Abstract
Release of the excitatory neurotransmitter glutamate and the excessive stimulation of N-methyl-d-aspartate (NMDA)-type glutamate receptors is thought to be responsible for much of the neuronal death that occurs following focal hypoxia-ischemia in the central nervous system. Our laboratory has identified endogenous sulfated steroids that potentiate or inhibit NMDA-induced currents. Here we report that 3α-ol-5β-pregnan-20-one hemisuccinate (3α5βHS), a synthetic homologue of naturally occurring pregnanolone sulfate, inhibits NMDA-induced currents and cell death in primary cultures of rat hippocampal neurons. 3α5βHS exhibits sedative, anticonvulsant, and analgesic properties consistent with an action at NMDA-type glutamate receptors. Intravenous administration of 3α5βHS to rats (at a nonsedating dose) following focal cerebral ischemia induced by middle cerebral artery occlusion significantly reduces cortical and subcortical infarct size. The in vitro and in vivo neuroprotective effects of 3α5βHS demonstrate that this steroid represents a new class of potentially useful therapeutic agents for the treatment of stroke and certain neurodegenerative diseases that involve over activation of NMDA receptors.
Exposure of neurons to the excitatory neurotransmitter glutamate causes an increase in the concentration of intracellular free Ca2+ (1) and initiates the process of excitotoxic cell death (2). There are at least three pharmacologically distinct ionotropic glutamate receptors that differ in their sensitivity to the selective agonists N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA), and kainate. Whereas excessive activation of NMDA, AMPA, or kainate receptors results in neurotoxicity, specific inhibition of NMDA receptors is sufficient to attenuate most of the neuronal death that develops after in vitro hypoxia, exposure to glutamate, in vivo ischemia, or hypoglycemia (3). As Ca2+ passes through the NMDA receptor operated ion channel, it is believed that this receptor subserves a crucial role in mediating excitotoxicity. Therefore, it has been proposed that over activation of NMDA receptors may be an obligatory phase preceding neuronal death that occurs following focal cerebral ischemia induced stroke in humans.
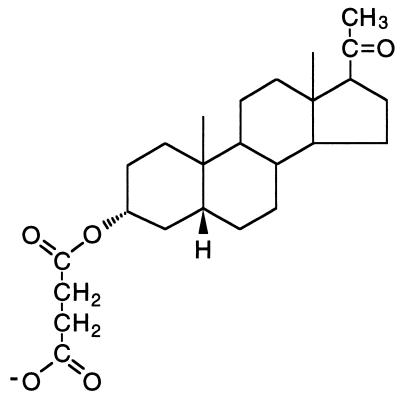
Neuroactive steroids have been shown to directly modulate excitatory and inhibitory amino acid receptor function (4–7). Therefore the possibility is raised that certain steroids might be useful neuroprotective agents. The endogenous neurosteroid pregnenolone sulfate probably acts as a positive allosteric modulator of the NMDA receptor by enhancing NMDA-induced inward currents and the subsequent rise in cytoplasmic free calcium (8, 9), whereas 3α-ol-5β-pregnan-20-one sulfate (3α5βS) is a negative modulator of NMDA-induced currents (6) and inhibits NMDA-stimulated increases in intracellular calcium (10). The present study examines the in vitro and in vivo neuroprotective activity of 3α-ol-5β-pregnan-20-one hemisuccinate (3α5βHS) (Fig. 1; a synthetic analog of the endogenous steroid 3α5βS) as an inhibitor of NMDA-induced currents. This study describes the neuroprotective as well as sedative, anticonvulsant, analgesic, activity produced by a steroid inhibitor of the NMDA receptor.
Figure 1.
Chemical structure of 3α5βHS. Sulfate replaces succinate at position 3 on the steriod A ring in 3α5βS.
MATERIALS AND METHODS
Tissue Culture.
Chicken spinal cord (11) and rat hippocampal and cortical (12, 13) cultures were prepared as described from 7-day chicken embryos and 18-day Sprague–Dawley rat embryos. Nonneuronal cell division was inhibited by exposure to 10−6 M cytosine arabinonucleoside (araC). araC was added to spinal cord cultures 36 h after plating. araC was added to rat hippocampal and cortical cultures 24 and 48 h after plating. After 24-h exposure to araC the medium from spinal cord cultures was replaced with a similar medium supplemented with 20.5 mM glucose, 18 mM KCl, and 2.5% chicken embryo extract. Fresh medium was added twice weekly and spinal cord neurons were used in electrophysiology experiments 2–4 weeks after plating. Forty-eight hours after exposure to araC, rat hippocampal and cortical cultures were maintained in serum-free DMEM plus defined components and used for experiments 14–18 days after plating.
Electrophysiology.
Whole-cell currents were recorded by the whole-cell variant of the patch-clamp technique (6). Stock solutions of steroids were prepared in dimethyl sulfoxide (DMSO). To obviate the possible effects of DMSO on NMDA-induced currents, all drug solutions and the external buffer (in the pressure pipette) contained 0.5% DMSO. The degree of modulation of the amino acid response by steroid, the percent change, is expressed as [(I′/I) − 1] × 100%, where I is the average of control responses obtained from the same cell before application and after washout of steroid and I′ is the agonist induced current in the presence of steroid.
In Vitro NMDA-Induced Cell Death.
Hippocampal cells were exposed to NMDA or glutamate with steroid, or DMSO vehicle for 15 min, at 37°C. Following exposure cells were washed three times using medium from sister cultures (conditioned medium) warmed to 37°C. NMDA and glutamate were dissolved in serum-free DMEM defined medium. Steroids were dissolved in DMSO. All exposure media contained 0.5% DMSO.
Cell Viability.
Cell viability was determined by the method of trypan blue exclusion (14). The identity of neurons was determined morphologically and confirmed by staining representative cultures with antibody to neuron-specific enolase and glial fibrillary acidic protein. The number of stained and unstained neurons were counted in four high power fields per culture well with an inverted phase contrast microscope using both bright field and phase settings. All assessments were performed blind to the treatment each culture well had received. Percent cell death was expressed as the number of trypan blue stained neurons ÷ (the number of trypan blue stained neurons + the number of unstained neurons) × 100. The degree of modulation of NMDA-induced cell death by steroid, the percent change, is expressed as [(D′/D) − 1] × 100%, where D is the percent cell death produced by NMDA alone and D′ is the percent cell death produced by NMDA in the presence of steroid.
NMDA-Induced Seizure.
Male CD-1 (Charles River Breeding Laboratories) mice (20–25 g) were injected i.v. with 3α5βS or 3α5βHS (12.5, 25, and 50 mg/kg, n = 10) or vehicle [0.1 M phosphate buffer pH 7.4/5% DMSO/10% cyclodextrin, n = 5] followed 2 min later by injection of NMDA (200 mg/kg i.p.), and the latency to tonic clonic seizure was recorded. A maximum latency period of 30 min was used.
Formalin-Induced Pain.
The formalin test was performed as described (15). Male CD-1 mice (30–25 g) were injected with 3α5βHS (15 mg/kg i.p., n = 8) or vehicle (n = 8) 5 min prior to 20 μl of formalin (1%) into the hind paw. The time spent licking the injected paw was then monitored for 25 min at 5-min intervals.
Middle Cerebral Artery (MCA) Occlusion.
MCA occlusion was performed under halothane anesthesia in male Wistar rats (290–320 g, Charles River Breeding Laboratory) as described (16). Briefly, a chronic in-dwelling catheter for the administration of steroid or vehicle was placed in the left jugular vein. Both common carotid arteries (CCA) were isolated, and a loose silk ligature was place around each artery. The left MCA was exposed, and after permanent ligation of the ipsilateral CCA, the MCA was coagulated from its origin to the olfactory tract. The contralateral CCA was occluded for a period of 2 h. Immediately or 30 min after occlusion of the contralateral CCA, rats were administered an i.v. loading dose 6.9 mg/kg 3α5βHS followed by i.v. infusion of 3α5βHS at 6.9 mg/kg per h until sacrifice. Control rats were treated with vehicle (0.1 M phosphate buffer, pH 7.4/5% DMSO/10% cyclodextrin). Twenty-four hours after MCA occlusion rats were killed, the brains sliced into 2 mm coronal sections, and stained with 2,3,5-triphenyl tetrazolium chloride. Cortical and subcortical infarct volumes were calculated by integration of the area of infarct and the distance between slices using an image analysis system (NIH image). Analysis was performed with the observer blind to the treatment group.
Statistics.
Results are expressed as mean ± SE. Statistical comparisons were carried out using paired two-tailed t tests. When data are expressed relative to control values statistical significance was determined by calculating of the 95% confidence limits.
Chemicals and Steroids.
3α5βHS and 3α5βS were purchased from Steraloids (Wilton, NH). All Other chemicals were purchased from Sigma.
RESULTS AND DISCUSSION
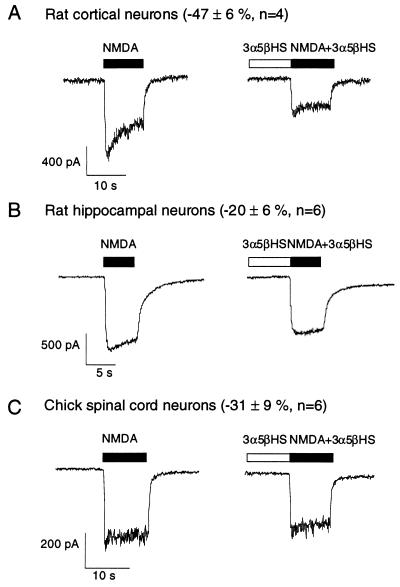
NMDA-induced whole cell currents were measured in rat neocortical, rat hippocampal, and chicken spinal cord neurons maintained in primary culture. 3α5βHS (100 μM) rapidly and reversibly inhibits 47 ± 6% of the current induced by 30 μM NMDA in rat cortical neurons (Fig. 2A). The inhibition produced by 3α5βHS in rat hippocampal (−20 ± 6%, n = 6; Fig. 2B) and chicken spinal cord (−31 ± 9%, n = 6; Fig. 2C) neurons is less than that observed in rat cortical neurons (−47 ± 6%, n = 4). 3α-ol-5β-pregnan-20-one has no effect on NMDA-induced currents (6) whereas 3α5βS and 3α5βHS inhibit NMDA-induced currents, suggesting that a negative charge at the C-3 position of the steroid A-ring may be important for inhibition. In 3α5βHS the negative charge is farther from the steroid A-ring than in 3α5βS, suggesting that some flexibility exists for placement of this negative charge.
Figure 2.
3α5βHS rapidly inhibits the NMDA-induced whole cell currents. VH = −70 mV. 3α5βHS (100 μM) inhibits the current induced by 30 μM NMDA in rat cortical neurons (A), rat hippocampal neurons (B), and chicken spinal cord neurons (C). The horizontal bar above each trace represents the period of drug application.
Studies have demonstrated that the sensitivity to modulation by glycine (17), spermine (18), and reducing agents (19) varies with the subunit composition of NMDA receptors. The differences in the sensitivity of neurons to 3α5βHS inhibition of NMDA-induced currents from different species and brain regions may reflect varying subunit compositions. The possibility that NMDA receptor subunit composition may determine sensitivity to inhibition by steroids is intriguing, and studies are ongoing to examine this possibility.
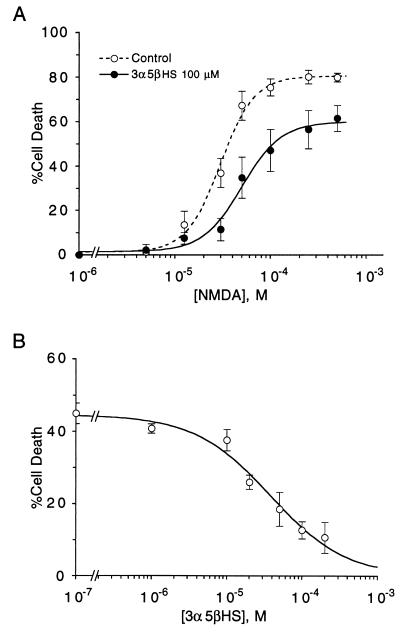
To determine whether 3α5βHS protects neurons maintained in primary culture against NMDA-induced cell death, neurons were exposed to NMDA in the presence and absence of 3α5βHS. A 15-min exposure to NMDA dose-dependently induces neuronal death in rat hippocampal cultures with an EC50 of 31 ± 2 μM and a maximum response of 80 ± 2%. 3α5βHS protects neurons by decreasing the potency of NMDA and reducing the maximum number of NMDA-sensitive neurons. 3α5βHS (100 μM) increases the NMDA EC50 to 50 ± 12 μM and reduces the maximum response to 67 ± 2%, suggesting a noncompetitive mechanism of inhibition similar to that observed for 3α5βS inhibition of NMDA-induced currents in chicken spinal cord neurons (6) (Fig. 3A). The effect of 3α5βHS is dose dependent (EC50 of 44 ± 14 μM), with significant neuroprotection detected at concentrations as low as 20 μM (Fig. 3B). When all experiments were pooled 100 μM 3α5βHS inhibited 51 ± 4% (P < 0.05, n = 15) of the cell death caused by brief exposure to 30 μM NMDA.
Figure 3.
3α5βHS protects neurons against acute NMDA-induced cell death by decreasing the potency and efficacy of NMDA as an excitotoxin. (A) Dose–response curves for NMDA-induced neuronal death were determined using 15-min exposure to NMDA. 3α5βHS or DMSO vehicle was present during NMDA exposure only. 3α5βHS (100 μM) increases the EC50 for NMDA-induced neuronal death from 31 ± 2 μM to 78 ± 28 μM (P < 0.05) and decreases the maximum neuronal death from 80 ± 2% to 68 ± 3% (P < 0.05); vehicle, n = 16; 3α5βHS, n = 5. (B) Dose–response curves for steroid modulation of NMDA-induced neuronal death were determined using 15-min exposure to 30 μM NMDA. 3α5βHS was present during NMDA exposure only. 3α5βHS inhibits NMDA-induced neuronal death with an EC50 of 44 ± 14 μM and a maximum inhibition of 78 ± 12% (n = 4). Results are expressed as mean % neuronal death-control ± SEM. Smooth curves were generated by nonlinear regression using the logistic equation. The break in the x axis represents a change from linear to log scale.
Neuroprotection was also seen when cell death was induced by glutamate. 3α5βHS (100 μM) significantly inhibits cell death caused by 15-min exposure to 30 μM glutamate (31 ± 6% inhibition; P < 0.05, n = 4). 3α5βHS has no effect on kainate-induced currents in chicken spinal cord neurons, indicating that the decreased ability of 3α5βHS to protect against glutamate toxicity may be due to activation of non-NMDA type glutamate receptors by glutamate.
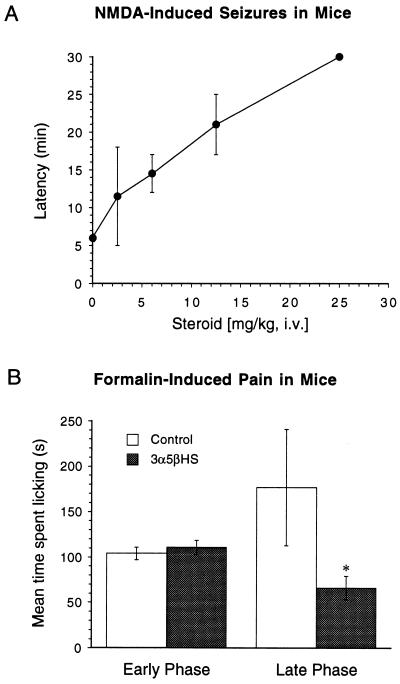
To examine whether 3α5βHS and 3α5βS can antagonize NMDA receptors in vivo we tested the ability of these steroids to prevent NMDA-induced seizures in mice. 3α5βHS causes rapid and reversible sedation in mice, such as is seen with NMDA receptor antagonists acting at the agonist or glycine sites. When administered (i.v. or i.p. at 25 mg/kg or higher) to mice, 3α5βHS causes initial sluggishness and ataxia followed by a complete lack of physical activity and responsiveness to external stimulation. 3α5βS inhibits NMDA-induced currents (6) and inhibits NMDA-induced increases in intracellular calcium (10). In addition, 3α5βS protects hippocampal neurons against NMDA-induced cell death (C.E.W. and D.H.F., unpublished data) but produced no sedation in mice at 25 mg/kg i.v. 3α5βHS causes an increase in the latency to seizure with significant anticonvulsant activity observed at about 7.5 mg/kg (Fig. 4A). Much higher concentrations of 3α5βS were required for anticonvulsant activity. The equipotent concentration to cause a 2-fold increase in the latency to seizure was 12.5 mg/kg for 3α5βHS and 50 mg/kg for 3α5βS. 3α5βHS (12.5 mg/kg i.v.) increased the latency to seizure from 6 to 20 min without sedation, and 25 mg/kg i.v. completely prevented seizures. 3α5βHS (15 mg/kg, i.p.) was also found to be analgesic in the late phase (10–25 min postinjection) of formalin-induced pain, significantly decreasing the time spent licking the injected paw from 177 ± 64 to 66 ± 13 min (P < 0.05, n = 8), while having no effect on the early phase (0–5 min postinjection) (Fig. 4B). Analgesia in the late phase, as opposed to the early phase, of formalin-induced pain is characteristic of NMDA receptor antagonists (15). GABAA receptor agonists produce apparent analgesia in both the early and late phase of formalin-induced pain, whereas positive modulators of the GABAA receptor, such as midazolam, have no effect on either the early or late phase in this pain model (20). Therefore, the analgesia produced by 3α5βHS is consistent with antagonism of spinal NMDA receptors.
Figure 4.
3α5βHS inhibits NMDA-induced seizures and inhibits the late phase of formalin-induced pain. (A) Mice were injected i.v. with steroid or vehicle control (n = 8) followed 2 min later by injection with NMDA (200 mg/kg, i.p.), and the latency to seizure was recorded. Animals were observed for a maximum of 30 min. 3α5βHS dose dependently increased the latency to NMDA-induced seizures. 3α5βHS at 25 mg/kg completely prevented seizures during the period of observation. 3α5βS (25 mg/kg) had no effect on the latency to seizure. 3α5βS (50 mg/kg) was required to increase the latency to convulse comparable to that seen with 12.5 mg/kg 3α5βHS. (B) Mice were injected i.p. with 3α5βHS (15 mg/kg i.p.) or vehicle control 5 min prior to injection of 20 μl of 1% formalin into the hind paw. The time spent licking the injected paw was monitored for 25 min at 5-min intervals. The early phase (0–5 min) was unaffected by administration of 3α5βHS, whereas the time spend licking the injected paw in the late phase (10–25 min) was significantly reduced from 177 ± 64 s to 66 ± 13 s (∗, P < 0.05, n = 8) when animals were treated with 3α5βHS.
The relative lack of in vivo efficacy of 3α5βS may be due to the unfavorable pharmacokinetics of sulfated steroid. The low pKa (≈2.5) of the sulfate group dictates that this compound will be ionized at physiological pH and will penetrate the blood brain barrier poorly. Approximately 100 times more 3α5βHS (pKa ≈ 4.5) will be in the un-ionized form, allowing it to reach the central nervous system more easily. 3α5βS produces greater protection of cultured neurons against NMDA-induced neuronal death under conditions of acute (15 min) as compared with chronic (16 h) NMDA exposure. The decreased ability of 3α5βS to protect against chronic NMDA exposure may be due to its metabolism by neuronal steroid sulfatases (21) during chronic exposure. Unlike 3α5βS, 3α5βHS reduces the death induced by short-term (15 min) and long-term (16 h) exposure of cultured neurons to NMDA to a similar degree. This may reflect the greater stability of the hemisuccinate toward steroid esterases as compared with the sulfate toward steroid sulfatases. Therefore, it is possible that the decreased in vivo efficacy of 3α5βS compared with 3α5βHS is related to rapid metabolism of 3α5βS by sulfatases. During stroke the blood brain barrier is likely to be less effective; however, the relative in vitro stability of 3α5βHS suggests that it has a significant therapeutic advantage. Both 3α5βHS and 3α5βS are biotransformed into endogenously occurring innocuous compounds and are unlikely to exhibit significant short-term or long-term side effects.
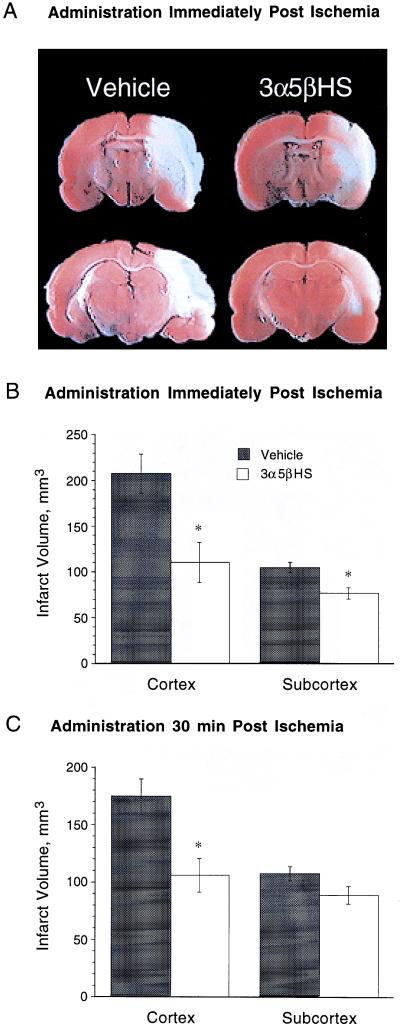
To determine the neuroprotective efficacy of 3α5βHS in an animal model for stroke, a nonsedative dose of 3α5βHS (6.9 mg/kg i.v. bolus followed by continuous i.v. infusion of 6.9 mg/kg per h) was administered to rats immediately or 30 min after permanent MCA occlusion. The cortical and subcortical infarct volumes were evaluated 24 h after the induction of ischemia. Administration of 3α5βHS immediately after the onset of ischemia reduced the volume of cortical and subcortical infarct by 47 ± 10% and 26 ± 6% respectively (Fig. 5 A and B). When infusion was delayed until 30 min after the onset of ischemia the volume of cortical infarct was reduced by 39 ± 7%, with no significant reduction in the subcortical region (Fig. 5C).
Figure 5.
3α5βHS is neuroprotective in an in vivo model of stroke. Rats were infused with vehicle or 6.9 mg/kg per h (6.9 mg/kg loading dose) of 3α5βHS, beginning immediately or 30 min after initiation of ischemia. Infusion of 3α5βHS was continued for an additional 22 h, at which time the rats were killed and their brains were stained with 2,3,5-triphenyl tetrazolium Cl. (A) Representative sections from animals receiving 3α5βHS and vehicle infusions immediately after initiation of ischemia. Infarct area appears pale. (B) When 3α5βHS was administered beginning immediately after the onset of ischemia the volume of cortical infarct was reduced from 206 ± 22 mm3 to 110 ± 21 mm3 (P < 0.005; vehicle, n = 9; 3α5βHS, n = 10). The subcortical infarct was reduced from 103 ± 6 mm3 to 76 ± 6 mm3 (∗, P < 0.005). (C) When 3α5βHS was administered 30 min after the onset of ischemia the volume of cortical infarct was reduced from 173 ± 15 mm3 to 106 ± 15 mm3 (∗, P < 0.005; n = 13), with no reduction apparent in the subcortical region.
CONCLUSIONS
The present study demonstrates that the steroid 3α5βHS inhibits NMDA induced currents, protects cultured neurons against exposure to NMDA, inhibits NMDA-induced seizures, and at a nonsedating dose reduces cortical and subcortical infarct size in the MCA occlusion model for stroke. These data suggest that the neuroprotective effects of 3α5βHS is through antagonism of NMDA receptor function. As a therapeutic for the treatment of stroke it is extremely important that neuroprotective agents be effective when given after the onset of ischemia. The observation that 3α5βHS is still neuroprotective when administered 30 min after the onset of focal cerebral ischemia in rodents indicates that 3α5βHS represents a potentially useful compound for the treatment of stroke. Experiments are underway to assess the degree of neuroprotection provided by 3α5βHS at longer times after the onset of ischemia and to evaluate the ability of 3α5βHS to prevent the loss of cognitive and behavioral function associated with focal cerebral ischemia.
Acknowledgments
We thank Dr. Gordon Letts for support and encouragement. We also thank Michele Land and Rhonda Kitabjian for expert technical assistance. This work was supported by the National Institute of Mental Health.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: 3α5βHS, 3α-ol-5β-pregnan-20-one hemisuccinate; 3α5βS, 3α-ol-5β-pregnan-20-one sulfate; NMDA, N-methyl-d-aspartate; MCA, middle cerebral artery; DMSO, dimethyl sulfoxide.
References
- 1.MacDermott A, Mayer M, Westbrook G, Smith S, Barker S. Nature (London) 1986;321:519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- 2.Choi D. Neurosci Lett. 1985;58:293–297. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]
- 3.Choi D, Koh J, Peters S. J Neurosci. 1988;8:185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puia G, Santi M, Vicini S, Pritchett D B, Purdy R H, Paul S M, Seeburg P H, Costa E. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- 5.Farb D H, Gibbs T T, Wu F-S, Gyenes M, Friedman L, Russek S J. In: Steroid Modulation of Amino Acid Neurotransmitter Receptors. Biggio G, Concas A, Costa E, editors. Vol. 47. New York: Raven; 1992. pp. 119–131. [PubMed] [Google Scholar]
- 6.Park-Chung M, Wu F-S, Farb D H. Mol Pharmacol. 1994;46:146–150. [PubMed] [Google Scholar]
- 7.Lambert J J, Belelli D, Hill-Venning C, Peters J A. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- 8.Wu F S, Gibbs T T, Farb D H. Mol Pharmacol. 1991;40:333–336. [PubMed] [Google Scholar]
- 9.Irwin R P, Maragakis N J, Rogawski M A, Purdy R H, Farb D H, Paul S M. Neurosci Lett. 1992;141:30–34. doi: 10.1016/0304-3940(92)90327-4. [DOI] [PubMed] [Google Scholar]
- 10.Irwin R, Lin S-Z, Rogawski M, Purdy R, Paul S. J Pharmacol Exp Ther. 1994;271:677–682. [PubMed] [Google Scholar]
- 11.Farb D H, Berg D K, Fischbach G D. J Cell Biol. 1979;80:651–661. doi: 10.1083/jcb.80.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brewer G J. Brain Res. 1989;494:65–74. doi: 10.1016/0006-8993(89)90144-3. [DOI] [PubMed] [Google Scholar]
- 13.Pike C J, Burdick D, Walencewicz A J, Glabe C G, Cotman C W. J Neurosci. 1993;13:1676–87. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawson V L, Dawson T M, London E D, Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaccarino A L, Marek P, Kest B, Weber E, Keana J F W, Liebeskind J C. Brain Res. 1993;615:331–334. doi: 10.1016/0006-8993(93)90045-o. [DOI] [PubMed] [Google Scholar]
- 16.Oliff H S, Weber E, Miyazaki B, Marek P. Brain Res. 1995;699:329–331. doi: 10.1016/0006-8993(95)01045-w. [DOI] [PubMed] [Google Scholar]
- 17.Katsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, Mishina M. Nature (London) 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Zheng X, Paupard M C, Wang A P, Santchi L, Friedman L K, Zukin R S, Bennett M V. Proc Natl Acad Sci USA. 1994;91:10883–10887. doi: 10.1073/pnas.91.23.10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohr G, Eckardt S, Luddens H, Moyer H, Seeburg P H. Neuron. 1994;12:1031–1040. doi: 10.1016/0896-6273(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 20.Akaike N, Maruyama T, Tokutomi N. J Physiol (London) 1987;394:85–98. doi: 10.1113/jphysiol.1987.sp016861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwamori M, Moser H W, Kishimoto Y. J Neurochem. 1976;27:1389–1395. doi: 10.1111/j.1471-4159.1976.tb02620.x. [DOI] [PubMed] [Google Scholar]