Abstract
Neuronal apoptosis was observed in the rat dentate gyrus in two experimental models of human limbic epilepsy. Five hours after one hippocampal kindling stimulation, a marked increase of in situ terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL) of fragmented DNA was observed in nuclei located within and on the hilar border of the granule cell layer and in the polymorphic region. Forty kindling stimulations with 5-min interval produced higher numbers of labeled nuclei compared with one stimulation. The increase of TUNEL-positive nuclei was prevented by the protein synthesis inhibitor cycloheximide but not affected by the N-methyl-d-aspartate receptor antagonist MK-801. Kainic acid-induced seizures lead to a pattern of labeling in the hippocampal formation identical to that evoked by kindling. A large proportion of cells displaying TUNEL-positive nuclei was double-labeled by the neuron-specific antigen NeuN, demonstrating the neuronal identity of apoptotic cells. Either 1 or 40 kindling stimulations also gave rise to a marked increase of the number of cells double-labeled with the mitotic marker bromodeoxyuridine and NeuN in the subgranular zone and on the hilar border of the dentate granule cell layer. The present data show that single and intermittent, brief seizures induce both apoptotic death and proliferation of dentate gyrus neurons. We hypothesize that these processes, occurring early during epileptogenesis, are primary events in the development of hippocampal pathology in animals and possibly also in patients suffering from temporal lobe epilepsy.
Keywords: kindling, epilepsy, rat, in situ DNA fragmentation, neurogenesis
Human temporal lobe epilepsy is frequently associated with a marked loss of hippocampal and dentate gyrus neurons. Although this degeneration, called hippocampal sclerosis, was recognized early on (1–3), it is still controversial whether neuronal death is directly involved in the pathophysiology of epilepsy or is just a secondary phenomenon following severe recurring or prolonged seizures. Recent experimental evidence suggests that cell loss can be the result of intense seizure activity and can promote further progression of the epileptic disease (4). It has been well documented that severe and sustained seizures, known as status epilepticus, lead to neuronal degeneration in the human hippocampus within hours from onset (5). Also, experimentally produced status epilepticus in animals consistently causes neuronal loss in a pattern mimicking that of the human condition (see ref. 6). Seizure-induced cell death has traditionally been discussed in the context of excitotoxic mechanisms and degeneration through necrosis (7, 8). More recently, however, it has been shown that apoptosis contributes to the degeneration following status epilepticus in rats (9–12). Even if longer periods of seizure activity, lasting for hours, invariably cause death of hippocampal neurons, it is unclear if single or intermittent seizures, with a duration in the range of minutes, can lead to neuronal damage. It seems highly warranted to explore this possibility because repeated, brief seizures are a feature of human temporal lobe epilepsy. Kindling, an animal model of this type of epilepsy, is particularly suitable for this purpose. In kindling, a permanent epileptic state is produced by a series of brief seizures induced by electrical stimulation (13). The progressive development of seizures in kindling, through well-defined stages, enables a detailed assessment of cellular degeneration during epileptogenesis. Previous studies using conventional methods for estimation of cell numbers have not detected any cell loss at early stages of kindling development (14, 15). At later stages, when hyperexcitability has developed and generalized seizures with tonic–clonic convulsions occur, a mild to moderate cell loss, starting in the dentate gyrus, has been observed (14, 15), although these findings remain controversial (16–20). However, the net effect of seizures on hippocampal cell numbers may be quite complex due to dentate granule cell neurogenesis, which occurs in adult rodents and nonhuman primates (21), and can be increased by status epilepticus (22).
The main objective of the present study was to clarify if brief, single, and recurrent epileptic seizures, induced by hippocampal kindling stimulations, can lead to apoptotic cell death as assessed by highly sensitive in situ DNA fragmentation analysis. In addition, we compared the pattern of apoptosis after kindling with that observed in another model of limbic epilepsy, systemic kainic acid treatment, which causes status epilepticus. Finally, we explored the possibility that kindling-evoked seizures influence neurogenesis in the dentate gyrus.
MATERIALS AND METHODS
Kindling Procedure.
Animal care procedures followed local health care guidelines. Male Sprague–Dawley rats (B&K Universal, Stockholm) weighing 280–300 g were anesthetized using Equithesin (3.0 ml/kg i.p.) and mounted in a Kopf stereotaxic frame. A twisted bipolar, plastic-coated stainless-steel electrode with an outer diameter of 0.28 mm (Plastics One, Roanoke, VA) was implanted into the right ventral hippocampal CA1 region at the following coordinates: tooth-bar at 0; 4.8 mm caudal to bregma, 5.2 mm lateral to midline, and 6.5 mm ventral to dura (23). A reference electrode was placed between the skull and the left temporal muscle. Electrodes were secured to the skull with screws and dental acrylate. Ten days after surgery, 12 rats were given one kindling stimulation (1 s, 100 Hz biphasic pulses of 1-ms duration, 400 μA peak-to-peak amplitude delivered by a Grass S-88 stimulator; Quincy, MA) and were killed 2, 5, and 24 h later (n = 4 at each time point). Four age-matched, electrode-implanted rats were connected to the stimulation/recording apparatus without receiving any stimulation and served as controls. Another 28 rats were given 40 stimulations (10 s, 10 Hz biphasic pulses of 1-ms duration at 400 μA) with 5-min interstimulus intervals according to the rapid kindling protocol of Lothman et al. (24). Behavioral seizures were scored as follows (25): grade 0, normal behavior, wet dog shakes, arrest; 1, facial twitches; 2, head nodding, chewing; 3, forelimb clonus; 4, rearing, falling on forelimbs; 5, falling on the side or back, hindlimb clonus. Seven groups of rats were decapitated 30 min, 2, 4, 12, 24, 72 h, and 7 days after the last stimulation (n = 4 at each time point). Twelve rats underwent electrode implantation and served as nonstimulated controls. Electroencephalographic activity was monitored by a Grass 79D recorder.
Drug Pretreatment.
In a separate experiment, cycloheximide (Sigma–Aldrich; 2.0 mg/kg s.c. in PBS, pH 7.4; volume 1.0 ml/kg) was given to four rats 30 min before a series of 40 rapid kindling stimulations. Another four rats received vehicle injections. Cycloheximide- and vehicle-pretreated rats together with 4 electrode-implanted, nonstimulated rats were killed 2 h after termination of stimulations. At the dose used in this study, cycloheximide effectively blocks ribosomal protein synthesis for more than 12 h (26). In another experiment, 7 rats received MK-801 (Research Biochemicals, Natick, MA; 1 mg/kg i.p.), and 7 animals were given vehicle injections 30 min before 40 kindling stimulations and were decapitated 2 h thereafter. In addition, MK-801 or vehicle was administered to electrode-implanted rats, which were nonstimulated (n = 4 in each group).
Kainic Acid Treatment.
Male Sprague–Dawley rats (90–110 g; n = 4) were given kainic acid (Sigma-Aldrich; 10 mg/kg) s.c. and observed for 1 h to confirm the presence of convulsions. Animals were decapitated 4 h after onset of convulsions.
In Situ Analysis of DNA Fragmentation.
After decapitation, brains were immediately frozen on powdered dry ice. Coronal cryostat sections (14 μm) were processed according to the terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL) Apoptag kit protocol (Oncor) modified from Gavrieli et al. (27). Briefly, after fixation in 10% neutral buffered formalin for 10 min at room temperature, sections were washed in PBS (two times for 5 min) and postfixed in ethanol/acetic acid (2:1) for 5 min at −20°C. After washing in PBS (two times for 5 min) and incubation in equilibration buffer for 5 min at room temperature, reaction buffer containing digoxigenin-11-dUTP and dATP as well as terminal deoxynucleotidyltransferase was applied for 60 min at 37°C. A set of sections was incubated in the absence of terminal deoxynucleotidyltransferase as a negative control. After incubation in stop buffer for 10 min followed by PBS (three times for 3 min), fluorescein conjugated antidigoxigenin antibody was applied together with blocking serum for 30 min at room temperature. After rinsing in PBS (three times for 5 min), intact DNA was stained by propidium iodide (ICN; 5 μg/ml) for 15 min at room temperature. Finally, sections were washed in PBS and briefly in destilled water before being dried and mounted using Vectashield (Vector Laboratories). From each animal, three to six sections taken at the level of the dorsal hippocampus were analyzed blindly and independently by two investigators using epifluorescence microscopy at ×200 magnification. The number of labeled nuclei was counted in an area defined medially, dorsally, and ventrally by the outer margins of the granule cell layer and laterally by a line through the lateral tips of the dorsal and ventral blades of the granule cell layer.
Double-Labeling for in Situ DNA Fragmentation and NeuN.
Subsequent to visualization of fragmented DNA using the TUNEL method, sections were incubated in 10% normal horse serum and 0.40% Triton X-100 in PBS for 1 h at room temperature. A mouse monoclonal anti-NeuN antibody (Chemicon) (28) at a dilution of 1:100 in 5% normal horse serum and 0.10% Triton X-100 was then applied overnight. After washing in PBS (three times for 10 min), sections were incubated with a biotinylated secondary horse-anti-mouse IgG antibody (1:100; Vector Laboratories) for 1 h at room temperature followed by three times for 10 min in PBS. Texas-red avidin D (1:100; Vector Laboratories) was applied for 1 h at room temperature, and sections were then finally rinsed in PBS and briefly in water before being dried and mounted in Vectashield. Visualization of intact nuclear DNA using propidium iodide (ICN) was omitted on sections double stained for apoptosis and NeuN. Sections were analyzed in conventional epifluorescence light as well as in a Bio-Rad confocal laser scanning microscope.
Silver Staining.
Six rats (280–300 g) were given kainic acid (7 mg/kg s.c.) and killed at 4 h (n = 3) or 12 h (n = 3) after the onset of convulsions. Two vehicle-injected animals served as controls (one at each time point). Another 8 animals were given 1 (n = 4) or 40 (n = 4) kindling stimulations and killed at 5 or 2 h, respectively, after the last stimulation. Visualization of degenerating cells was performed according to Nadler and Evenson (29). Briefly, rats were perfused transcardially with 50 ml of 0.9% NaCl followed by 250 ml paraformaldehyde in 0.1 M phosphate buffer (pH 7.2). One hour after perfusion, the brains were removed from the skull and postfixed in the same solution for at least 24 h. After cryoprotection of the tissue in 30% sucrose, 40-μm-thick sections were cut on a freezing microtome. Sections were washed in 0.1 M Tris buffer (pH 7.6) followed by washing three times for 5 min in dH2O. After pretreatment in 4.5% NaOH and 8% NH4NO3, sections were incubated in impregnation solution (5.4% NaOH/6.4% NH4NO3/0.2% AgNO3 in dH2O) for 10 min and then washed in 31.6% ethanol/0.5% Na2CO3/0.12% NH4NO3. The staining was developed in 0.05% citric acid/0.55% formaldehyde/9.5% ethanol/0.12% NH4NO3 for 3–5 min. Finally, sections were rinsed in 0.1 M Tris buffer (pH 7.6), mounted, dehydrated, coverslipped, and analyzed by light microscopy. The number of labeled cells within the dentate gyrus granule cell layer and hilus was quantified by two independent observers.
Analysis of Cell Proliferation.
Eighteen electrode-implanted rats were either left unstimulated or given 1 or 40 kindling stimulations (n = 6 in each group). Five days later, all rats received four injections of bromodeoxyuridine (BrdU; Sigma–Aldrich; 37.5 mg/kg i.p.) with 8-h intervals. Two weeks later, animals were perfused and brains were processed for double-label fluorescent immunocytochemistry with antibodies against NeuN and BrdU. In brief, free-floating sections were denatured by incubation for 2 h in 50% formamide/2× SSC (0.3 M NaCl/0.03 M sodium citrate) at 65°C, rinsed for 5 min in 2× SSC, followed by incubation in 2 M HCl for 30 min at 37°C. Sections were rinsed three times for 10 min in PBS, preincubated in blocking solution (0.25% Triton X-100/2% normal donkey serum/2% normal horse serum in PBS) for 1 h at room temperature and incubated overnight at 4°C with pooled primary antibodies (rat anti-BrdU, 1:100, Harlan Sera-lab, Crawley Down, U.K.; and mouse anti-NeuN, 1:100, Chemicon). The sections were then rinsed three times for 10 min in blocking solution and two secondary antibodies conjugated to Cy3 (donkey anti-rat-IgG, 1:400, Jackson ImmunoResearch) and biotin (horse anti-mouse, 1:200, Chemicon), respectively, were applied for 2 h in the dark at room temperature. After rinsing three times for 5 min in PBS, fluorescein avidin D (1:250, Vector Laboratories) diluted in PBS with 0.25% Triton X-100 was applied for 2 h in the dark. The sections were rinsed three times for 10 min in PBS, mounted, coverslipped with Vectashield, and analyzed by conventional epifluorescence light and confocal laser scanning microscopy.
The total number of BrdU-labeled cells and the number of BrdU-positive, NeuN-positive cells within the dentate gyrus granule cell layer and subgranular zone was counted in two to five sections per animal.
Statistics.
The number of nuclei positive for DNA fragmentation in the different groups and the effect of cycloheximide and MK-801 on DNA fragmentation were analyzed statistically using one-way ANOVA followed by Bonferroni–Dunn post-hoc test. Comparisons of the number of nuclei positive for DNA fragmentation between the left and right side, of the number of proliferating cells between the groups, and of seizure characteristics were performed using Student’s paired or unpaired t test. All values are given as means ± SEM.
RESULTS
In each section from electrode-implanted but nonstimulated control animals, a mean of 1.1 ± 0.2 nuclei with fragmented DNA, as visualized using the TUNEL technique, was observed within the granule cell layer or immediately infragranular in the polymorphic layer of the hilus (left and right side pooled; Figs. 1A and 2A). This observation is consistent with previous findings showing turnover of granule neurons at a low rate in the normal adult hippocampus and elimination of cells through apoptosis (30, 31). In regions outside the dentate gyrus, no consistent in situ DNA fragmentation was seen.
Figure 1.
Schematic drawing of the distribution of individual nuclei positive for fragmented DNA (dots) as assessed by TUNEL technique in the dentate gyrus and hilus from three control animals (A), three rats subjected to one hippocampal kindling stimulation and killed 5 h thereafter (B), three animals decapitated 2 h after 40 kindling stimulations (C), and three rats killed 4 h after systemic kainic acid (D). A micrograph of the boxed area is shown in Fig. 3A. (Bar = 800 μm.)
Figure 2.
Number of nuclei positive for fragmented DNA as assessed by the TUNEL technique bilaterally in the dentate gyrus and hilus at various time points after a single hippocampal kindling stimulation (A), 40 kindling stimulations (B), and 4 h after systemic kainic acid (KA) injection (C). Mean ± SEM. ∗∗, P < 0.001, and ∗, P < 0.05 compared with control; one-way ANOVA followed by Bonferroni–Dunn post-hoc test.
One kindling stimulation produced focal epileptiform activity in the hippocampus, so-called afterdischarge, lasting for 82 ± 7 s. No behavioral convulsions were observed (grade 0). Five hours after the stimulus-evoked seizure, a significant increase in the number of nuclei displaying DNA fragmentation was seen bilaterally in the dentate gyrus. The number of labeled nuclei was 214 ± 32% of control (left and right side pooled; P < 0.05; Fig. 2A). The increased labeling in the dentate gyrus after one stimulation was only observed at 5 h (Fig. 2A) and no change of the number of labeled cells occurred in other regions. Labeled nuclei were preferentially located along the hilar border of the dorsal and ventral blade of the dentate granule cell layer (Fig. 1B). A proportion of nuclei showing DNA fragmentation was also seen within the granule cell layer, and a few labeled cells were found within the hilar region of the dentate gyrus. Labeled nuclei displayed morphological features characteristic of apoptotic cells (Figs. 3 B and C). In addition to being intensely labeled using the TUNEL technique, nuclear profiles considered to be positive fulfilled at least one of the following criteria: shrinkage compared with propidium iodide-stained intact nuclei, irregular, or lobulated shape, association with smaller nuclear fragments, apoptotic bodies. Furthermore, positive nuclei often displayed a clear central zone surrounded by condensed chromatin. In control sections, processed for TUNEL histochemistry in the absence of terminal deoxynucleotidyltransferase enzyme, no positive signal was detected.
Figure 3.
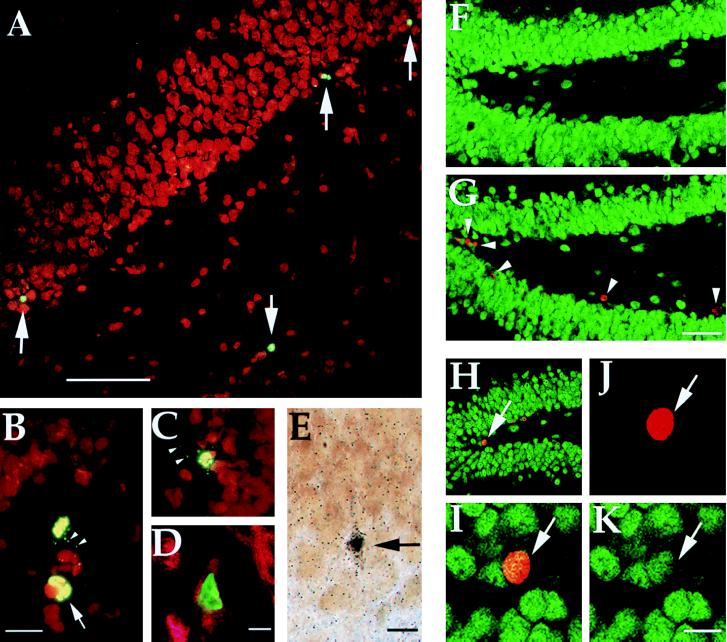
(A) Nuclei labeled for fragmented DNA (arrows) along the hilar border of the granule cell layer 2 h after 40 hippocampal kindling stimulations. (B and C) High-power photomicrographs of labeled nuclei with morphological features characteristic of apoptosis, such as condensation and lobulation (arrow), following kindling stimulation. Note numerous apoptotic bodies (arrowheads). (D) Confocal scanning laser image showing nuclear TUNEL (green) and cytoplasmic NeuN immunolabeling (red) demonstrating the neuronal identity of a degenerating cell 2 h after 40 hippocampal kindling stimulations. (E) Light photomicrograph of an argyrophilic, degenerating neuron in the dentate gyrus granule cell layer 2 h following 40 rapid kindling stimulations. (F and G) Low-power confocal images of sections labeled by NeuN (green) and BrdU (red) immunofluorescence showing the medial dentate gyrus in a control rat (F) and in an animal 19 days after 40 kindled seizures (G). Arrowheads in G denote nuclei labeled by BrdU injections 5 days following stimulation. (H–K) Confocal images of a cell (arrow; same cell in all four views) double-labeled by NeuN (green) and BrdU (red) in the dentate gyrus after 40 kindled seizures. Individual signals for BrdU (J) and NeuN (K) are shown as well as composite images (H and I). I–K are 1 μm optical sections. (Bars: A = 80 μm; B = 15 μm; D, E, and K = 10 μm; G = 16 μm; magnification in C is the same as in B; magnification in H–J is the same as in K; magnification in F is the same as in G.)
Forty rapid kindling stimulations delivered with 5-min interstimulus interval resulted in 2.3 ± 0.8 generalized (grade 4–5) seizures. The mean afterdischarge duration in the hippocampus was 35 ± 5 s. Higher numbers of labeled nuclei were observed within the dentate gyrus after forty seizures as compared with one seizure (Figs. 1C, 2B, and 3A). Two hours after the last stimulation, the number of nuclei displaying fragmented DNA was 415 ± 87% of control (left and right side pooled; P < 0.001). A significant increase in the number of labeled cells was only observed at this time point, although a trend toward higher numbers was seen at 0.5 and 4 h after the last seizure. Interestingly, because the 40 stimulations were given during 3 h and 15 min, the increase seen at 2 h following the last seizure coincides with that observed at 5 h following a single stimulation. Also after 40 seizures, increased numbers of labeled nuclei were confined to the dentate gyrus granule cell layer and hilus. There were no significant differences between the left and right dentate gyrus in the number of labeled nuclei after either 1 or 40 kindling-evoked seizures. This finding indicates that the increased labeling was due to seizure activity and not caused by the electrical current from the unilateral stimulating electrode.
The N-methyl-d-aspartate receptor antagonist MK-801 did not influence the number of labeled nuclei in the dentate gyrus after 40 kindling-induced seizures. As compared with nonstimulated control, the increase of labeled nuclei in the dentate granule cell layer and hilus was 181 ± 52% and 214 ± 64% in MK-801-treated and vehicle-injected rats, respectively. However, the animals given MK-801 did not develop generalized seizures, and the duration of seizure activity was significantly shorter as compared with that in vehicle-injected controls (data not shown).
The increase in nuclear profiles labeled for fragmented DNA in the dentate gyrus at 2 h after 40 kindling-induced seizures was completely prevented by pretreatment with the protein synthesis inhibitor cycloheximide. The vehicle-treated, kindled animals exhibited a 340 ± 34% increase in the number of labeled nuclei in the dentate granule cell layer and hilus compared with electrode-implanted, nonstimulated animals (left and right side pooled; P < 0.05). In contrast, in cycloheximide-treated kindled rats the number of labeled nuclei was only 67 ± 12% of that in nonstimulated controls (P < 0.01 compared vehicle-injected kindled rats). There was no difference between the cycloheximide- and vehicle-treated groups in the duration of seizure activity, mean seizure grade, number of generalized seizures, and number of secondary afterdischarges in response to the 40 kindling stimulations.
Similar to kindling, kainic acid-evoked seizures rapidly produced elevated numbers of nuclei with fragmented DNA in the dentate gyrus (Fig. 1D). Four hours after the onset of convulsions, the number of labeled nuclei within the dentate gyrus was 253 ± 20% of that in vehicle-injected control rats (P < 0.05; Fig. 2C). Also after kainic acid, labeled nuclei were preferentially located along the hilar border of the granule cell layer.
Confocal microscopic or conventional double immunofluorescence analysis of sections processed for combined TUNEL histochemistry and neuron-specific NeuN immunolabeling was performed to evaluate whether the apoptotic cells were neurons. Approximately 50% of TUNEL-positive cells in the dentate gyrus granule cell layer and hilus were double labeled by NeuN or immediately associated with NeuN positive cellular debris in control rats and at 2 h following 40 kindling stimulations (Fig. 3D). This finding provides evidence for neuronal identity of a large proportion of cells undergoing apoptosis in response to kindling-induced seizures. Presumably, only a fraction of apoptotic neurons is labeled by the NeuN antibody because at late stages of the degenerative process, degradation of the NeuN antigen is likely to occur with a concomitant disappearance of immunoreactivity. The number of NeuN positive apoptotic cells is therefore likely to be an underestimation of the actual number of degenerating neurons.
Dying neurons were also demonstrated with the silver staining technique early after kindling- and kainic acid-evoked seizures. No positively stained cells were found in the dentate gyrus of control rats. At 2 h after 40 kindling stimulations, scattered argyrophilic neurons (0–2/section) were detected in the dentate granule cell layer and hilus, with a distribution similar to that of TUNEL-positive cells (Fig. 3E). Kainic acid injection produced argyrophilic neurons in these areas at both 4 and 12 h and, in addition, argyrophilic neurons were seen in the hippocampal pyramidal cell layer, the cingulate, parietal and piriform cortices, the amygdaloid complex, and thalamus. At 5 h after one kindled seizure (lasting 87 ± 7 s), no argyrophilic cells were detected in the dentate gyrus. This lack of labeled cells may indicate that the sensitivity of the silver staining technique was too low to allow for the visualization of the fewer neurons detected with the TUNEL technique after one compared with 40 stimulations. Also, the optimal time point for demonstrating cytoplasmic modifications (with silver staining) and nuclear changes (with TUNEL) during the process of apoptotic cell death may differ (10).
To explore the possibility that single and rapidly recurring kindled seizures increase cell proliferation and neurogenesis in the dentate gyrus, the mitotic marker BrdU was administered 5 days after 1 or 40 hippocampal stimulations. The total number of BrdU-labeled cells within the granule cell layer as well as the number of cells double labeled by NeuN and BrdU were counted 14 days later. In electrode-implanted nonstimulated rats only 2 ± 1 out of a total of 18 ± 8 BrdU-labeled cells per section were labeled by both markers. Forty rapid kindling stimulations lead to a marked increase in the number of both proliferating, NeuN-positive cells (574 ± 169% of control; P < 0.05; Fig. 3 F–K) and BrdU-positive, NeuN-negative cells (650 ± 190% of control; P < 0.01). In animals given a single kindling stimulation, the corresponding increases were 283 ± 82% and 317 ± 43%, respectively. The increase in BrdU-labeled nuclei was largely confined to the granule cell layer and the subgranular zone, preferentially the medial tip of the ventral blade of the dentate gyrus. Cells double labeled for NeuN and BrdU were most often seen in the subgranular zone or on the hilar border of the granule cell layer. In most instances, they showed less NeuN immunoreactivity as compared with mature granule cells.
DISCUSSION
The present study provides evidence that single and intermittent, brief epileptic seizures can lead to neuronal death. Epileptic brain injury has previously been demonstrated only after continuous and prolonged seizure activity or after a large number of severe intermittent seizures (2, 9–12, 14, 15, 32). Using specific in situ labeling of DNA fragmentation, inhibition of protein synthesis, and morphological criteria, our study demonstrates apoptosis of a subset of cells confined to the granule cell layer and the polymorphic region bilaterally in the dentate gyrus after brief, single, or recurring kindling-evoked seizures. The degeneration was correlated to the severity and duration of epileptic activity because 40 seizure events, including generalized convulsions, gave rise to a higher number of apoptotic cells within the dentate gyrus as compared with one focal hippocampal seizure. A similar distribution pattern of apoptotic cells in the dentate gyrus was also observed early after the onset of status epilepticus in the kainic acid model of limbic epilepsy. Immunolabeling for the neuron-specific antigen NeuN in combination with TUNEL histochemistry showed that a large proportion of cells at early stages of apoptosis following seizures were neurons. The neuronal identity of the dying cells was also supported by the demonstration of argyrophilic, degenerating neurons in the dentate gyrus after both kindling- and kainic acid-induced seizures.
Epileptic brain damage has long been attributed to the mechanism of excitotoxicity-induced necrosis. Necrosis usually deletes groups or clusters of cells and includes loss of membrane integrity, cellular swelling, and lysis as well as activation of brain microglia and astrocytes. However, recent studies have shown that neurons also die via apoptosis following excitotoxic injury (9–12, 33–38). Apoptosis involves specific deletion of single cells and is characterized by cell shrinkage, plasma, and nuclear membrane blebbing and budding off of fragments known as apoptotic bodies, chromatin condensation, and endonuclease-mediated DNA cleavage (39–41). In contrast to necrosis, apoptosis usually requires de novo protein synthesis (42, 43). Seizures rapidly and markedly increase the intracellular calcium concentration, which in certain experimental systems is a powerful trigger for apoptotic chromatin fragmentation (44–47). Although the protein synthesis inhibitor cycloheximide effectively blocked kindling-induced apoptosis, the rapid induction of apoptotic DNA fragmentation already at 5 h after 1 kindled seizure or after the onset of 40 rapid kindling stimulations indicates that the intracellular regulators and executors of this type of apoptosis may be constitutively expressed (48). The rapid time course of degeneration is in agreement with previous studies showing apoptosis of cultured cerebellar granule cells within 6 h following exposure to kainic acid or glutamate (37, 38). However, in contrast to the glutamate-induced apoptotic death of cerebellar granule cells (37), MK-801 did not prevent the seizure-evoked apoptosis of dentate gyrus neurons, suggesting that this degeneration was not triggered by activation of N-methyl-d-aspartate receptor-operated channels. The number of apoptotic cells in the present study may be an underestimation of the actual values since apoptotic cells are rapidly cleared by phagocytosis (12). In accordance, at 2 h after maximum degeneration following 40 kindling stimulations, most apoptotic cells had already disappeared.
Both kindling and systemic kainic acid produce experimental seizures with many similarities to human temporal lobe epilepsy. However, the two seizure paradigms likely model different aspects of epileptogenesis. Rapid hippocampal kindling is comparable in many ways to traditional kindling in which daily, repeated electrical stimulation of limbic areas during 2–4 weeks progressively leads to permanent hyperexcitability (24). In rapid kindling, the epileptic condition develops gradually over about 4 weeks following a series of 40 hippocampal stimulations delivered during 3 h and 15 min (49). Each stimulation during rapid kindling evokes a relatively brief epileptiform response lasting for 30–40 s, and the total duration of stimulus-evoked seizure activity is limited to ≈25–30 min. Status epilepticus is not induced by this type of kindling stimulation. In contrast, within 1 h after kainic acid injection, severe limbic status epilepticus develops which lasts for 2–4 h. After this initial intense seizure activity subsides, a secondary state of hyperexcitability develops with a high incidence of spontaneous seizures. The finding of apoptosis in a similar pattern in these two different models of temporal lobe epilepsy indicates that early death of specific granular and hilar neurons through apoptosis might constitute one of the primary events in seizure-induced hippocampal pathology. Postmortem analysis of humans with chronic idiopathic epilepsy suggests that neuronal damage is cumulative and related to the frequency of seizures and duration of the disease (50). This implies that even if the cell loss induced by each brief seizure episode may be modest, as demonstrated here, the apoptosis of dentate gyrus neurons following repeated seizures over several years could lead to severe pathological changes, possibly also hippocampal sclerosis in patients.
Cells labeled for DNA fragmentation were located within the granule cell layer or on its hilar border as well as in the polymorphic region of the dentate gyrus. Recent data demonstrate that dentate granule cells exhibit morphological features indicative of apoptosis in response to excessive excitation or traumatic head injury, whereas dentate hilar neurons die through necrosis in response to these insults (12, 51). Based on these findings, it seems likely that at least part of the dying neurons in the present study are granule cells. However, whether a neuron will die via apoptosis or necrosis is probably dependent on the severity of the insult (52, 53). Therefore, the apoptotic cells observed here could also be hilar interneurons or a mixture of granule cells and interneurons (54, 55). In support of this, interneurons are highly vulnerable in a wide variety of epilepsy models that induce longer or more intense seizures (6, 12, 14, 56–63).
Our data also indicate that, in addition to apoptotic neuronal death, brief, single, and intermittent seizures can stimulate proliferation of both neurons and nonneuronal cells in the dentate gyrus. In a parallel study (22), 3–5 h of pilocarpine-induced status epilepticus or 6 or 24 h of continuous hippocampal seizure activity evoked by perforant path stimulation was reported to increase dentate granule cell neurogenesis. These findings suggest that the number of neurons in the dentate gyrus following periods of seizure activity is dependent on the relative magnitude of neuronal apoptosis and proliferation. It is conceivable, though, that these two processes are differentially regulated and that the balance between them could be influenced by various factors—e.g., age (64) or physiological parameters during seizure activity. Whether the rapid induction of apoptotic death and proliferation of neurons demonstrated here following brief periods of seizure activity has a pathophysiological role in epileptogenesis remains to be elucidated. It seems highly warranted to clarify in future studies how apoptosis and neurogenesis are regulated by seizures and under which conditions apoptotic neuronal death is the predominant process. However, even without a significant loss of the total number of dentate gyrus neurons following seizures, this structure could be functionally abnormal if the apoptotic cells have been replaced by newly born neurons which are immature, ectopically located or have formed aberrant connections.
Acknowledgments
We thank Tadeusz Wieloch and Michael Drake for valuable discussions and Monica Lundahl, Mats Ekstrand, Johan Fält, and Andreas Arvidsson for technical assistance. Stefan Seth provided advice in the use of confocal microscopy. This work was supported by the Swedish Medical Research Council; the Magnus Bergvall, Crafoord, Rut and Erik Hardebo, Greta and Johan Kock, Elsa Schmitz, Elsa and Thorsten Segerfalk, Åke Wiberg, and Thelma Zoega Foundations; the Royal Physiographic Society; the Swedish Association of Neurologically Disabled; and the Society of Swedish Physicians. J.B. was supported by a fellowship from the Swedish Society for Medical Research.
ABBREVIATIONS
- BrdU
bromodeoxyuridine
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling
References
- 1.Bratz F. Arch Psychiat Nervenkr. 1899;31:820–936. [Google Scholar]
- 2.Sommer W. Arch Erkrank Nervenheilk. 1880;10:631–675. [Google Scholar]
- 3.Spielmeyer W. Z Dtsch Ges Neurol Psychiat. 1927;109:501–520. [Google Scholar]
- 4.McNamara J O. Trends Neurosci. 1992;15:357–359. doi: 10.1016/0166-2236(92)90178-b. [DOI] [PubMed] [Google Scholar]
- 5.Hauser W A. Adv Neurol. 1983;34:3–14. [PubMed] [Google Scholar]
- 6.Sperk G. Prog Neurobiol. 1994;42:1–32. doi: 10.1016/0301-0082(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 7.Wasterlain C G, Fujikawa D G, Penix L, Sankar R. Epilepsia. 1993;34:S37–53. doi: 10.1111/j.1528-1157.1993.tb05905.x. [DOI] [PubMed] [Google Scholar]
- 8.Olney J W. Adv Exp Med Biol. 1986;203:631–645. doi: 10.1007/978-1-4684-7971-3_48. [DOI] [PubMed] [Google Scholar]
- 9.Pollard H, Cantagrel S, Charriaut-Marlangue C, Moreau J, Ben-Ari Y. NeuroReport. 1994;5:1053–1055. doi: 10.1097/00001756-199405000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Pollard H, Charriaut-Marlangue C, Cantagrel S, Represa A, Robain O, Moreau J, Ben-Ari Y. Neuroscience. 1994;63:7–18. doi: 10.1016/0306-4522(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 11.Weiss S, Cataltepe O, Cole A J. Neuroscience. 1996;74:541–551. doi: 10.1016/0306-4522(96)00148-0. [DOI] [PubMed] [Google Scholar]
- 12.Sloviter R S, Dean E, Sollas A L, Goodman J H. J Comp Neurol. 1996;366:516–533. doi: 10.1002/(SICI)1096-9861(19960311)366:3<516::AID-CNE10>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 13.Goddard G V, McIntyre D C, Leech C K. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- 14.Cavazos J E, Sutula T P. Brain Res. 1990;527:1–6. doi: 10.1016/0006-8993(90)91054-k. [DOI] [PubMed] [Google Scholar]
- 15.Cavazos J E, Das I, Sutula T P. J Neurosci. 1994;14:3106–3121. doi: 10.1523/JNEUROSCI.14-05-03106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crandall J E, Bernstein J J, Boast C A, Zornetzer S F. Behav Neural Biol. 1979;27:516–522. doi: 10.1016/s0163-1047(79)92124-1. [DOI] [PubMed] [Google Scholar]
- 17.Khurgel M, Switzer R C, III, Teskey G C, Spiller A E, Racine R J, Ivy G O. Neurobiol Dis. 1995;2:23–35. doi: 10.1006/nbdi.1995.0003. [DOI] [PubMed] [Google Scholar]
- 18.Represa A, Le Gal La Salle G, Ben-Ari Y. Neurosci Lett. 1989;99:345–350. doi: 10.1016/0304-3940(89)90471-0. [DOI] [PubMed] [Google Scholar]
- 19.Sutula T, He X X, Cavazos J, Scott G. Science. 1988;239:1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- 20.Bertram E H d, Lothman E W. Brain Res. 1993;603:25–31. doi: 10.1016/0006-8993(93)91295-4. [DOI] [PubMed] [Google Scholar]
- 21.Gould E, McEwen B S, Tanapat P, Galea L A M, Fuchs E. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parent J M, Yu T W, Leibowitz R T, Geschwind D H, Sloviter R S, Lowenstein D H. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 24.Lothman E W, Hatlelid J M, Zorumski C F, Conry J A, Moon P F, Perlin J B. Brain Res. 1985;360:83–91. doi: 10.1016/0006-8993(85)91223-5. [DOI] [PubMed] [Google Scholar]
- 25.Racine R J. Electroencephalog Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 26.Ch’ih J J, Procyk R, Devlin T M. J Biochem. 1977;162:501–507. doi: 10.1042/bj1620501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavrieli Y, Sherman Y, Ben-Sasson S A. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullen R J, Buck C R, Smith A M. Development (Cambridge, UK) 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 29.Nadler J V, Evenson D A. Methods Enzymol. 1983;103:393–400. doi: 10.1016/s0076-6879(83)03027-x. [DOI] [PubMed] [Google Scholar]
- 30.Cameron H A, Woolley C S, McEwen B S, Gould E. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 31.Gould E, Woolley C S, McEwen B S. J Comp Neurol. 1991;304:408–418. doi: 10.1002/cne.903040306. [DOI] [PubMed] [Google Scholar]
- 32.Nevander G, Ingvar M, Auer R, Siesjo B K. Ann Neurol. 1985;18:281–290. doi: 10.1002/ana.410180303. [DOI] [PubMed] [Google Scholar]
- 33.Kure S, Tominaga T, Yoshimoto T, Tada K, Narisawa K. Biochem Biophys Res Commun. 1991;179:39–45. doi: 10.1016/0006-291x(91)91330-f. [DOI] [PubMed] [Google Scholar]
- 34.Linnik M D, Zobrist R H, Hatfield M D. Stroke. 1993;24:2002–2008. doi: 10.1161/01.str.24.12.2002. [DOI] [PubMed] [Google Scholar]
- 35.MacManus J P, Hill I E, Huang Z G, Rasquinha I, Xue D, Buchan A M. NeuroReport. 1994;5:493–496. doi: 10.1097/00001756-199401120-00031. [DOI] [PubMed] [Google Scholar]
- 36.MacManus J P, Buchan A M, Hill I E, Rasquinha I, Preston E. Neurosci Lett. 1993;164:89–92. doi: 10.1016/0304-3940(93)90864-h. [DOI] [PubMed] [Google Scholar]
- 37.Ankarcrona M, Dypbukt J M, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton S A, Nicotera P. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 38.Simonian N A, Getz R L, Leveque J C, Konradi C, Coyle J T. Neuroscience. 1996;74:675–684. doi: 10.1016/0306-4522(96)00141-8. [DOI] [PubMed] [Google Scholar]
- 39.Kerr J F, Wyllie A H, Currie A R. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyllie A H, Kerr J F, Currie A R. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 41.Clarke P G. Anat Embryol. 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- 42.Wyllie A H, Morris R G, Smith A L, Dunlop D. J Pathol. 1984;142:67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- 43.Ratan R R, Murphy T H, Baraban J M. J Neurosci. 1994;14:4385–4392. doi: 10.1523/JNEUROSCI.14-07-04385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaiser N, Edelman I S. Proc Natl Acad Sci USA. 1977;74:638–642. doi: 10.1073/pnas.74.2.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martikainen P, Isaacs J. Prostate. 1990;17:175–187. doi: 10.1002/pros.2990170302. [DOI] [PubMed] [Google Scholar]
- 46.McConkey D J, Nicotera P, Hartzell P, Bellomo G, Wyllie A H, Orrenius S. Arch Biochem Biophys. 1989;269:365–370. doi: 10.1016/0003-9861(89)90119-7. [DOI] [PubMed] [Google Scholar]
- 47.McConkey D J, Hartzell P, Amador-Perez J F, Orrenius S, Jondal M. J Immunol. 1989;143:1801–1806. [PubMed] [Google Scholar]
- 48.Chow S C, Peters I, Orrenius S. Exp Cell Res. 1995;216:149–159. doi: 10.1006/excr.1995.1019. [DOI] [PubMed] [Google Scholar]
- 49.Elmér E, Kokaia M, Kokaia Z, Ferenz I, Lindvall O. Brain Res. 1996;712:19–34. doi: 10.1016/0006-8993(95)01424-1. [DOI] [PubMed] [Google Scholar]
- 50.Dam A M. Epilepsia. 1980;21:617–629. doi: 10.1111/j.1528-1157.1980.tb04315.x. [DOI] [PubMed] [Google Scholar]
- 51.Colicos M A, Dash P K. Brain Res. 1996;739:120–131. doi: 10.1016/s0006-8993(96)00824-4. [DOI] [PubMed] [Google Scholar]
- 52.Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton S A. Proc Natl Acad Sci USA. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charriaut-Marlangue C, Margaill I, Represa A, Popovici T, Plotkine M, Ben-Ari Y. J Cereb Blood Flow Metab. 1996;16:186–194. doi: 10.1097/00004647-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Amaral D G. J Comp Neurol. 1978;182:851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- 55.Amaral D G, Ishizuka N, Claiborne B. Prog Brain Res. 1990;83:1–11. doi: 10.1016/s0079-6123(08)61237-6. [DOI] [PubMed] [Google Scholar]
- 56.Sutula T P, Cavazos J E, Woodard A R. Hippocampus. 1994;4:254–258. doi: 10.1002/hipo.450040305. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell J, Gatherer M, Sundstrom L E. Acta Neuropathol. 1995;89:425–430. doi: 10.1007/BF00307647. [DOI] [PubMed] [Google Scholar]
- 58.Freund T F, Ylinen A, Miettinen R, Pitkanen A, Lahtinen H, Baimbridge K G, Riekkinen P J. Brain Res Bull. 1992;28:27–38. doi: 10.1016/0361-9230(92)90227-o. [DOI] [PubMed] [Google Scholar]
- 59.Sloviter R S. Hippocampus. 1991;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- 60.Nitecka L, Tremblay E, Charton G, Bouillot J P, Berger M L, Ben-Ari Y. Neuroscience. 1984;13:1073–1094. doi: 10.1016/0306-4522(84)90289-6. [DOI] [PubMed] [Google Scholar]
- 61.Griffiths T, Evans M C, Meldrum B S. Neuroscience. 1984;12:557–567. doi: 10.1016/0306-4522(84)90073-3. [DOI] [PubMed] [Google Scholar]
- 62.Dragunow M, Preston K. Brain Res Rev. 1995;21:1–28. doi: 10.1016/0165-0173(95)00003-l. [DOI] [PubMed] [Google Scholar]
- 63.Obenaus A, Esclapez M, Houser C R. J Neurosci. 1993;13:4470–4485. doi: 10.1523/JNEUROSCI.13-10-04470.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuhn H G, Dickinson-Anson H, Gage F H. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]