Abstract
Calretinin (Cr) is a Ca2+ binding protein present in various populations of neurons distributed in the central and peripheral nervous systems. We have generated Cr-deficient (Cr−/−) mice by gene targeting and have investigated the associated phenotype. Cr−/− mice were viable, and a large number of morphological, biochemical, and behavioral parameters were found unaffected. In the normal mouse hippocampus, Cr is expressed in a widely distributed subset of GABAergic interneurons and in hilar mossy cells of the dentate gyrus. Because both types of cells are part of local pathways innervating dentate granule cells and/or pyramidal neurons, we have explored in Cr−/− mice the synaptic transmission between the perforant pathway and granule cells and at the Schaffer commissural input to CA1 pyramidal neurons. Cr−/− mice showed no alteration in basal synaptic transmission, but long-term potentiation (LTP) was impaired in the dentate gyrus. Normal LTP could be restored in the presence of the GABAA receptor antagonist bicuculline, suggesting that in Cr−/− dentate gyrus an excess of γ-aminobutyric acid (GABA) release interferes with LTP induction. Synaptic transmission and LTP were normal in CA1 area, which contains only few Cr-positive GABAergic interneurons. Cr−/− mice performed normally in spatial memory task. These results suggest that expression of Cr contributes to the control of synaptic plasticity in mouse dentate gyrus by indirectly regulating the activity of GABAergic interneurons, and that Cr−/− mice represent a useful tool to understand the role of dentate LTP in learning and memory.
The maintenance of low intracellular free calcium concentration ([Ca2+]i) is of vital importance for the cell and is performed by a variety of plasma membrane pumps and exchangers, organelle sequestration, and cytoplasmic Ca2+ binding proteins (1). In neurons, these different systems control both resting [Ca2+]i and the variations of [Ca2+]i that follow cell activation by environmental signals or electrical activity (1). Among Ca2+ binding proteins, calbindin D28k and calretinin (Cr)—two members of the calmodulin superfamily—are believed to play an important role in calcium homeostasis (2). These proteins, which share 58% amino acid identity, are the only known members of a subfamily characterized by the presence of six “EF hand” Ca2+ binding domains (3–5). Recent reports have shown that calbindin D28k controls Ca2+ homeostasis by buffering Ca2+ and regulating Ca2+ channel activity (6). Overexpression of calbindin D28k in cultured cells affects the time course and spatial spreading of intracellular Ca2+ transients and induces modifications in Ca2+-triggered signals (6–8). Marked changes of synaptically evoked postsynaptic Ca2+ transients have also been observed in Purkinje cells of calbindin D28k-deficient mice, resulting in an unusual type of ataxia (9). At the presynaptic level, calbindin D28k overexpression did not modify the evoked neurotransmitter release in cultured hippocampal pyramidal neurons but suppressed posttetanic potentiation (10). Calbindin D28k has also been implicated in synaptic plasticity. In mice with reduced amount of calbindin, long-term potentiation (LTP) could not be always evoked in the CA1 area of hippocampus (11).
To investigate further the physiological role of Cr in vivo, we have generated Cr-deficient (Cr−/−) mice by homologous recombination. A large number of morphological, biochemical, and behavioral parameters were found unaffected in Cr−/− mice. Because in the normal mouse hippocampus Cr is expressed in hilar mossy cells and in widely distributed subsets of GABAergic (GABA = γ-aminobutyric acid) interneurons (12), we analyzed synaptic transmission and plasticity in dentate gyrus and CA1 area of mutant mice as a physiological correlate for the absence of Cr expression in these cells.
MATERIALS AND METHODS
Targeting Vector Construction.
A DNA fragment including the second exon of the murine Cr gene was isolated from a 129 phage genomic library (Stratagene) after screening with a probe encompassing the first 40 nt of the human corresponding exon (5). To construct the targeting vector, a large XmnI–BamHI fragment including the last 42 nt of the second exon and ≈10.9 kb of genomic sequence 3′ of this exon was linked to the herpes simplex virus thymidine kinase cassette. Then, a SalI–EcoRI fragment containing 1.3 kb of the first intron and the first 4 nt of the second exon was produced by PCR and ligated to the EcoRI–HindIII pKJ1 neomycine resistance cassette, generating a SalI–HindIII fragment. Finally, the SalI–HindIII pKJ1-containing fragment was introduced into unique restriction sites at the 5′ end of the 10.9-kb genomic fragment to create the targeting vector.
Generation of Cr-Deficient Mice.
129/Ola-derived E14 embryonic stem cells were electroporated with the SalI-linearized targeting vector and selected in the presence of G418 and gancyclovir. Genomic DNA of resistant clones was digested with EcoRV and BamHI and hybridized with probe A, a 0.27-kb StuI–ClaI DNA fragment located outside the targeting vector. The homologous recombination events were confirmed by hybridization with probe B, a 1.6-kb XbaI–BamHI DNA fragment included in the construct. The absence of nonhomologous integration in the recombinant embryonic stem clones was checked using a 0.8-kb PstI–BamHI fragment of the neomycine resistance cassette as a probe. Chimaeric mice were obtained after aggregation with CD-1 morulae. Experimental mice, on a 129/Ola × C57BL/6 genetic background, were derived from F2–F5 intercross generations. C57BL/6JIco and 129/OlaHsd mice were purchased from Iffa Credo and Harlan France Sarl, respectively.
Northern Blot Analysis.
Total brain RNA (12 μg/lane) was separated on a 1% agarose gel and transferred to a nylon membrane. The blot was hybridized with the human Cr or calbindin D28k cDNAs as probes (4, 5).
Western Blot and Two-Dimensional Gel Electrophoretic Analysis.
Supernatants of brain and/or ocular globe tissue homogenates were analyzed. For Western blot analysis, proteins (100 μg/lane) were separated by SDS/PAGE and transferred to nitrocellulose sheets. Saturation, incubation with rabbit anti-calbindin D28k (1:1000, SWant, Bellinzona, Switzerland), anti-Cr (1:1000, SWant), or anti-parvalbumin (1:1000, SWant) antiserum, and staining were performed as described by the manufacturer. Two-dimensional gel electrophoretic analysis were done according to standard procedures.
Histology and in Situ Hybridization Histochemistry.
Mice were ether-anesthetized, killed by decapitation, and their brains quickly removed. For routine histological examination, brains were fixed in 10% formol and embedded in paraffin following a standard procedure. Serially cut 7-μm-thick parasagital sections were hematoxylin/eosin-stained. In situ hybridization was performed using Cr (5′-CATGCCAGAACCCTTCCTTGCCTTCTCCAGCTCCTGGAAGAAG-3′) and calbindin D28k (5′-CAATCCAGCCTTCTTTCGCGCCTGCAGAAGCTCCTGGATCA-3′) oligonucleotide probes complementary to part of the corresponding murine exon 2.
Immunohistochemistry.
Brains were fixed in 4% paraformaldehyde and cryopreserved according to standard procedures. Serially cut 35-μm-thick sagital cryosections were processed as free-floating, saturated, overnight incubated with the primary antibodies referred to above, and thereafter with swine anti-rabbit Igs (Dakopatts) and rabbit peroxidase-antiperoxidase complex. Cr- and calbindin D28k-immunoreactivity were thereafter detected using 3,3′-diaminobenzidine (Vector Laboratories) as chromogen.
Electrophysiology.
Hippocampus slices (500–700 μm thick) were disposed into a submersion-type recording chamber through which artificial cerebrospinal fluid (ACSF: 124 mM NaCl/3 mM KCl/1.25 mM NaH2PO4/2 mM MgSO4/2 mM CaCl2/26 mM NaHCO3/10 mM glucose; Mg2+ was increased to 3.3 mM for CA1 recordings) was continuously perfused at 2.5–3 ml/min. ACSF was bubbled with a mixture of 95% O2/5% CO2 and maintained at 32°C (13). Test stimulations (0.1 ms duration) were delivered at constant voltage every 30 s through bipolar concentric stainless steel electrodes placed in the molecular layer of the dentate gyrus or in the stratum radiatum of the CA1 area on either the subicular or the hilar side. Extracellular field excitatory postsynaptic potentials (EPSPs) were recorded with a monopolar tungsten electrode placed in the dendritic field of each region. Synaptic efficacy was monitored at half-maximal stimulation strength as determined from input/output curve construction. LTP was induced by theta burst stimulation (TBS) consisting in 10 trains of 100 pulses at 250 Hz for dentate gyrus (50 pulses at 250 Hz for CA1) with 5-s intertrain intervals.
Behavioral Analysis.
All tests were performed on age- and sex-matched animals. The open field was an arena (40 × 40 × 15 cm high) divided in 16 equal squares. Latency to move (in seconds), horizontal activity (number of squares crossed) over a 3.5-min period, and the number of rearing events (vertical activity) over the same period were recorded. The water maze consisted in a circular pool (90 cm diameter for 6- to 8-week-old mice and 150 cm for 7-month-old mice) surrounded by posters bearing large geometric figures to serve as spatial landmarks. Water was maintained at room temperature and made opaque by a nontoxic white colorant (Lytron 621, Geneva, Switzerland). A white platform (5 × 5 × 14 cm) was hidden in a fixed position ≈1 cm beneath the water surface. Mice swimming in the pool were detected as dark spots on a white surface divided into four quadrants by a computerized video-tracking system (Videotrack; Imetronic, Lyon, France). Training consisted of 10 consecutive daily sessions of four trials separated by a 10- to 15-min interval for 6- to 8-week-old mice, and of three consecutive daily sessions of six trials for 7-month-old mice. Each trial started from a different position (N, S, E, and W in a random order). A trial began by placing the mice in the pool facing the wall and ended when they climbed onto the platform (SE quadrant) or after 60 s. Then, mice were left or gently guided on the platform for 15 s. Twenty-four hours after the last training session, mice were subjected to a probe test in which the platform was removed. Mice started from the NW position and were left to search for the platform for 30 s. In the hot plate test, mice were placed on a plate (15 × 15 cm) heated at 55°C, and the latency to escape the plate was recorded. Motor coordination was tested for 5 min on a rotarod: mice were placed onto a rod of 90 mm in diameter, and the rod was rotated at 10 rpm. The retention time of mice on the rod was recorded. Five trials per day were given for 2 consecutive days.
RESULTS
Targeted Inactivation of the Cr Gene.
To inactivate the Cr gene, a targeting vector was designed that replaces 31 of the 77 bp second exon by the pKJ1 neomycine resistance cassette (Fig. 1 A and B). Cr−/− mice on a 129 × C57BL/6 genetic background were born at a frequency not significantly different from the expected Mendelian transmission of an autosomal gene, suggesting that disruption of both Cr alleles does not cause embryonic lethality.
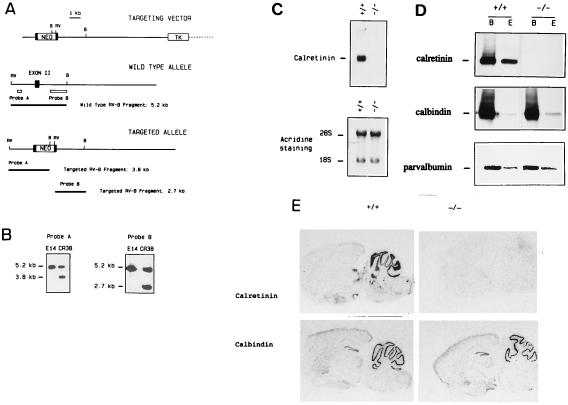
Figure 1.
Cr gene inactivation. (A) The targeting vector, the wild-type allele, and the targeted allele expected following homologous recombination (exon, closed box). RV, EcoRV; B, BamHI. (B) Southern blot analysis of genomic DNA isolated from recombinant clone Cr38. DNA was digested with EcoRV and BamHI and hybridized with probe A and B. The 5.2-, 3.8-, and 2.7-kb fragments represent the wild-type and the mutated alleles after hybridization with probe A and B, respectively. (C) Northern blot analysis. Total brain RNA isolated from Cr+/+ and Cr−/− mice was hybridized with a Cr cDNA probe. A single 2.0-kb signal is observed in Cr+/+ mice. Acridine orange staining confirms that an equal amount of RNA was effectively loaded for each genotype. (D) Western blot analysis. Proteins isolated from brain (lane B) and ocular globes (lane E) of Cr+/+ and Cr−/− mice were probed with anti-Cr, anti-calbindin D28k, or anti-parvalbumin antibodies. (E) In situ hybridization analysis on sagital sections from Cr+/+ and Cr−/− brains with Cr- or calbindin D28k-specific probes.
To verify that disruption of the Cr gene indeed created a null mutation, the corresponding RNA and protein were assayed in brain and retina from adult mutant mice. No RNA transcript could be detected in the brain of Cr−/− mice by Northern blot analysis after hybridization with a human Cr cDNA probe (Fig. 1C), and Western blot analysis confirmed the complete absence of Cr immunoreactivity in brain and ocular globe of Cr−/− mice (Fig. 1D).
Northern and Western blotting, in situ hybridization, as well as two-dimensional gel electrophoretic analysis showed that the loss of Cr gene product was selective and did not result in the obvious up- or down-regulation of other proteins, including calbindin D28k and parvalbumin (Fig. 1 D and E, and data not shown). Routine histological analysis performed on hematoxylin/eosin-stained sections showed that the general anatomy of Cr−/− brains was normal: there were no obvious histological abnormalities or cell loss. In particular, the morphology of the cerebral cortex, the hippocampal formation, the cerebellum, the retina, and the brainstem appeared normal (Fig. 2 A and B, and data not shown).
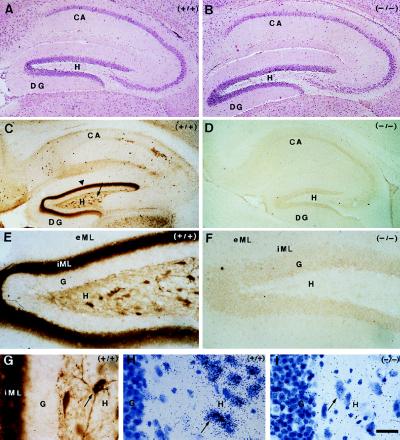
Figure 2.
Histological analysis of hippocampus from adult Cr+/+ and Cr−/− mice. Hippocampal formation of Cr+/+ (n = 5) and Cr−/− (n = 5) mice stained with hematoxylin/eosin (A and B, respectively) and with anti-Cr antibody (C, E, and G, and D and F, respectively): widespread distribution of Cr-immunoreactive (IR) interneurons in all regions of the hippocampus, and very high level of Cr expression in hilar mossy cells soma (arrow) and terminals (arrowhead). Hilar mossy cells are Cr-IR in Cr+/+ mice (E and G), but not in Cr−/− mice (F). Detection of Cr mRNA was by in situ hybridization in hilar mossy cells (arrow) of Cr+/+ mice (H), but not of Cr−/− mice (I). Note the persistence of a normal density of mossy cells in Cr−/− as compared with Cr+/+ mice (compare A and B, and H and I). CA, Ammon’s horn; DG, dentate gyrus, eML, external two-thirds of molecular layer; G, granule cells layer; H, hilus; iML, inner third of molecular layer. (Bar = 200 μm in A–D, 50 μm in E and F, and 25 μm in G–I.)
General Appearance of Cr Mutant Mice.
Cr−/− mice grew normally, were fertile, and had a normal life expectancy; their body weight at 2 and 6 weeks of age was similar to Cr+/+ mice (Table 1). Behavioral analysis of ≈6-week-old animals showed that lack of Cr expression did not significantly alter exploratory activity in the open field test, pain sensitivity in the hot plate test, and motor coordination on the rotorod apparatus (Table 1).
Table 1.
In vivo analysis of Cr−/− mice
| Parameter | Cr+/+ | Cr−/− |
|---|---|---|
| Body weight at 6 weeks, g | ||
| Male | 27 ± 4 | 26 ± 3 |
| Female | 22 ± 3 | 21 ± 3 |
| Open field exploratory test | ||
| Latency to move, s | 6 ± 3 | 5 ± 3 |
| Horizontal activity,*n | 81 ± 15 | 83 ± 17 |
| Vertical activity,*n | 18 ± 5 | 16 ± 6 |
| Hot plate test† | ||
| Latency to escape, s | 22 ± 7 | 25 ± 10 |
| Rotorod (retention time),‡ s | 150 ± 30 | 135 ± 37 |
In the open field exploratory test, Cr+/+ (n = 10) and Cr−/− mice (n = 10) were placed in an arena divided in 16 equal squares. The number of squares crossed over a 3.5-min period was recorded as horizontal activity. The number of rearing events over the same period was recorded as vertical activity. Values are the means ± SEM.
Cr+/+ (n = 7) and Cr−/− mice (n = 7) were placed on a plate heated at 55°C, and the latency to escape the plate was recorded. The values are the means ± SEM.
The rotating rod task was carried out for 5 min and the retention time on the rotating rod was counted for Cr+/+ (n = 8) and Cr−/− mice (n = 8). Five trials per day were given for 2 consecutive days; results are the means ± SEM for the second day.
Cr in the Hippocampal Formation of Cr+/+ and Cr−/− Mice.
In the hippocampus, Cr is expressed by a subset of GABAergic interneurons that have a widespread distribution in Ammon’s horn (CA1 to CA3) and in dentate gyrus (Fig. 2C; ref. 12). However, high levels of Cr were also detected in other neuronal populations of the dentate gyrus. In this area, large multipolar neurons in the hilus were Cr-IR and a dense band of punctate labeling was seen in the inner third of the molecular layer (Fig. 2C). The Cr-positive cells had all the characteristics of the hilar mossy cells (Fig. 2 E and G; ref. 12). The supragranular zone—the inner third of the molecular layer—is known as the target of ipsi- and contralateral mossy cells giving rise to the commissural pathway (14, 15). The dense band of punctate Cr-IR was therefore attributed to mossy cell terminals. The detection of Cr mRNA in large hilar cells of Cr+/+ dentate gyrus confirmed that mossy cells indeed expressed Cr (Fig. 2H). It is noteworthy that neither dentate granule cells nor fibers of the perforant pathway ending in the middle and the outer third of the molecular layer were Cr-IR (Fig. 2 C and E). In the dentate gyrus of Cr−/− mice, no Cr protein (Fig. 2 D and F) or mRNA (Fig. 2I) were detected, although large multipolar cells were still present at a normal density (Fig. 2 B and I).
Induction of LTP Is Impaired in the Dentate Gyrus of Cr−/− Mice.
It has recently been shown that excitatory hilar mossy cells are part of a local pathway ending on dentate granule cells (16). Because mossy cells are strongly Cr-IR, we examined synaptic transmission and plasticity in dentate gyrus of Cr−/− mice as a tentative physiological correlate for the absence of Cr expression in these cells. Mice from parental 129 or C57BL/6 genetic background were also included as controls in the following experiments, but no significant difference was observed as compared with hybrid Cr+/+ mice.
Extracellular field EPSPs were recorded in hippocampal slices maintained in vitro in response to electrical stimulations delivered to the perforant path input to granule cells. Baseline synaptic excitability in dentate gyrus was not affected by the absence of Cr expression. Application of single test stimuli of graded strength showed that the overall size and shape of the EPSPs in Cr−/− mice were similar to that seen in Cr+/+ mice (data not shown). EPSP slope values at half maximal stimulation voltage were not significantly different between genotypes [Cr+/+: 0.133 ± 0.019 V/s (mean ± SEM); Cr−/−: 0.178 ± 0.029 V/s; unpaired Student’s t test: t = 1.274, P > 0.21]. To induce LTP, repeated brief trains of high frequency stimulations following a protocol known as TBS were delivered. Whereas TBS enhanced dentate synaptic transmission in Cr+/+ mice by about 30% for at least 1 h, Cr−/− mice showed a reduced posttetanic potentiation that decayed progressively over time (Fig. 3A). In slices from Cr+/+ mice, the EPSPs were potentiated to 131 ± 3% (n = 12 slices from 10 mice; range, 112–142%) of baseline levels 1 h after TBS while in slices from Cr−/− mice, the EPSPs 1 h after TBS were 93 ± 7% (n = 13 slices, 7 animals; range, 73–126%). Unpaired Student’s t test indicated that these differences were highly significant (t = 5.462, P < 0.0001).
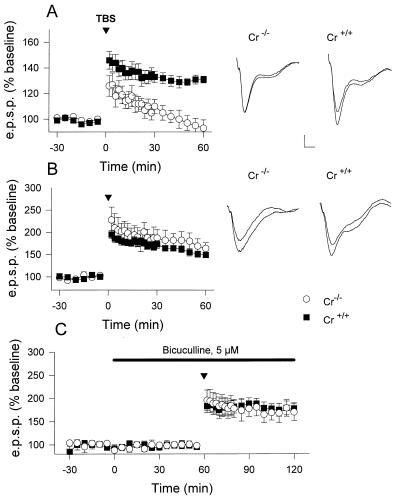
Figure 3.
Synaptic plasticity in Cr−/− mice. Time-course of changes in EPSP slope following TBS to the perforant path-granule cell (A) and the Schaffer commissural-CA1 pyramidal cell (B) synapses in slices from Cr+/+ (▪) and Cr−/− mice (○). (C) rescue of deficient dentate gyrus LTP in the presence of the GABAA receptor antagonist bicuculline applied during the time materialized by the bar. In all graphs, slope values were normalized with respect to a 30-min baseline recording before application of TBS (arrowhead). Values are the means ± SEM. Traces are superimposed EPSPs collected before and 60 min following TBS during a representative experiment for the indicated genotype. (Bars: vertically = 0.1 mV for dentate gyrus and 0.2 mV for CA1; horizontally = 4 ms for all traces.)
To test whether this deficiency in dentate LTP induction was specific to the absence of Cr expression in hilar mossy cells, we analyzed for comparison LTP at the Schaffer-commissural input to CA1 pyramidal cells. In CA1 area, Cr is only expressed in a subset of GABAergic interneurons also present in the dentate gyrus. In contrast to the dentate gyrus, LTP was normally induced in CA1 region of Cr−/− mice (Fig. 3B). Indeed, EPSPs reached 149 ± 7% (n = 13 slices, 11 mice; range, 124–189%) and 164 ± 13% (n = 8 slices, 5 mice; range, 128–212%) of baseline 1 h after TBS for Cr+/+ and Cr−/− mice, respectively (t = 1.053, P > 0.30).
Because Cr-IR excitatory mossy cells in the dentate gyrus are integrated in a local inhibitory circuitry that innervates granule cells via GABAergic interneurons (16), LTP induction was reexamined in Cr−/− mice in the presence of 5 μM bicuculline, a GABAA receptor antagonist. In these conditions, LTP could be induced in Cr−/− mice: its magnitude in the presence of bicuculline (170 ± 18%, 6 slices from 4 mice; range, 110–226%) was not significantly different from Cr+/+ mice in the same conditions (178 ± 12%, 5 slices from 4 mice; range, 137–200%; t = 0.393, P > 0.70) (Fig. 3C). LTP in slices from Cr+/+ mice was enhanced when compared with the conditions where bicuculline was absent (131 ± 3%, 12 slices from 10 mice; range, 112–142%; t = 3.914, P < 0.05). Together, these results support the idea that an exagerated endogenous GABAergic inhibitory tonus interferes with the induction of LTP in Cr−/− mice.
Spatial Memory Is Not Impaired in Cr−/− Mice.
LTP in the hippocampus has been associated with learning and memory (17). Learning capabilities of Cr−/− mice were therefore analyzed using a water maze spatial learning procedure known to be sensitive to pharmacological disruption of LTP (18). Six- to 8-week-old Cr+/+ (n = 10) and Cr−/− (n = 10) mice were trained to locate a hidden platform using spatial clues in the maze environment. The distance swum until the mice escaped on the platform gradually decreased over a 10-day training period in all groups of animals, indicating that the absence of Cr in mutant mice did not impair spatial learning in this model (Fig. 4A). Indeed, a repeated measure ANOVA revealed a highly significant within subject effect of days of training [F(9,162) = 30.11, P < 0.0001], but no significant difference between mutants and controls [F(1,18) = 3.12, P > 0.09].
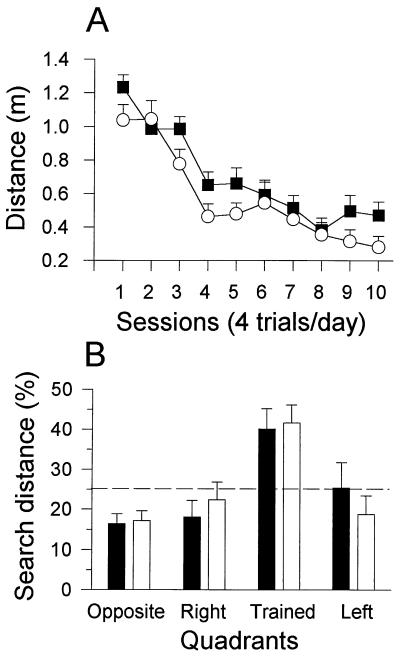
Figure 4.
Spatial learning is normal in Cr−/− mice. Cr−/− (open symbols/patterns; n = 10) and Cr+/+ mice (filled symbols/patterns; n = 10) were tested in a water maze. The dotted lines indicate chance level. (A) Training: mutant and control mice showed a significant decrease of the distance swum to escape on a platform hidden in a fixed position in the water maze over 10 consecutive daily sessions. The distance presented is the mean ± SEM for the four trials forming a session. (B) Probe test: mutant and control mice swam for significantly longer distances searching for the removed platform in the trained quadrant than in the three other quadrants (P < 0.05, Newman–Keuls). The mean percentages of total distance (±SEM) swam in each quadrant during a 30-s test are represented.
When subjected 24 hr after the last training session to a probe test in which the platform was removed from the pool, all mutant and control mice [F(1,18) = 2.0, P > 0.17] showed a highly significant bias to search the platform in the trained quadrant with respect to the others [F(3,54) = 8.93, P < 0.0001; opposite, left, right vs. trained quadrant: P < 0.0008, Newman–Keuls], indicating that they actively remembered the former position of the platform (Fig. 4B). Similar results were obtained in the water maze when 7-month-old Cr+/+ (n = 10) and Cr−/− (n = 10) mice were analyzed: the time spent in the goal quadrant (P > 0.20) as well as the probe test (P > 0.15) was not significantly different between the two genotypes (data not shown).
DISCUSSION
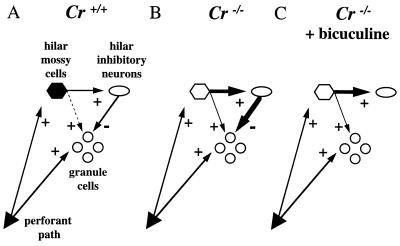
Using classical gene knock-out strategy, we have generated a mouse strain deficient for the calcium binding protein Cr. These Cr−/− mice grow and reproduce normally, have no increased mortality rate, and do not present obvious behavioral abnormalities. Because Cr is mainly expressed in specific subpopulations of neurons (3), particular attention was taken to the analysis of the central nervous system of Cr−/− mice. However, neither gross histological abnormalities nor cell loss were observed in brain or retina, suggesting either that Cr does not play a fundamental role during development of these structures or that the absence of Cr is compensated by overexpression of other calcium binding proteins. Two-dimensional gel electrophoresis, Northern and Western blot analyses, as well as in situ hybridization analysis did not reveal modifications in the expression level of other proteins—including the Cr-related calbindin D28k—in the brain of Cr−/− mice, suggesting the lack of compensatory mechanisms for the absence of Cr. However, it is not excluded that the normal levels of other abundant neuronal calcium binding proteins can fulfill the function(s) of Cr and compensate for its loss in various neuron populations. Crossing our Cr mutant mice with calbindin D28k- or parvalbumin-deficient mice may reveal more dramatic phenotypes than observed in single knock-out mice. In this report, we have focused our experiments on the hippocampus and have shown that the absence of Cr expression interferes with the induction of LTP in dentate gyrus of Cr−/− mice. LTP was specifically impaired in the region of the mouse hippocampus where high levels of Cr are present in wild-type mice, and not in the CA1 region that only contains few scattered Cr-positive interneurons. We and others (12) could ascribe Cr expression in mouse dentate gyrus to hilar mossy cells. In recent reports, it has been shown that these large multipolar neurons innervate dentate granule cells and GABAergic interneurons, that in turn inhibit dentate granule cells (Fig. 5A, adapted from ref. 16 and references therein; and refs. 19–21). Because hilar mossy cells excite granule cells weakly relative to inhibitory interneurons, stimulation of mossy cells results in IPSPs in dentate granule cells (16, 21, 22). Moreover, during perforant pathway stimulation, mossy cells are excited prior to dentate granule cells, reflecting their lower activation threshold (23). Based on the properties of this local inhibitory neuronal circuitry, our results in Cr-deficient mice suggest that during tetanic stimulation of the perforant pathway, excitatory output from hilar mossy cells is increased when Cr is absent in these cells. This increase leads to a net excess of GABA release on dentate granule cells and inhibition of LTP induction (Fig. 5B). Incubation of hippocampal slices from Cr−/− mice with the GABAA receptor antagonist bicuculline not only restored LTP induction in mutant mice, but potentiation reached levels not significantly different from those observed in slices from Cr+/+ mice in the same conditions (Fig. 5C).
Figure 5.
Increased GABAergic tonus on dentate granule cells in Cr−/− mice: a working model for the inhibition of LTP induction (adapted from ref. 16). (A) In Cr+/+ mice, stimulation of excitatory hilar mossy cells and inhibitory interneurons evoked EPSPs and inhibitory postsynaptic potentials (IPSPs) in dentate granule cells, respectively. However, hilar mossy cells excite dentate granule cells weakly as compared with inhibitory interneurons and thus, the resultant on the granule cells is an IPSP. During perforant pathway stimulation, mossy cells are excited before dentate granule cells because of their lower activation threshold. (B) After TBS, no LTP is detected in Cr−/− mice, because excitatory output from Cr-deficient hilar mossy cells is increased. This increase leads to a net excess of inhibitory tonus from GABAergic interneurons to dentate granule cells which interferes with LTP induction. (C) In the presence of bicuculline, dentate granule cells are relieved from the excess of inhibitory tonus and LTP is restored in Cr−/− mice, reaching levels similar to Cr+/+ mice.
We cannot exclude a more direct role of the few Cr-IR inhibitory interneurons located in the dentate gyrus to explain the absence of LTP induction in Cr−/− mice. However, the regional inhibition of LTP in these mice contrasts with the widespread distribution of Cr-IR interneurons in all areas and layers of the hippocampus (Fig. 2C; ref. 12). An elegant way to definitely distinguish between hilar mossy cells versus inhibitory interneurons as the primary cause of LTP inhibition would be to generate a mouse strain lacking Cr specifically in one of these specific subpopulations of neurons.
Whatever the cell primarily responsible for the inhibition of LTP induction in Cr−/− mice, neurotransmitter release from dentate Cr-deficient cells seems enhanced only when tetanic stimulation was applied. Single test stimulus delivered to the perforant pathway induced EPSPs of similar size and shape in granule cells of mutant and normal mice, confirming that basal synaptic transmission was not affected. The molecular mechanisms responsible for evoked transmitter release after a single test stimulus involve the establishment of Ca2+ microdomains directly beneath the voltage-sensitive Ca2+ channels in the terminal membrane (24). As recently discussed by Chard et al. (10) in experiments where the structurally related calbindin-D28k Ca2+ buffer protein was expressed in cultured neurons, the establishment of these Ca2+ microdomains is probably not affected by Cr or calbindin levels because the Ca2+ binding kinetics of these proteins are too slow. By contrast, the absence of Cr protein probably decreases the Ca2+ buffering capacity in presynaptic terminals of Cr−/− neurons, and increases the residual intraterminal Ca2+ concentration ([Ca2+]) after a first action potential (10). The increased residual [Ca2+] modifies neurotransmitter release probably through binding to specific vesicular or cytoplasmic proteins involved in storing vesicles in nerve terminals and in docking vesicles to active zones of exocytosis (24). During a tetanic stimulation, these events could therefore increase the amount of neurotransmitter released in the synaptic cleft, resulting in an enhanced inhibitory tonus on dentate granule cells and LTP inhibition in Cr−/− mice (Fig. 5).
The regional inhibition of LTP induction in Cr−/− mice allowed us to examine the correlation between spatial memory and site of hippocampal LTP. In Cr−/− mice, no deficit in spatial learning was observed in the water maze despite the selective absence of LTP induction in the dentate gyrus. Our results are reminiscent of those observed in mice lacking the neuronal glycoprotein Thy-1 (25). In these knock-out mice, perforant pathway-dentate LTP, but not CA1 LTP, is selectively inhibited in anaesthetized animals, and this inhibition is not correlated with spatial memory deficit. Although some degree of dentate LTP persists in Thy-1 knock-out mice when recorded from in vitro experiments or from awake animals that may suffice to support some learning (26), most of the available knock-out models exhibiting regio-selective impairment of LTP (i.e., CA1, CA3, or dentate gyrus) indicate that spatial memory alteration correlates with blockade of Schaffer collateral/commissural CA1 LTP (27, 28). Because hippocampus has been implicated in other forms of learning and memory than purely spatial learning (29), our Cr-deficient mice could represent a useful model to investigate the specific role of perforant path-dentate gyrus LTP in these functions.
Acknowledgments
The continuous support and interest of Drs. S. Laroche and J.-J. Vanderhaeghen is deeply acknowledged. We thank A. Berns and S. Refetoff for providing us with E14 embryonic stem cells and the 129 phage genomic library, respectively. The expert assistance of Michel Reibaud (Rhône-Poulenc Rorer S.A., France) in behavioral studies as well as Roberte Menu and Michèle Authelet for immunohistochemistry and in situ hybridization is also acknowledged. This work was supported by the Belgian Programme on Interuniversity Poles of Attraction (no. 9 and 22). This work was also supported by the Fondation Médicale Reine Elisabeth, the Fonds de la Recherche Scientifique Médicale of Belgium, and Boehringer Ingelheim. S.S. was partially supported by the Fondation Horlait-Dhapsens and the Fondation R.O.S.E. contre le cancer. S.S. and S.N.S. are chercheurs qualifiés of the Fonds National de la Recherche Scientifique, Belgium.
ABBREVIATIONS
- LTP
long-term potentiation
- Cr
calretinin
- GABA
γ-aminobutyric acid
- EPSP
excitatory postsynaptic potential
- IR
immunoreactive
- TBS
theta burst stimulation
- IPSP
inhibitory postsynaptic potential
References
- 1.Clapham D E. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 2.Christakos S, Gabrielides C, Rhoten W B. Endocr Rev. 1989;10:3–26. doi: 10.1210/edrv-10-1-3. [DOI] [PubMed] [Google Scholar]
- 3.Rogers J H. J Cell Biol. 1987;105:1343–1353. doi: 10.1083/jcb.105.3.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parmentier M, Lawson D E M, Vassart G. Eur J Biochem. 1987;170:207–215. doi: 10.1111/j.1432-1033.1987.tb13688.x. [DOI] [PubMed] [Google Scholar]
- 5.Parmentier M, Lefort A. Eur J Biochem. 1991;196:79–85. doi: 10.1111/j.1432-1033.1991.tb15788.x. [DOI] [PubMed] [Google Scholar]
- 6.Lledo P-M, Somasundaram B, Morton A J, Emson P C, Mason W T. Neuron. 1992;9:943–954. doi: 10.1016/0896-6273(92)90246-a. [DOI] [PubMed] [Google Scholar]
- 7.Mattson M P, Rychlik B, Chu C, Christakos S. Neuron. 1991;6:41–51. doi: 10.1016/0896-6273(91)90120-o. [DOI] [PubMed] [Google Scholar]
- 8.Chard P S, Bleakman D, Christakos S, Fullmer C S, Miller R J. J Physiol (London) 1993;472:341–357. doi: 10.1113/jphysiol.1993.sp019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Airaksinen M S, Eilers J, Garaschuk O, Thoenen H, Konnerth A, Meyer M. Proc Natl Acad Sci USA. 1997;94:1488–1493. doi: 10.1073/pnas.94.4.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chard P S, Jordan J, Marcuccilli C J, Miller R J, Leiden J M, Roos R P, Ghadge G D. Proc Natl Acad Sci USA. 1995;92:5144–5148. doi: 10.1073/pnas.92.11.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molinari S, Battini R, Ferrari S, Pozzi L, Killcross A S, Robbins T W, Jouvenceau A, Billard J-M, Dutar P, Lamour Y, Baker W A, Cox H, Emson P C. Proc Natl Acad Sci USA. 1995;93:8028–8033. doi: 10.1073/pnas.93.15.8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Fujise N, Kosaka T. Exp Brain Res. 1996;108:389–403. doi: 10.1007/BF00227262. [DOI] [PubMed] [Google Scholar]
- 13.Böhme G A, Bon C, Lemaire M, Reibaud M, Piot O, Stutzmann J-M, Doble A, Blanchard J-C. Proc Natl Acad Sci USA. 1993;90:9191–9194. doi: 10.1073/pnas.90.19.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckmaster P S, Strowbridge B W, Kunkel D D, Schmiege D L, Schwartzkroin P A. Hippocampus. 1992;2:349–362. doi: 10.1002/hipo.450020403. [DOI] [PubMed] [Google Scholar]
- 15.Deller T, Leranth C. J Neurophysiol. 1990;300:433–447. doi: 10.1002/cne.903000312. [DOI] [PubMed] [Google Scholar]
- 16.Scharfman H E. Neuroscience. 1996;72:655–668. doi: 10.1016/0306-4522(95)00569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bliss T V, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 18.Davis S, Butcher S P, Morris R G M. J Neurosci. 1992;12:21–34. doi: 10.1523/JNEUROSCI.12-01-00021.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scharfman H E. Neurosci Lett. 1993;156:61–66. doi: 10.1016/0304-3940(93)90440-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scharfman H E. Neurosci Lett. 1994;168:29–33. doi: 10.1016/0304-3940(94)90408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scharfman H E. J Neurophysiol. 1995;74:179–194. doi: 10.1152/jn.1995.74.1.179. [DOI] [PubMed] [Google Scholar]
- 22.Douglas R M, McNaughton B L, Goddard G V. J Comp Neurol. 1983;219:285–294. doi: 10.1002/cne.902190304. [DOI] [PubMed] [Google Scholar]
- 23.Scharfman H E. J Neurosci. 1991;11:1660–1673. doi: 10.1523/JNEUROSCI.11-06-01660.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zucker R S. Neuron. 1996;17:1049–1055. doi: 10.1016/s0896-6273(00)80238-x. [DOI] [PubMed] [Google Scholar]
- 25.Nosten-Bertrand M, Errington M L, Murphy K P S J, Tokugawa Y, Barboni E, Kozlova E, Michalovich D, Morris R G M, Silver J, Stewart C L, Bliss T V P, Morris R J. Nature (London) 1996;379:826–829. doi: 10.1038/379826a0. [DOI] [PubMed] [Google Scholar]
- 26.Errington M L, Bliss T V P, Morris R J, Laroche S, Davis S. Nature (London) 1997;387:666–667. doi: 10.1038/42625. [DOI] [PubMed] [Google Scholar]
- 27.Tsien J Z, Huerta P T, Tonegawa S. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 28.Chen, C. & Tonegawa, S. (1997) Annu. Rev. Neurosci., in press. [DOI] [PubMed]
- 29.Bunsey M, Eichenbaum H. Nature (London) 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]