R. Cancer Res Suppl. 1992;52:2024s–2029s. [PubMed] [Google Scholar]
- 9.Steinmetz K A, Potter J D. Cancer Causes Control. 1991;2:325–357. doi: 10.1007/BF00051672. [DOI] [PubMed] [Google Scholar]
- 10.Steinmetz K A, Potter J D. J Am Diet Assoc. 1996;96:1027–1039. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 11.Prochaska H J, Santamaria A B, Talalay P. Proc Natl Acad Sci USA. 1992;89:2394–2398. doi: 10.1073/pnas.89.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verhoeven D T H, Goldbohm R A, van Poppel G, Verhagen H, van den Brandt P A. Cancer Epidemiol Biomarkers Prev. 1996;5:733–748. [PubMed] [Google Scholar]
- 13.Verhoeven D T H, Verhagen H, Goldbohm R A, van den Brandt P A, van Poppel G. Chem-Biol Interact. 1997;103:79–129. doi: 10.1016/s0009-2797(96)03745-3. [DOI] [PubMed] [Google Scholar]
- 14.Prochaska H J, Santamaria A B. Anal Biochem. 1988;169:328–336. doi: 10.1016/0003-2697(88)90292-8. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Talalay P, Cho C-G, Posner G H. Proc Natl Acad Sci USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Kensler T W, Cho C-G, Posner G H, Talalay P. Proc Natl Acad Sci USA. 1994;91:2147–2150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerhäuser C, You M, Liu J, Moriarty R M, Hawthorne M, Mehta R G, Moon R C, Pezzuto J M. Cancer Res. 1997;57:272–278. [PubMed] [Google Scholar]
- 18.Rosa E A S, Heaney R K, Fenwick G R, Portas C A M. Hortic Rev. 1997;19:99–215. [Google Scholar]
- 19.Prestera T, Fahey J W, Holtzclaw W D, Abeygunawardana C, Kachinski J L, Talalay P. Anal Biochem. 1996;239:168–179. doi: 10.1006/abio.1996.0312. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Cho C-G, Posner G H, Talalay P. Anal Biochem. 1992;205:100–107. doi: 10.1016/0003-2697(92)90585-u. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Wade K L, Prestera T, Talalay P. Anal Biochem. 1996;239:160–167. doi: 10.1006/abio.1996.0311. [DOI] [PubMed] [Google Scholar]
- 22.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Talalay P. Cancer Res Suppl. 1994;54:1976s–1981s. [PubMed] [Google Scholar]
- 24.Prochaska H J, Talalay P. Cancer Res. 1988;48:4776–4782. [PubMed] [Google Scholar]
- 25.Lewis J A, Fenwick G R. Food Chem. 1987;25:259–268. [Google Scholar]
- 26.McDanell R, McLean A E M, Hanley A B, Heaney R K, Fenwick G R. Food Chem Toxicol. 1988;26:50–70. doi: 10.1016/0278-6915(88)90042-7. [DOI] [PubMed] [Google Scholar]
- 27.Barcelo S, Gardiner J M, Gescher A, Chipman J K. Carcinogenesis. 1996;17:277–282. doi: 10.1093/carcin/17.2.277. [DOI] [PubMed] [Google Scholar]
- 28.Bjeldanes L F, Kim J-Y, Grose K R, Bartholomew J C, Bradfield C A. Proc Natl Acad Sci USA. 1991;88:9543–9547. doi: 10.1073/pnas.88.21.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim D J, Han B S, Ahn B, Hasegawa R, Shirai T, Ito N, Tsuda H. Carcinogenesis. 1997;18:377–381. doi: 10.1093/carcin/18.2.377. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Wormke M, Safe S H, Bjeldanes L F. J Natl Cancer Inst. 1994;86:1758–1765. doi: 10.1093/jnci/86.23.1758. [DOI] [PubMed] [Google Scholar]
- 31.Wattenberg L W, Hanley A B, Barany G, Sparnins V L, Lam L K T, Fenwick G R. In: Diet, Nutrition and Cancer. Hayashi Y, et al., editors. Tokyo: Japan Sci. Soc. Press; 1986. pp. 193–203. [Google Scholar]