Abstract
Chronic exposure to cocaine leads to prominent, long-lasting changes in behavior that characterize a state of addiction. The striatum, including the nucleus accumbens and caudoputamen, is an important substrate for these actions. We previously have shown that long-lasting Fos-related proteins of 35–37 kDa are induced in the striatum by chronic cocaine administration. In the present study, the identity and functional role of these Fos-related proteins were examined using fosB mutant mice. The striatum of these mice completely lacked basal levels of the 35- to 37-kDa Fos-related proteins as well as their induction by chronic cocaine administration. This deficiency was associated with enhanced behavioral responses to cocaine: fosB mutant mice showed exaggerated locomotor activation in response to initial cocaine exposures as well as robust conditioned place preference to a lower dose of cocaine, compared with wild-type littermates. These results establish the long-lasting Fos-related proteins as products of the fosB gene (specifically ΔFosB isoforms) and suggest that transcriptional regulation by fosB gene products plays a critical role in cocaine-induced behavioral responses. This finding demonstrates that a Fos family member protein plays a functional role in behavioral responses to drugs of abuse and implicates fosB gene products as important determinants of cocaine abuse.
Repeated administration of psychomotor stimulants (e.g., cocaine and amphetamine) causes long-lasting behavioral plasticity. An important neural substrate for this plasticity is the nucleus accumbens, the ventral part of the striatum (1, 2). Several members of the Fos and Jun families of transcription factors are induced in this brain region by acute or chronic cocaine exposure (3–6). However, it has been difficult to determine the role of any particular transcription factor in cocaine action.
We have shown that chronic administration of cocaine or other drugs of abuse results in the induction of a long-lasting activator protein 1 (AP-1) DNA-binding complex in nucleus accumbens and caudoputamen (dorsal part of the striatum) (5–7). AP-1 refers to the DNA site to which Fos- and Jun-related proteins bind. This AP-1 complex is associated with the persistent expression of 35- to 37-kDa Fos-related proteins, which have been termed chronic FRAs (Fos-related antigens) (6–8). The identity of the chronic FRAs is unknown, although a relationship to ΔFosB, a splice variant of FosB with a truncated C terminus (9, 10), has been suggested (6, 11). The recent development of a fosB mutant mouse (12) has made it possible to study directly the potential involvement of fosB gene products in this cocaine action. Therefore, we used fosB mutant mice, which have a targeted mutation at exon 2 of the fosB gene (12), to first determine whether the chronic FRAs are products of the fosB gene. The results reported here provide definitive evidence that the chronic FRAs are products of the fosB gene, specifically isoforms of ΔFosB.
Second, given the induction of the ΔFosB variants in the striatum, including the nucleus accumbens, we used fosB mutant mice to study directly a possible relationship between these ΔFosB variants and behavioral responses to cocaine. Repeated exposure to cocaine induces a progressive increase in hyperactivity, a phenomenon called locomotor (or behavioral) sensitization (13). Behavioral sensitization in rodents has been proposed as a model of an initial stage of cocaine addiction in humans, without which severe cocaine dependence does not develop, as well as a model of cocaine-induced psychosis in human addicts (2, 14, 15). Repeated exposure to cocaine also elicits a distinct behavior, termed conditioned locomotor activity. This occurs when animals experience repeated cocaine injections in the same environment and consequently exhibit hyperactivity in that environment even in the absence of an additional cocaine challenge (13). Conditioned locomotor activity has been proposed as a model of the conditioned cue effects of cocaine, also prominent in cocaine addicts (16, 17). Cocaine also can establish preference for an environment associated with its exposure in rodents. The conditioned place preference (CPP) paradigm provides a measure of a drug’s rewarding and presumably abusive properties (18). We directly tested the roles of fosB gene products in these cocaine-induced behavioral changes. The results show abnormal locomotor and CPP responses, but normal conditioned locomotor activity, in the fosB mutant mice, and thereby establish a role for fosB gene products in specific behavioral responses to cocaine.
MATERIALS AND METHODS
Mice.
We used male fosB mutant mice and their age-matched littermates (2–3 months old). The fosB mutation was bred into 129sv inbred and 129sv × BALB/c mixed background mice, and subsequent generations were bred by crossing heterozygote males and females. c-fos mutant mice and wild-type littermates were obtained from The Jackson Laboratory.
Gel Shifts and Immunoblotting.
Mice were treated with saline (+/+, n = 15; −/−, n = 11) or cocaine (20 mg/kg i.p.; +/+, n = 23; −/−, n = 21) for 6–7 days and were sacrificed 18–24 hr after the last injection. Another group of mice was treated with saline for 6 days and sacrificed 2 hr after an additional injection of saline (+/+, n = 2; −/−, n = 2) or a single injection of cocaine (20 mg/kg, +/+, n = 3; −/−, n = 3). The two groups (2 hr and 18–24 hr) of saline-treated animals yielded equivalent results. Striatal tissue, including the nucleus accumbens and caudoputamen, was isolated by gross dissection. The 20 mg/kg dose of cocaine was used because it optimally induces the 35- to 37-kDa FRAs (6, 19). Striatal extracts (containing 20, 50, or 255 μg of protein) were used for gel mobility shift assay, Western blotting, and two-dimensional gel electrophoresis, respectively. For Western blotting, the following antibodies were used: a FRA antiserum (1:4,000, M. J. Iadarola, National Institutes of Health, Bethesda, MD) raised against a sequence highly conserved in all known Fos family member proteins, an N terminus FosB antibody (which recognizes ΔFosB and FosB) (1:1,000, Y. Nakabeppu, Kyushu University, Fukuoka, Japan), a C-terminus FosB antibody (which recognizes only FosB) (1:1,000, Y. Nakabeppu), a dopamine transporter (DAT) mAb (1:10,000, Chemicon), a tyrosine hydroxylase (TH) polyclonal antibody (1:10,000, EugeneTech, Allendale, NJ), a glutamate decarboxylase polyclonal antibody (1:2,000, Chemicon), and a dopamine- and cAMP-regulated phosphoprotein-32 kDa (DARPP-32) mAb (1:5,000, a gift of P. Greengard, The Rockefeller University, New York). Omission of the primary antibodies or preadsorption of the primary antibodies with the respective immunogens eliminated specific bands. Electrophoresis and blotting conditions are described elsewhere (6). For gel mobility shift assay, we used a double-stranded consensus AP-1 sequence from the human metallothionein II gene, 5′-TCGACGTGACTCAGCGCGC-3′, exactly as described (6).
Immunohistochemistry.
Mice were treated with saline (+/+, n = 4; −/−, n = 7) or cocaine (+/+, n = 4; −/−, n = 5, 20 mg/kg, i.p.) for 5–6 days and were perfused 18–20 hr after the last injection with 0.9% saline followed by 4% paraformaldehyde. Brain sections were stained with a polyclonal FosB antibody (1:4,000, Santa Cruz Biotechnology) or a monoclonal calbindin-D28K antibody (1:1,000, Sigma). The FosB antibody was raised against an N-terminus portion of FosB that is present in both full-length FosB and ΔFosB. FosB-like proteins were detected using standard nickel-intensified diaminobenzidine, and calbindin was detected using the standard avidin biotin complex-diaminobenzidine method, as reported previously (20). For detection of striatal neuropeptides and TH, immunohistochemistry was performed using standard streptavidin or diaminobenzidine procedure (20, 21). The specificity of antisera against met-enkephalin (1:2,000, Incstar, Stillwater, MN), dynorphin A (1:8,000, L. Terenius, Karolinska Institute, Stockholm, Sweden), substance P (1:20,000, J.-S. Hong, National Institute on Environmental Health Sciences, Research Triangle Park, NC), and TH (1:2,000, EugeneTech) has been well characterized (20, 21).
Behavioral Analysis.
Locomotor activity. Mice (24–28 g) were tested blindly at the same time each day. The locomotor activity chamber was a plastic cage (28 × 17 × 12 cm) with 10 pairs of photocell beams dividing the box into 11 rectangular fields. Horizontal movement was counted as locomotor activity. For the first 3 days, mice (+/+, n = 30; −/−, n = 22) were placed in the chambers immediately after saline injections and locomotor activity was measured for 10 min. From the fourth to ninth day, mice were given either saline or cocaine and were placed in the chambers. On day 4, mice were tested for acutely induced locomotor activity (10 mg/kg: +/+, n = 11; −/−, n = 9; 20 mg/kg: +/+, n = 20; −/−, n = 17; vehicle: +/+, n = 19; −/−, n = 12). From day 4 to day 9, mice were given cocaine injections once a day, and their locomotor activity was measured (cocaine, 20 mg/kg: +/+, n = 20; −/−, n = 16; saline: +/+, n = 10; −/−, n = 5). This experimental procedure was designed to avoid complications of locomotor activity measurements by the appearance of stereotypy. Pilot studies showed that during repeated cocaine injections (20 mg/kg i.p.) locomotor activity increased in the first 10 min, after which time the locomotor activity began to be replaced by stereotypy (20–30 min) in both wild-type and mutant mice. Therefore, to maximize the intensity of locomotor activity and the temporal correlation between locomotor activation and the exposure to the test chamber, mice were placed in the test chambers for only 10 min. This procedure has been shown to optimize conditioning effects in rats (22), and pilot studies showed that this procedure established more reliable and robust locomotor sensitization and conditioned locomotor activity than longer exposure time (30 min) in mice at the dose of cocaine used. Locomotor activity predominates in this procedure, and focused stereotypy (which was rated blindly) was not observed over the six cocaine sessions in either the wild-type or mutant mice. On day 10, all the mice received saline injections and were placed in the test chambers, and their locomotor activity was measured for 10 min (cocaine: +/+, n = 20; −/−, n = 16; saline: +/+, n = 10; −/−, n = 5). After completion of the 10-day sessions, we again measured locomotor activity for 30 min after an additional cocaine dose (20 mg/kg); the peak locomotor activity was still in the first 10 min in both wild-type and mutant mice (data not shown).
CPP.
The apparatus used was a Plexiglas box that was composed of three distinct compartments. Two large chambers (24 × 18 × 33 cm) had distinguishable visual and tactile cues and were separated by a central chamber (12 × 18 × 33 cm). Each of the two large chambers had three pairs of photocells to detect the presence of an animal. During the first session, mice were allowed to explore the three chambers freely for 15 min. On a group basis, neither mutant mice nor wild-type littermates showed a bias to either of the two large chambers. The following four sessions were used for conditioning: mice were alternatively confined to either of the two large chambers with either cocaine (10 or 20 mg/kg, i.p.) or saline for 20 min. On the sixth session the mice were placed in the central chamber and were allowed to move freely in the three chambers for 15 min. The time they spent in the cocaine-paired chamber minus that in the saline-paired chamber provides a measure of CPP.
Statistical Analysis.
Gel shift assays and Western blots were quantified by image analysis. Gel shifts were analyzed by ANOVA and Newman–Keuls posthoc tests; Western blots were analyzed by t test. Behavioral data were analyzed by ANOVA. Each ANOVA was followed by Newman–Keuls posthoc tests, as needed. The number of FosB-immunoreactive nuclei was counted in the shell and core divisions of the nucleus accumbens, as described (20). The data were analyzed by t test.
RESULTS AND DISCUSSION
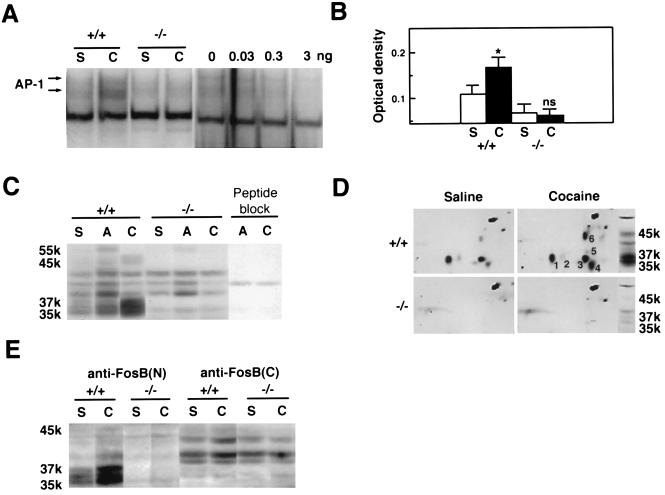
Repeated cocaine treatment induced an AP-1 binding complex and FRAs of 35–37 kDa, and to a lesser extent a FRA of ≈45 kDa, in the striatum (including both nucleus accumbens and caudoputamen) of wild-type mice (Fig. 1 A–C). No other long-lasting FRAs were up-regulated by chronic exposure to cocaine. In marked contrast to wild-type mice, fosB mutant mice failed to show an increase in AP-1 binding activity after repeated cocaine administration (Fig. 1 A and B), and the 35- to 37-kDa FRAs, as well as the ≈45-kDa FRA, were completely absent under both basal and cocaine-treated conditions (Fig. 1C). Two-dimensional Western blotting confirmed these results: both the 35- to 37-kDa FRAs, which exist as several variants (Fig. 1D, nos. 1–5), and the ≈45-kDa FRA (Fig. 1D, no. 6) were absent in the fosB mutant mice. In addition, both the 35- to 37-kDa and ≈45-kDa FRAs were recognized by an antibody directed against the N-terminal portion of FosB, whereas only the ≈45-kDa FRA was recognized by an antibody directed against the C-terminal portion of FosB (Fig. 1E). Together, these results establish that the 35- to 37-kDa chronic FRAs, and the ≈45-kDa FRA, are products of the fosB gene, presumably ΔFosB variants and FosB, respectively, and that fosB gene products predominate, among Fos-related proteins, in the striatum after chronic cocaine treatment.
Figure 1.
Induction of AP-1 binding activity and FRAs in mouse striatum by cocaine. (A) Gel mobility shift assay with a consensus AP-1 sequence. S, repeated saline treatment; C, repeated cocaine treatment. +/+, wild-type littermates; −/−, fosB mutant mice. (Right) The autoradiogram demonstrates specificity of the AP-1 binding activity, by showing competition of the activity in striatum from a cocaine-treated wild-type mouse with a nonradioactive AP-1 probe (0, 0.03, 0.3, or 3 ng). (B) Levels of AP-1 activity. ∗, P < 0.05, statistically significant difference between saline- and cocaine-treated groups; ns, not statistically different. S+/+, n = 11; C+/+, n = 10; S−/−, n = 10; C−/−, n = 11. (C) Western blotting with the anti-FRA antiserum (ref. 4, see Materials and Methods). S, saline; A, acute (2 hr) cocaine treatment; C, repeated cocaine treatment. Peptide block, preadsorption of the FRA antiserum with the M-peptide immunogen (4) eliminated FRA bands. The band at ≈40 kDa is a nonspecific band. (D) Two-dimensional Western blotting with the anti-FRA antiserum. (E) Western blotting with antibodies directed against the N terminus of FosB [anti-FosB(N), which recognizes ΔFosB and FosB] or the C terminus of FosB [anti-FosB(C), which recognizes FosB only].
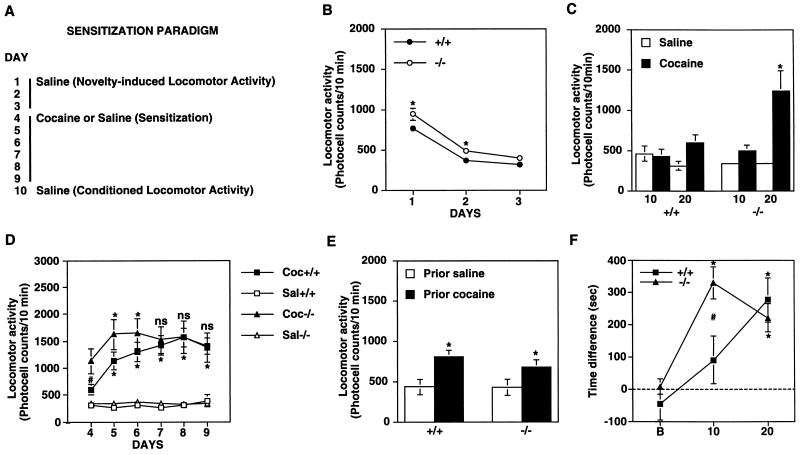
We next studied behavioral responses to cocaine in fosB mutant and wild-type mice. Mutant mice showed slightly higher spontaneous locomotor activity when they were first introduced into test chambers, but this hyperactivity gradually decreased with habituation and reached the same level as that exhibited by wild-type littermates by day 3 (Fig. 2B). An acute cocaine injection induced significantly more locomotor activity in mutant mice than in wild-type littermates (Fig. 2C). However, wild-type mice showed a consistent and large (3-fold) increment in cocaine-induced hyperactivity over 6 days (i.e., sensitization), whereas the mutant mice failed to exhibit clear sensitization (Fig. 2D). The mutant mice did show small increases in cocaine-induced locomotor activity on days 5 and 6, but did not show reliable increases on days 7, 8, and 9, compared with day 4. The mutant and wild-type mice did not differ in locomotor activity during a course of saline treatment (P > 0.05) (Fig. 2D). Repeated cocaine administration can elicit another type of movement, called stereotypy (repetitive, nonpurposeful movements), which can complicate measures of ambulatory locomotor activity (13). However, the locomotor activity findings of the present study were not complicated by the presence of stereotypy, because significant stereotypy was not observed in the wild-type or mutant mice under the experimental conditions used (see Materials and Methods). The wild-type and mutant mice showed equivalent conditioned locomotor activity (Fig. 2E): when, after a course of previous cocaine injections on days 4–9, animals were given saline injections and placed in the test chambers on day 10, equivalent locomotor activity was seen in the wild types and mutants. The fact that the fosB mutant mice developed normal conditioned locomotor activity suggests that the abnormality in locomotor activity in these mice was not due primarily to abnormalities in sensory, motivational, or motor functions. Previous work has shown that the fosB mutant mice also are normal with respect to several other general behavioral measures, including hippocampus-dependent learning, sensory perception, and certain motivational behaviors (12).
Figure 2.
Regulation of the locomotor and rewarding effects of cocaine in wild-type and fosB mutant mice by cocaine. (A) The experimental protocol used to assess spontaneous locomotor activity, locomotor activity induced acutely by cocaine, locomotor sensitization to repeated cocaine administration, and conditioned locomotor activity. (B) Locomotor activity counts of wild-type littermates (+/+) and fosB mutant mice (−/−) on 3 consecutive days of saline treatment. ∗, statistically significant differences between wild-type and mutant mice (P < 0.05). (C) Locomotor activity induced by a single, acute cocaine injection (10 or 20 mg/kg i.p.). ∗, statistically significant differences between saline- and cocaine-treated groups (P < 0.05). (D) Locomotor sensitization to repeated cocaine injections (20 mg/kg i.p.) in wild-type and mutant mice. The upper and lower symbols [∗, (P < 0.05) and ns (not statistically different)] indicate differences from day 4 in the mutant and wild-type mice, respectively. #, statistically significant difference between the mutant and wild-type mice. (E) Conditioned locomotor activity in wild-type (+/+) and mutant (−/−) mice. On day 10, mice that previously had received saline or cocaine were given an additional saline injection and locomotor activity was measured. ∗, statistically significant differences between prior-cocaine and prior-saline groups (P < 0.05). (F) CPP established by cocaine (10 or 20 mg/kg, i.p.). Time difference, obtained by subtracting time animals spent in the saline-paired side from time they spent in the cocaine-paired side. B, preconditioning baseline. ∗, statistically significant preference. #, statistically significant difference between +/+ and −/− mice.
FosB mutant mice also showed abnormalities in the CPP paradigm. This paradigm assesses the ability of a drug of abuse to establish a conditioned preference for a previously drug-paired environment (18). As shown in Fig. 2F, the mutant mice showed dramatically more robust CPP at 10 mg/kg than wild-type littermates. In contrast, the mutant and wild-type mice showed equivalent CPP at a higher dose of cocaine (20 mg/kg).
The most straightforward interpretation of these behavioral data is that the fosB mutant mice show increased inherent sensitivity to the locomotor-activating and rewarding effects of cocaine. Thus, in the locomotor assays, the mutant animals appeared “presensitized” to cocaine: they exhibited exaggerated responses to the initial cocaine exposure but failed to show consistent further increases in activity after repeated cocaine exposures (i.e., sensitization) possibly due to a ceiling effect. Similarly, in the CPP paradigm, the mutant animals developed place conditioning to a lower dose of cocaine than wild-type mice, with no difference in place conditioning seen at a higher cocaine dose. The abnormal behavioral responses of the mutant mice to even initial cocaine exposures is consistent with an important role exerted by the significant levels of ΔFosB isoforms expressed uniquely in striatal regions under basal conditions (Fig. 1 C–E; see also ref. 19).
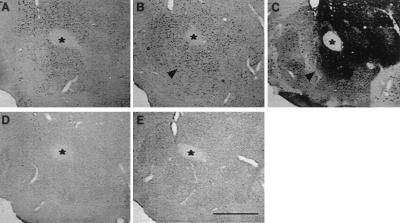
Having established the importance of fosB gene products for specific behavioral effects of cocaine exposure, we next investigated where in the brain the proteins are expressed in an effort to gain insight into the mechanism of fosB action. Previous immunohistochemical studies have examined only c-Fos or used a pan-FRA antibody that recognizes all Fos-like proteins in tissue sections. Therefore, we performed immunohistochemistry with an anti-N-terminus FosB antibody to specifically characterize cocaine induction of long-lasting FosB-related proteins in a subregion of the striatum (the nucleus accumbens), which is known to play a critical role in the sensitizing and rewarding actions of cocaine (1, 2, 23, 24). FosB-like proteins were detected in the absence of cocaine exposure in the nucleus accumbens (Fig. 3A), consistent with the Western blotting data (see Fig. 1 C–E). Repeated cocaine treatment significantly increased the number of immunoreactive nuclei in cell bodies of the calbindin-rich, core division of the nucleus accumbens (Fig. 3 B and C). The cocaine-induced increase in the number of FosB-positive nuclei was statistically significant in the nucleus accumbens core division [55%, t(32) = 3.32, P < 0.05], but not in the calbindin-poor, shell division [1%, t(32) = 0.25, P > 0.05]. FosB-like immunoreactivity was absent in the mutant mice after saline (Fig. 3D) or cocaine (Fig. 3E) treatment. The two divisions of the nucleus accumbens have different connections with other brain regions and are thought to subserve different aspects of striatal function (23, 24). The selective induction of FosB-like proteins in the accumbens core, the pattern of which resembles expression of the DAT (21, 25), could reflect the site where cocaine interacts maximally with dopamine terminals to increase dopaminergic transmission. Although this finding is at odds with the importance of the shell division of the accumbens in certain acute effects of cocaine, our data suggest that at least some prominent adaptations to chronic cocaine occur in the core division.
Figure 3.
Distribution of FosB-like proteins in the ventral striatum of wild-type and fosB mutant mice after chronic cocaine treatment. (A, B, D, and E) FosB-like immunoreactivity. (C) Calbindin-like immunoreactivity. (A and D) Saline-treated groups. (B and E) Chronic cocaine-treated groups (20 mg/kg i.p.). (A–C) +/+. (D and E) −/−. ∗, anterior commissure. Arrow in B points at induction of FosB-like proteins. Arrow in C points at the corresponding position in an adjacent section stained with calbindin-D28K. (Scale bar = 500 μm.)
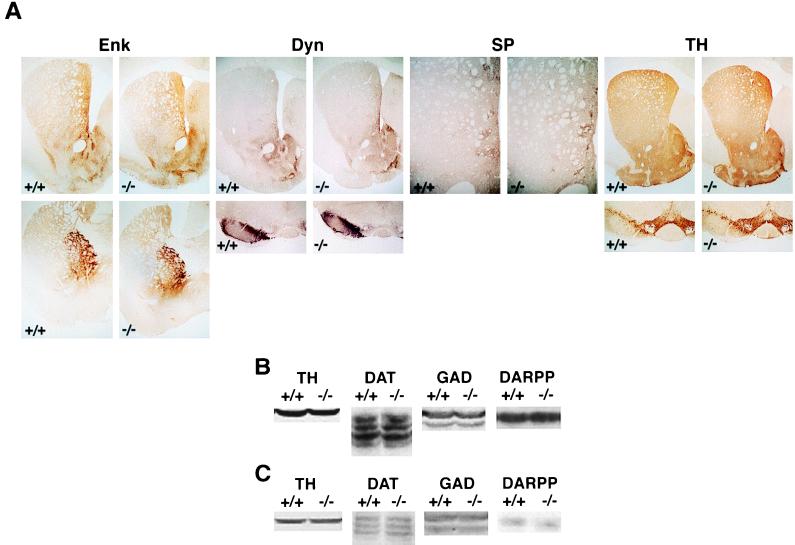
The abnormal behavioral responses to repeated cocaine administration observed in fosB mutant mice could reflect an abnormal response in an otherwise developmentally intact neural system. Alternatively, the abnormal behavioral responses could reflect general abnormalities in the striatum that occurred during development (e.g., see ref. 21). To study these possibilities, we examined the striatal cytoarchitecture of fosB mutant mice. Whereas the striatum of fosB mutant mice was in general slightly smaller than that of age-matched, wild-type littermates, this did not cause generalized striatal dysfunction, because the fosB mutant mice displayed normal conditioned locomotor activity (Fig. 2E). In addition, immunohistochemical analyses revealed no detectable abnormalities in striatal efferents, afferents, or compartments (Fig. 4), suggesting that the behavioral abnormality in fosB mutant mice is due to a defect in an adaptive response rather than a defect in development. A marker of the striatal “indirect” pathway (26, 27), met-enkephalin, is enriched in the matrix compartment in mice (21). This neuropeptide showed apparently normal distribution in the caudoputamen and nucleus accumbens as well as in the globus pallidus and ventral pallidum, projection areas of the striatal “indirect” pathway. A marker of the striatal “direct” pathway (26, 27), dynorphin, also showed apparently normal distribution in the caudoputamen and nucleus accumbens as well as in the substantia nigra and ventral tegmental area, midbrain projection areas of the direct pathway. Substance P-immunoreactive neurons and fibers in both wild-type and mutant mice showed clustered immunoreactivity in patches in the caudoputamen, consistent with their striosomal organization (26). The major striatal afferent with which cocaine interacts to induce locomotor activity and sensitization as well as its rewarding effects are dopaminergic neurons (23, 28–31). TH (the rate-limiting enzyme in the biosynthesis of dopamine) (Fig. 4A) showed apparently normal distributions in the striatum and midbrain. Moreover, levels of TH and DAT (markers of dopaminergic neurons and their terminals), and glutamate decarboxylase (GAD) and DARPP-32 (markers of nucleus accumbens and caudoputamen medium spiny neurons and their terminals), were quantitatively equivalent in wild-type and mutant mice (Fig. 4 B and C). Analysis of optical density of each protein showed that the wild-type and mutant mice did not differ (P > 0.05) in levels of: TH [B, t(34) = 0.46; C, t(25) = 0.55], DAT [B, t(26) = 0.01; C, t(17) = 1.12], GAD [B, t(10) = 0.75; C, t(18) = 0.09], or DARPP-32 [B, t(54) = 0.52; C, t(26) = 1.26]. These results indicate that fosB mutant mice exhibit apparently normal compartmental organization in striatal regions, that striatal neurons in which ΔFosB variants are induced by chronic cocaine administration are grossly normal, and that dopaminergic terminals, on which cocaine acts to produce its behavioral effects, are also normal. Thus, fosB gene products are not required for essentially normal development of the striatum. Rather, the absence of fosB gene products results in an abnormality in the ability of striatal neurons to respond to cocaine exposure.
Figure 4.
Striatal cytoarchitecture of wild-type and fosB mutant mice. (A) Immunohistochemistry of wild-type littermates (+/+) and fosB mutant mice (−/−) using antisera against met-enkephalin (Enk), dynorphin A (Dyn), substance P (SP), or TH. (Upper) Striatum (Enk, Dyn, and TH). (Lower) Globus pallidus-ventral pallidum (Enk) or midbrain (Dyn and TH). The SP panel shows the medial caudoputamen. The results shown are representative of the analysis of four wild-type and seven mutant mice. (B and C) Levels of TH, DAT, glutamate decarboxylase (GAD), and DARPP-32. (B) Nucleus accumbens and caudoputamen. (C) Ventral tegmental area and substantia nigra. +/+, wild-type littermates; −/−, fosB mutant mice. +/+, n = 7–37; −/−, n = 5–21.
In the present study, we show that the absence of fosB gene products causes enhanced behavioral responses to cocaine. These results suggest that induction of ΔFosB isoforms by chronic cocaine administration represents a compensatory adaptation in striatal neurons that opposes cocaine’s actions. Such an adaptation could contribute to behavioral abnormalities seen in cocaine withdrawal states that are thought to contribute to relapse in human addicts. Such a role for fosB gene products contrasts with the apparent lack of involvement of another Fos family member, c-Fos. We observed no difference in acute locomotor responses to cocaine, or in locomotor sensitization, in c-fos mutant mice compared with wild-type controls (data not shown). This finding highlights the selectivity of the deficiency in behavioral plasticity to cocaine observed in the fosB mutant mice. Although c-Fos is acutely induced in a compartment-specific manner in the striatum by stimulants (3), this does not seem to be required for normal behavioral responses to cocaine.
Because fosB gene products function as transcription factors, it will be important to identify the specific target genes that mediate the effects of these transcription factors on behavioral adaptations to cocaine. Putative target genes, which warrant further study, include those that encode certain intracellular messenger proteins, glutamate and dopamine receptors, and dynorphin. Chronic exposure to cocaine alters the levels of these signaling proteins in the nucleus accumbens and caudoputamen, and such alterations have been implicated in cocaine-induced behavioral plasticity (23, 29–31). Moreover, the genes for these signaling proteins are known to contain AP-1 sites.
Finally, the chronic FRAs have been shown to accumulate in a region-specific manner in brain in response to several chronic perturbations, including chronic seizures, chronic antipsychotic drug administration, and kainic acid and other lesions (6, 19, 20, 32–35). By establishing the identity of these proteins as fosB gene products, the results of the present study contribute to understanding the general role these transcription factors play in mediating long-term adaptations in the brain.
Acknowledgments
We thank T. Kosten and C. Moore for assistance with the behavioral analyses, and J.-S. Chen, W. Carlezon, and M. Kelz for their valuable suggestions during the course of the experiments. We also thank R. Duman and M. Picciotto for comments on the manuscript. This work was supported by grants from the National Institute on Drug Abuse and the Abraham Ribicoff Research Facilities, State of Connecticut Department of Mental Health and Addiction Services. M.E.G. was supported by National Institute of Child Health and Human Development Grant HD18655 and National Institute of Neurological Disorders and Stroke Grant NS28829.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: AP-1, activator protein 1; DARPP-32, dopamine- and cAMP-regulated phosphoprotein-32 kDa; CPP, conditioned place preference; FRA, Fos-related antigen; TH, tyrosine hydroxylase; DAT, dopamine transporter.
References
- 1.Koob G F, Bloom F E. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 2.Wise R A, Leeb K. Behav Pharmacol. 1993;4:339–349. [PubMed] [Google Scholar]
- 3.Graybiel A M, Moratalla R, Robertson H A. Proc Natl Acad Sci USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young S T, Porrino L J, Iadarola M J. Proc Natl Acad Sci USA. 1991;88:1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hope B T, Kosofsky B, Hyman S E, Nestler E J. Proc Natl Acad Sci USA. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hope B T, Nye H E, Kelz M B, Self D W, Iadarola M J, Nakabeppu Y, Duman R S, Nestler E J. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 7.Nye H E, Nestler E J. Mol Pharmacol. 1996;49:636–645. [PubMed] [Google Scholar]
- 8.Moratalla R, Elibol B, Vallejo M, Graybiel A M. Neuron. 1996;17:147–156. doi: 10.1016/s0896-6273(00)80288-3. [DOI] [PubMed] [Google Scholar]
- 9.Mumberg D, Lucibello F C, Schuermann M, Muller R. Genes Dev. 1991;5:1212–1223. doi: 10.1101/gad.5.7.1212. [DOI] [PubMed] [Google Scholar]
- 10.Nakabeppu Y, Nathans D. Cell. 1991;64:751–759. doi: 10.1016/0092-8674(91)90504-r. [DOI] [PubMed] [Google Scholar]
- 11.Chen J S, Nye H E, Kelz M B, Hiroi N, Nakabeppu Y, Hope B T, Nestler E J. Mol Pharmacol. 1995;48:880–889. [PubMed] [Google Scholar]
- 12.Brown J R, Ye H, Bronson R T, Dikkes P, Greenberg M E. Cell. 1996;86:297–309. doi: 10.1016/s0092-8674(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 13.Post R M, Rose H. Nature (London) 1976;260:731–732. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- 14.Robinson T E, Berridge K C. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 15.Koob G F. Neuron. 1996;16:893–896. doi: 10.1016/s0896-6273(00)80109-9. [DOI] [PubMed] [Google Scholar]
- 16.Stewart J, deWit H, Eikelboom R. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- 17.Pert A, Post R, Weiss S R B. In: National Institute on Drug Abuse Research Monograph. Erinoff L, editor. Washington, D.C.: U. S. Department of Health and Human Services; 1990. pp. 208–241. [Google Scholar]
- 18.White N M, Hiroi N. Sem Neurosci. 1993;5:329–336. [Google Scholar]
- 19.Nye H E, Hope B T, Kelz M B, Iadarola M J, Nestler E J. J Pharmacol Exp Ther. 1995;275:1671–1680. [PubMed] [Google Scholar]
- 20.Hiroi N, Graybiel A M. J Comp Neurol. 1996;374:70–83. doi: 10.1002/(SICI)1096-9861(19961007)374:1<70::AID-CNE5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Xu M, Moratalla R, Gold L H, Hiroi N, Koob G F, Graybiel A M, Tonegawa S. Cell. 1994;79:729–742. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]
- 22.Hiroi N, White N M. Pharmacol Biochem Behav. 1989;32:249–258. doi: 10.1016/0091-3057(89)90241-4. [DOI] [PubMed] [Google Scholar]
- 23.White F J, Hu X-T, Henry D J, Zhang X-F. In: Neurobiology of Cocaine. Hammer R P Jr, editor. New York: CRC; 1995. pp. 99–119. [Google Scholar]
- 24.Pierce R C, Bell K, Duffy P, Kalivas P W. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freed C, Revay R, Vaughan R A, Kriek E, Grant S, Uhl G R, Kuhar M J. J Comp Neurol. 1995;359:340–349. doi: 10.1002/cne.903590211. [DOI] [PubMed] [Google Scholar]
- 26.Graybiel A M. Trends Neurosci. 1990;13:244–253. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 27.Gerfen C R. Trends Neurosci. 1992;15:133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- 28.Kuhar M J, Ritz M C, Boja J W. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 29.Nestler E J, Hope B T, Widnell K. Neuron. 1993;11:955–1006. doi: 10.1016/0896-6273(93)90213-b. [DOI] [PubMed] [Google Scholar]
- 30.Hyman S E. Neuron. 1996;16:901–904. doi: 10.1016/s0896-6273(00)80111-7. [DOI] [PubMed] [Google Scholar]
- 31.Kalivas P W. In: Neurobiology of Cocaine. Hammer R P Jr, editor. New York: CRC; 1995. pp. 81–98. [Google Scholar]
- 32.Pennypacker K R, Hong J-S, McMillian M K. Trends Pharmacol Sci. 1995;16:317–321. doi: 10.1016/s0165-6147(00)89061-6. [DOI] [PubMed] [Google Scholar]
- 33.Doucet J P, Nakabeppu Y, Bedard P J, Hope B T, Nestler E J, Jasmin B J, Chen J S, Iadarola M J, St-Jean M, Wigle N, Blanchet P, Grondin R, Robertson G S. Eur J Neurosci. 1996;8:365–381. doi: 10.1111/j.1460-9568.1996.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Kelz M B, Hope B T, Nakabeppu Y, Nestler E J. J Neurosci. 1997;17:4933–4941. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandelzys A, Gruda M A, Bravo R, Morgan J I. J Neurosci. 1997;17:5407–5415. doi: 10.1523/JNEUROSCI.17-14-05407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]