Abstract
Hippocampal slices are used to show that, as a temporal input pattern of activity flows through a neuronal layer, a temporal-to-spatial transformation takes place. That is, neurons can respond selectively to the first or second of a pair of input pulses, thus transforming different temporal patterns of activity into the activity of different neurons. This is demonstrated using associative long-term potentiation of polysynaptic CA1 responses as an activity-dependent marker: by depolarizing a postsynaptic CA1 neuron exclusively with the first or second of a pair of pulses from the dentate gyrus, it is possible to “tag” different subpopulations of CA3 neurons. This technique allows sampling of a population of neurons without recording simultaneously from multiple neurons. Furthermore, it reflects a biologically plausible mechanism by which single neurons may develop selective responses to time-varying stimuli and permits the induction of context-sensitive synaptic plasticity. These experimental results support the view that networks of neurons are intrinsically able to process temporal information and that it is not necessary to invoke the existence of internal clocks or delay lines for temporal processing on the time scale of tens to hundreds of milliseconds.
Sensory stimuli produce spatio-temporal patterns of activity on the peripheral sensory layers of the nervous system. Depending on the nature of the stimuli and of the task, the spatial and/or temporal features of these activity patterns are used by the central nervous system to make perceptual and behavioral judgments. In temporal processing, information is encoded in the temporal pattern of activity on the sensory layer (i.e., by interval, duration, and order of different stimulus features). Determining whether two flashes of light or two brief tones are separated by 100 or 150 ms is an example of a temporal task (1, 2). Temporal processing is important for sensory processing in most sensory modalities but is perhaps most important in speech processing. Speech is rich in temporal structure, particularly temporal features on the time scale of tens to hundreds of milliseconds (3, 4). Indeed, recent data suggest that certain elements of speech can be identified based primarily on temporal cues (5). Furthermore, deficits in temporal processing may underlie certain types of learning disabilities (6–8).
In spatial tasks, information is encoded in the spatial pattern of active sensory afferents. Orientation selectivity or the formation of topographic maps are examples that require the formation of selective neuronal responses based on the spatial arrangement of input fibers. We have a relatively good understanding of how neurons develop responses to specific orientations (e.g., ref. 9). In contrast, we have little understanding of how neurons develop selective responses to simple temporal patterns, such as two tones separated by either a 100- or 150-ms interval. Both stimuli will activate the same hair cells in the cochlea but with a different temporal pattern. For central neurons to respond selectively to each stimulus or to generate two different behavioral responses, the nervous system must perform a temporal-to-spatial transformation. That is, information initially encoded in the temporal pattern of inputs must ultimately be encoded by different populations of neurons.
We have previously proposed that networks of neurons are intrinsically able to implement temporal-to-spatial transformations as a result of short-term forms of plasticity and slow synaptic events (10). Specifically, if a 100-ms interval is bounded by two input pulses, the first pulse will activate a subpopulation of units and trigger a series of processes such as paired-pulse facilitation (PPF) and slow inhibitory postsynaptic potentials. Even though the second pulse is identical to the first, it will activate a different subpopulation of units, because the network is in a different state as a result of the occurrence of the first pulse; some synapses will be facilitated while some cells will be inhibited. Differences in population responses to the first and second pulses can be viewed as a temporal-to-spatial transform and used to code intervals or order. A prediction that emerges from this model is that it should be possible to record differences in the activity of a population of neurons in response to the first versus a second pulse. In the present paper we tested this prediction by using hippocampal slices to study the transformations that take place as activity flows through a network of neurons.
MATERIALS AND METHODS
Experiments were performed on 500-μm-thick transverse hippocampal slices from Sprague–Dawley rats (15–28 days). The hippocampus was removed following anesthesia with pentobarbital and decapitation. Slices were cut and submerged in oxygenated medium composed of 119 mM NaCl, 2.5 mM KCl, 1.3 mM MgSO4, 1.0 mM NaH2PO4, 26.2 mM NaCO3, 2.5 mM CaCl2, and 10 mM glucose. After an equilibrium period of at least 1 hr, slices were transferred to a recording chamber, perfused at a rate of 2 ml/min, and maintained at a temperature of 30–31°C. Intracellular recordings from CA1 or CA3 pyramidal neurons were made with 40–100 MΩ electrodes filled with 3 M KAc.
Polysynaptic postsynaptic potentials were recorded in CA1 pyramidal neurons in response to paired-pulse stimulation of the dentate gyrus (DG). Stainless-steel bipolar electrodes were placed in the granule or molecular layer of the DG, generally in the suprapyramidal blade. The interpulse interval for paired-pulse stimulation was 100 or 200 ms. Stimulation intensity was between 50 and 200 μA, with 100-μs-long pulses. By stimulating the DG and recording in CA1 we tapped into the hippocampal trisynaptic circuit: the perforant path projects to the DG, which projects to CA3, which in turn projects to CA1. Because we stimulated the DG and recorded activity in a single CA1 neuron, the CA3 region essentially represents a “hidden layer,” and the CA1 neuron represents the output or read-out of the average activity of that layer.
To determine whether the first and second pulses activated the same population of CA1 neurons, associative LTP was used to differentially “tag” the neurons that responded to the first or second pulse. By depolarizing the CA1 neuron in conjunction with either the first or second of a pair of pulses it was possible to preferentially potentiate CA3-CA1 synapses that were activated by a particular pulse. A “sparse” pairing protocol was used to induce LTP (11, 12). Throughout the baseline, conditioning, and test phases the DG was stimulated with paired pulses every 15–20 sec. During conditioning, postsynaptic depolarizing pulses of 80–100 ms and 2–4 nA were used over 15–25 trials. For data analyses we analyzed the response ratio (RR), which was defined as the ratio of the second excitatory postsynaptic potential EPSP (EPSP2) over the first EPSP (EPSP1). The change in RR was defined as the value of RR after training minus RR before training. Because our goal was to use LTP specifically as a marker of synaptic activity, for data analysis only, cells that exhibited facilitation of at least 20% of either EPSP1 or EPSP2 20 min after conditioning were included. Some cells underwent reversal conditioning, in which after the first phase of training the pulse that was not paired with postsynaptic depolarization was then paired with depolarization. For analysis of the reversal data the change is measured relative to the RR 20 min after the first phase of conditioning. For the reversal data, only cells in which either EPSP underwent a further increase in amplitude in relation to the first posttest were analyzed.
The hippocampal trisynaptic circuit represents the primary flow of activity through the hippocampus; however, the circuitry is considerably more complex and alternate paths exist (13, 14). Because our interest is in understanding how neural activity is altered as it flows through this hidden layer, it is important to determine that the EPSPs recorded in CA1 are indeed primarily polysynaptic rather than monosynaptic. Two potential sources of monosynaptic input to CA1 that could arise from DG stimulation are the perforant path (which also projects to CA1) and CA3 axons activated in the DG. We do not believe there was a significant monosynaptic component in the present study for the following reasons. (i) The perforant path to CA1 projection is distinct from the perforant path to CA3 projection, and cuts made along the hippocampal fissure did not alter the probability of eliciting polysynaptic EPSPs. (ii) Only cells with EPSP latencies above 8 ms (mean, 10 ms) were used. (iii) As expected, the EPSP variability was larger than that observed by direct Schaffer collateral stimulation. (iv) More importantly, if the input was significantly contaminated by monosynaptic inputs, it would mask any order- or context-specific effect; thus, the results presented here represent a conservative estimate of the magnitude of the temporal-to-spatial transformation.
RESULTS
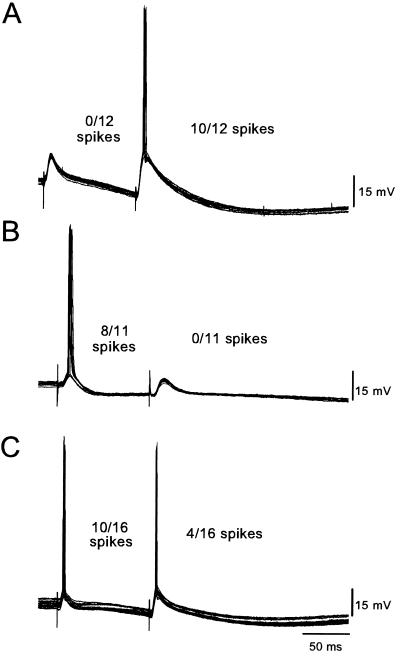
It is well known that, due to short-term forms of plasticity and slow synaptic events, the first and second of a pair of pulses can produce different postsynaptic responses. The CA3 region has been shown to exhibit a variety of paired-pulse effects, including paired-pulse facilitation (15), and effects mediated by pre- and postsynaptic GABAB receptors (16–18). If the resultant interaction between these different neural mechanisms is such that the first EPSP results in a larger degree of depolarization, spiking in response to the first pulse is favored. In contrast, if more depolarization is produced in response to the second EPSP, firing in response to the second pulse is favored. Although most studies with paired-pulse stimulation do not generally study neural responses at or near threshold, it is clear that cells can spike selectively to specific temporal patterns of stimulation (19). Indeed, Fig. 1 shows that CA3 neurons can exhibit order-sensitive responses in response to paired-pulse stimulation of the DG (100-ms interpulse interval). The cells in Fig. 1 A and B can be considered temporally selective because they fired an action potential exclusively to either the second or first pulse, respectively. The cell in Fig. 1C spiked in response to both pulses but exhibited a higher probability of firing to the first pulse. Including subthreshold responses, for a 100-ms interval, approximately 60% (n = 12) of cells exhibited their peak response or fired to the second pulse and 40% exhibited a peak response or fired to the first pulse.
Figure 1.
Examples of order-sensitive CA3 neurons. Intracellular recording from CA3 neurons in response to paired-pulse stimulation of the DG with an interpulse interval of 100 ms. (A) An order-sensitive CA3 pyramidal cell that spiked exclusively to the second pulse. (B) An order-sensitive cell that spiked exclusively to the first pulse. (C) Example of a neuron that spiked in response to both the first and second pulses but exhibited a higher probability of spiking in response to the first pulse. Numbers reflect the number of times the cell spiked in response to the first or second pulse over the number of sweeps shown. (Sweeps collected over a 10- to 15-min period.)
A question of interest is whether different populations of CA3 neurons are consistently activated by the first or second pulse, and whether these differences can be used by neurons downstream. If so, neurons downstream could potentially develop order- or context-selective responses by selectively potentiating the appropriate inputs. To examine this issue we have used hippocampal slices to study network dynamics. The hippocampus provides an excellent system for analyzing circuit and dynamic properties because its circuitry is relatively well understood. The three principal hippocampal areas, the DG, CA3, and CA1, are connected in series: DG → CA3 → CA1. To determine whether different populations of CA3 neurons are consistently activated by the first and second pulses, we recorded polysynaptic EPSPs from a single CA1 neuron in response to paired-pulse stimulation of the DG.
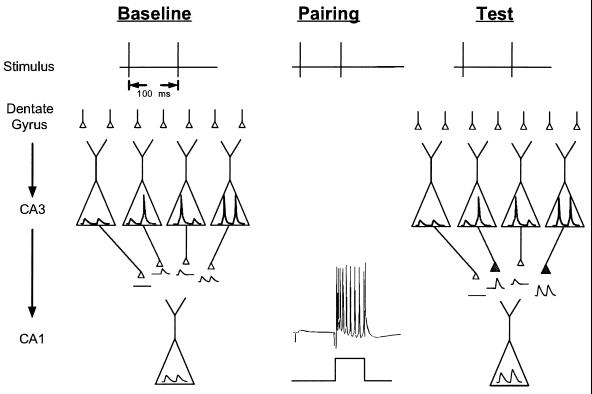
If the first and second pulses to the DG activate different subpopulations of CA3 pyramidal cells, then the first and second polysynaptic EPSP recorded in a CA1 neuron will have arisen from different CA3 neurons. To determine whether the EPSPs were from different subpopulations of CA3 neurons, we used associative LTP as an activity-dependent marker to “tag” synapses from CA3 neurons that were active during each pulse (see Materials and Methods). A schematic of this approach is shown in Fig. 2. CA3 neurons can exhibit four types of responses: not fire to either input pulse, fire to both, or fire to the first or second pulse (see Fig. 1). Assume, in the extreme case, that only two CA3 neurons were present: “A,” which fires in response to the first pulse and produces the first EPSP (EPSP1) in the CA1 cell, and cell “B,” which fires to the second pulse and produces the second EPSP (EPSP2) in the CA1 neuron. If depolarization of the CA1 neuron is explicitly paired with either the first (or second) pulse to the DG, associative LTP will selectively increase the strength of the synapses from A (or B) and will indicate that different cells are responsible for the postsynaptic response to each pulse. In the more realistic case, multiple populations of CA3 neurons contribute to each CA1 response. If the population of CA3 neurons that contributed to each pulse was identical, then the facilitation of the first and second CA1 response should be independent of whether depolarization was explicitly paired with the first or second pulse. If, however, some CA3 cells fire exclusively, or preferentially, during the first pulse, then postsynaptic depolarization during the first (second) pulse should produce more facilitation of the CA1 response to the first pulse. Using LTP as an activity-dependent marker in this manner allows sampling of a population of neurons without recording simultaneously from many different neurons. Moreover, it extends studies of synaptic plasticity into the temporal domain. That is, rather than selectively potentiating the EPSPs from one pathway and not from a second pathway, it is possible to preferentially potentiate either the first or second EPSPs from the same pathway. This method reflects a biologically plausible mechanism by which neurons could learn to respond selectively to a particular interval or stimulus combination.
Figure 2.
Schematic diagram of the use of associative plasticity to potentiate different populations of CA3 neurons. Paired-pulse stimulation of the DG can potentially activate four different subtypes of CA3 neurons (classified according to which input pulses they fire in response to). A sample of the CA3 activity can be recorded by the EPSPs produced in a CA1 neuron (Left). Pairing depolarization of the CA1 neuron exclusively with the second (or first) input pulse (Center) potentiates the synapses from CA3 neurons that were activated by the pulse (Right). A change in the ratio of the second and first EPSP suggests that some CA3 neurons were preferentially active during the first or second pulse.
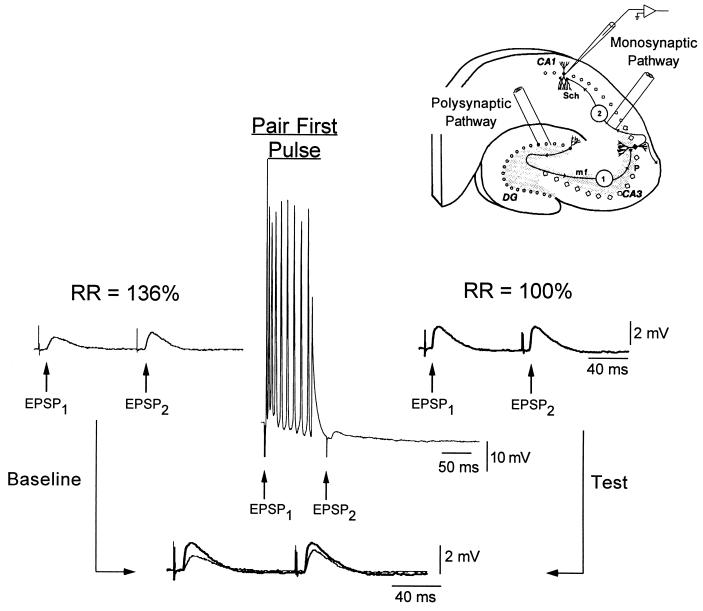
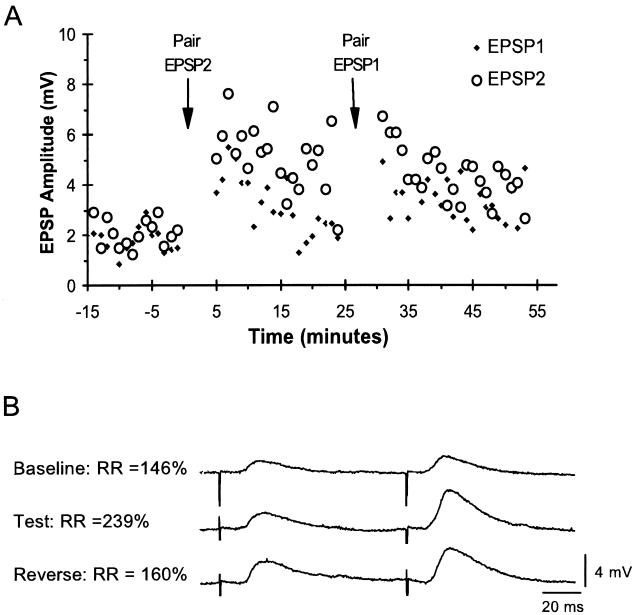
Two experimental groups were used: a First-Pulse Paired and a Second-Pulse Paired group. The only difference between the groups was which pulse was initially paired with depolarization of the CA1 pyramidal cell. Fig. 3 shows an example of an experiment from the First-Pulse Paired group. After a 10-min baseline period in which paired pulses were presented to the DG every 15–20 sec, there was a 5- to 6-min conditioning period in which the first pulse was paired with depolarization. After conditioning, a test period of 20 min was obtained. Data were analyzed by comparing the ratio of the amplitude of EPSP2 to EPSP1 (RR) before and 20 min after conditioning. Note that after pairing EPSP1 with postsynaptic depolarization, a potentiation of both EPSP1 and EPSP2 was observed. However, the potentiation of the paired pulse (EPSP1) was larger, resulting in a decrease in the RR. Thus, some but not all of the EPSPs activated by the second pulse had also been activated by the first. In some cells a second phase of reverse conditioning was performed. During reverse conditioning, the pulse that was not paired during the first phase of conditioning was paired. This made it possible to rule out any changes in the RR that might be independent of which pulse was paired, as well as any possible ceiling effects. Fig. 4A shows the time course and results of an experiment from the Second-Pulse Paired group with a reverse conditioning phase. As a result of initially pairing EPSP2, the RR increased, due to a larger increase in the amplitude of EPSP2. The increase in RR was then partially reversed by pairing EPSP1 with depolarization. Because the increase or decrease in the RR corresponded with whether the second or first pulse was paired, respectively, these results indicate that during reverse conditioning a separate subpopulation of synapses was being potentiated.
Figure 3.
Example of a context-sensitive synaptic plasticity experiment. By stimulating the DG, EPSPs are elicited in CA1 through a polysynaptic path DG → CA3 → CA1 (Inset). Paired pulse stimulation of the DG with an interpulse interval of 100 ms elicited two polysynaptic EPSPs (EPSP1 and EPSP2) with latencies above 8 ms. Data are analyzed in relation to the response ratio (RR = amplitude of EPSP2/EPSP1). Conditioning consisted of 15 trials in which the first pulse was paired with an 80-ms depolarizing pulse (note spikes), whereas the second pulse remained unpaired (note EPSP2). As a result of conditioning the RR decreased from 136 to 100%. By overlapping the baseline and test traces, a clear difference in the potentiation of EPSP1 vs. EPSP2 can be seen.
Figure 4.
Example of an experiment from the Second-Pulse Paired group. (A) Time course. EPSP amplitudes reflect the peak amplitude of the first and second polysynaptic EPSP. During conditioning, EPSP2 was initially paired 15 times with postsynaptic depolarization. Pairing resulted in a larger potentiation in EPSP2. During reverse conditioning, EPSP1 was paired with depolarization; in contrast to the first phase, this pairing resulted in an increase in EPSP1. Note increased overlap between circles and squares. (B) Average sweeps showing the EPSP and RRs before, 20 min after conditioning, and 20 min after reverse conditioning. Initial postsynaptic depolarization in conjunction with the EPSP2 resulted in an increase in RR. After reverse conditioning, there was an increase in the amplitude of EPSP1 as compared with Test and a small decrease in EPSP2. Note the long latencies of the EPSPs due to the polysynaptic nature of the input.
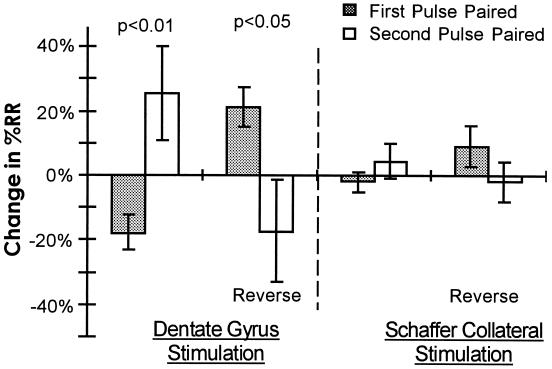
To establish a causal relationship between the direction of the change in the RR and which pulse was paired, we looked at the average change in RR for both groups. Fig. 5 shows that there was a significant difference in the change in RR between the First-Pulse and Second-Pulse groups (t17 = 2.88, P < 0.01). For the First-Pulse group, RR decreased 18 ± 7%, whereas for the Second-Pulse group, RR increased 26 ± 15%. Similarly, there was a group-specific difference between both groups after reverse conditioning (t13 = 2.5, P < 0.05). In interpreting these results, it is necessary to establish that the changes in RR are occurring as a result of facilitation of synapses arising from different subpopulations of CA3 neurons (i.e., changes are due to a circuit property rather than to some novel form of plasticity that could cause PPF to be differentially modified depending on whether the EPSP1 or EPSP2 was paired with depolarization. Although PPF is generally reported not to change with LTP (e.g., ref. 20), there have been reports of PPF either increasing or decreasing with LTP (21). However to account for the results reported here it would be necessary for the changes in PPF to exhibit a form of “Hebbian” plasticity (i.e., PPF would have to decrease if the first EPSP had been paired with depolarization, and increase if the second EPSP had been paired). To control for this possibility and other possible interpretations, we applied the same experimental protocol while directly stimulating the Schaffer collateral axons, which provide monosynaptic input to CA1 from CA3 neurons. Our results showed that, in contrast to the polysynaptic pathway, there was no significant difference between the change in the RR between the First- and Second-Pulse Paired groups (Fig. 5). These results are consistent with our previous findings (12) in which there was no relationship between whether the first or second pulse was paired and changes in PPF.
Figure 5.
Average change in the RR. The average RR decreased in the First-Pulse Paired Group and increased in the Second-Pulse Paired group. The difference between the groups was significant (P < 0.01). Similarly, there was a significant difference between the groups after reverse conditioning (P < 0.05). (Right) Average data for similar experiments performed on the monosynaptic Schaffer collateral pathway. Change in RR corresponds to RRtest − RRbaseline or RRreversal − RRtest. The number of data points for each bar (in order) is 11, 8, 9, 6, 15, 7, 7, 4. (Error bars = SEM.)
The average results shown in Fig. 5 establish that the degree of potentiation is distinct for the first and second EPSP in a manner causally related to the training protocol, and that such a relationship is not observed when a monosynaptic pathway is stimulated.
DISCUSSION
Our results suggest that networks of neurons are intrinsically able to perform temporal-to-spatial transformations. Specifically, the first and second of a pair of pulses activate different populations of neurons; thus, a particular temporal pattern of inputs is transformed into the firing of distinct neurons (i.e., into a spatial code). We have demonstrated that a temporal-to-spatial transformation might take place by showing that when a pattern of neural activity flows through a “hidden” layer, it is possible to preferentially potentiate the first or second of a pair of EPSPs. In other words, the presence or absence of a preceding input pulse establishes “context”; the degree of facilitation is dependent on whether or not the stimulus was preceded by an input pulse 100 ms earlier.
By using associative synaptic plasticity as an activity-dependent marker of inputs from a “hidden” layer, we were able to determine that different neurons responded to the first and second pulses without recording simultaneously from a large population of CA3 neurons. Recording downstream from an intermediary layer also represents the physiological flow of neural activity in a circuit and exemplifies a biologically realistic method by which the nervous system may generate temporally combination-sensitive neurons. Furthermore, by indirectly sampling the activity of CA3 neurons, the responses are more likely to reflect the actual neuronal behavior. Intracellular recordings with sharp electrodes introduce a somatic shunt conductance that can alter the input resistance (RN) and time constants (τ) of a cell (22). Thus, when recording suprathreshold responses with sharp microelectrodes it is possible that the recorded temporal response characteristics differ from the actual responses.
Although using associative plasticity, an activity-dependent marker, should reflect naturally occurring events in the neural circuits, other factors could potentially confound the interpretation of our results. The interpulse interval used here was 100 ms. It has been previously reported that a 100-ms interval between pre- and postsynaptic activity can result in a small degree of potentiation (11). Thus, pairing of the second pulse could possibly potentiate CA3 inputs activated by the first pulse. Due to the small magnitude of potentiation at 100 ms (11), we believe this potential “contamination” is not significant in our experiments. If, however, potentiation at a delay of 100 ms was significant, it would mask rather than increase the context-sensitive plasticity observed here. In our experiments, tetanic stimulation or a “massed” pairing protocol could not be used to induce LTP because they involve changes in the temporal pattern of stimulation. We used an associative protocol, which involved 15–25 single pairings (approximately 5 min). This protocol is not always effective in inducing long-term plasticity. However, our goal was not specifically to induce LTP but to use associative plasticity to differentially potentiate different subpopulations of CA3 neurons. Because a pairing protocol was used, any plasticity that was induced was associative; thus, the duration of the plasticity should not affect the interpretation of our results.
It seems likely that previously described time-dependent properties in CA3, such as paired-pulse facilitation (15), paired-pulse depression of fast inhibitory postsynaptic potentials (16–18), and slow GABAB-mediated inhibitory postsynaptic potentials (17, 23), can account for the differential responses of neurons to the first and second of a pair of input pulses. For example, as a result of paired-pulse facilitation, a neuron can fire selectively to the second pulse if cell threshold is between the amplitude of the first and second EPSP. Conversely, if the first EPSP was suprathreshold and activated a strong GABAB-mediated hyperpolarizing current, the second pulse may generate a subthreshold response. It is clear that on a cell-by-cell basis the balance of different opposing forms of time-dependent properties will determine the temporal response characteristics of each cell. Thus, the computational function of short-term forms of plasticity and slow synaptic events may be precisely to change the state of a network in a time-dependent manner to generate a range of different temporal response characteristics (10). Recently other functional roles for short-term forms of plasticity have been suggested. Carew and colleagues (24, 25) have shown that short-term synaptic enhancement between Aplysia interneurons provides a mechanism for “on-line” modulation of the siphon withdrawal reflex. It has also been suggested that paired-pulse depression of cortical synapses may play a role in gain control by amplifying transient changes in firing rates (26, 27). Given the heterogeneity in both the different forms and the sites of short-term plasticity, it is seems likely that there are multiple functional roles for short-term plasticity, including those mentioned above.
The experimental results presented here, together with previous theoretical results (10), support the view that networks of neurons are intrinsically able to process temporal information on the time scale of tens to hundreds of milliseconds. Thus, it may not be necessary to invoke the existence of internal clocks (28) or delay lines (29, 30) for temporal processing on this time scale. Temporal–spatial transformations, coupled with associative synaptic plasticity as used here, may provide a neural mechanism for temporal processing and the emergence of temporal combination-selective neurons, including interval- and vocalization-sensitive neurons in monkeys (31–33), song-sensitive neurons in birds (34–36), and word-selective neurons in humans (37).
Acknowledgments
The work was supported by Office of Naval Research Grant N00014–96-1–0206, National Institute of Mental Health Fellowship F32 MH10431, and National Institutes of Health Grant 10414. We thank Allison Doupe, Henry Mahncke, Ken Miller, Jennifer Raymond, and Michael Stryker for reading an earlier version of this manuscript.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: DG, dentate gyrus; LTP, long-term potentiation; RR, response ratio; EPSP, excitatory postsynaptic potential.
References
- 1.Rammsayer T H, Lima S D. Percep Psychophys. 1991;50:565–574. doi: 10.3758/bf03207541. [DOI] [PubMed] [Google Scholar]
- 2.Wright B A, Buonomano D V, Mahncke H W, Merzenich M M. J Neurosci. 1997;17:3956–3963. doi: 10.1523/JNEUROSCI.17-10-03956.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lisker L, Abramson A S. Word. 1964;20:384–422. [Google Scholar]
- 4.Tallal P. In: Temporal Coding in the Brain. Buzsáki G, Llinas R, Singer W, Berthoz A, Christen Y, editors. Berlin: Springer; 1994. pp. 291–299. [Google Scholar]
- 5.Shannon R V, Zeng F G, Kamath V, Wygonski J, Ekelid M. Science. 1995;270:303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- 6.Tallal P, Piercy M. Nature (London) 1973;241:468–469. doi: 10.1038/241468a0. [DOI] [PubMed] [Google Scholar]
- 7.Merzenich M M, Jenkins W M, Johnston P, Schreiner C, Miller S L, Tallal P. Science. 1996;271:77–81. doi: 10.1126/science.271.5245.77. [DOI] [PubMed] [Google Scholar]
- 8.Tallal P, Miller S L, Bedi G, Byma G, Wang X, Nagarajan S S, Schreiner C, Jenkins W M, Merzenich M M. Science. 1996;271:81–84. doi: 10.1126/science.271.5245.81. [DOI] [PubMed] [Google Scholar]
- 9.Hubel D. Nature (London) 1996;380:197–198. doi: 10.1038/380197a0. [DOI] [PubMed] [Google Scholar]
- 10.Buonomano D V, Merzenich M M. Science. 1995;267:1028–1030. doi: 10.1126/science.7863330. [DOI] [PubMed] [Google Scholar]
- 11.Gustafsson B, Wigström H, Abraham W C, Huang Y Y. J Neurosci. 1987;7:774–780. doi: 10.1523/JNEUROSCI.07-03-00774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buonomano D V, Merzenich M M. J Neurophysiol. 1996;76:631–636. doi: 10.1152/jn.1996.76.1.631. [DOI] [PubMed] [Google Scholar]
- 13.Amaral D G, Witter M P. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- 14.Yeckel M F, Berger T W. Proc Natl Acad Sci USA. 1990;87:5832–5836. doi: 10.1073/pnas.87.15.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zalutsky R A, Nicoll R A. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- 16.Lambert N A, Wilson W A. J Neurophysiol. 1993;69:630–635. doi: 10.1152/jn.1993.69.2.630. [DOI] [PubMed] [Google Scholar]
- 17.Gaiarsa J-L, Tseeb V, Ben-Ari Y. J Neurophysiol. 1995;73:246–255. doi: 10.1152/jn.1995.73.1.246. [DOI] [PubMed] [Google Scholar]
- 18.Buonomano D V, Hickmott P W, Merzenich M M. Soc Neurosci Abstr. 1996;22:1743. [Google Scholar]
- 19.Segundo J P, Moore G P, Stensaas L J, Bullock T H. J Exp Biol. 1963;40:643–667. doi: 10.1242/jeb.40.4.643. [DOI] [PubMed] [Google Scholar]
- 20.Manabe T, Wyllie D J A, Perkel D J, Nicoll R A. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- 21.Schulz P E, Cook E P, Johnston D. J Neurosci. 1994;14:5325–5337. doi: 10.1523/JNEUROSCI.14-09-05325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spruston N, Johnston D. J Neurophysiol. 1992;67:508–529. doi: 10.1152/jn.1992.67.3.508. [DOI] [PubMed] [Google Scholar]
- 23.Hablitz J J, Thalmann R H. J Neurophysiol. 1987;58:160–179. doi: 10.1152/jn.1987.58.1.160. [DOI] [PubMed] [Google Scholar]
- 24.Fischer T M, Carew T J. J Neurosci. 1995;15:762–773. doi: 10.1523/JNEUROSCI.15-01-00762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher S A, Fischer S A, Carew T J. Trends Neurosci. 1997;20:170–177. doi: 10.1016/s0166-2236(96)01001-6. [DOI] [PubMed] [Google Scholar]
- 26.Abbott L F, Varela J A, Kamal S, Nelson S B. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- 27.Tsodyks M V, Markram H. Proc Natl Acad Sci USA. 1997;94:719–723. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Church R M. Ann N Y Acad Sci. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- 29.Jeffress L A. J Comp Physiol Psychol. 1948;41:35–39. doi: 10.1037/h0061495. [DOI] [PubMed] [Google Scholar]
- 30.Carr C E. Annu Rev Neurosci. 1993;16:223–243. doi: 10.1146/annurev.ne.16.030193.001255. [DOI] [PubMed] [Google Scholar]
- 31.Riquimaroux R. Trans Tech Comm Psychol Physiol Acoust (H) 1994;94–28:1–8. [Google Scholar]
- 32.Rauschecker J P, Tian B, Hauser M. Science. 1995;268:111–114. doi: 10.1126/science.7701330. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Merzenich M M, Beitel R, Schreiner C E. J Neurophysiol. 1995;74:2685–2706. doi: 10.1152/jn.1995.74.6.2685. [DOI] [PubMed] [Google Scholar]
- 34.Margoliash D. J Neurosci. 1983;3:1039–1057. doi: 10.1523/JNEUROSCI.03-05-01039.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doupe A J, Konishi M. Proc Natl Acad Sci USA. 1991;88:11339–11343. doi: 10.1073/pnas.88.24.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewicki M S, Arthur B J. J Neurosci. 1996;16:6987–6998. doi: 10.1523/JNEUROSCI.16-21-06987.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Creutzfeldt O, Ojemann G, Lettich E. Exp Br Res. 1989;77:451–475. doi: 10.1007/BF00249600. [DOI] [PubMed] [Google Scholar]