The crystallization and X-ray diffraction analysis of the fourth FAS1 domain of human BigH3 are reported.
Keywords: BigH3, FAS1, corneal dystrophy
Abstract
The protein BigH3 is a cell-adhesion molecule induced by transforming growth factor-β (TGF-β). It consists of four homologous repeat domains known as FAS1 domains; mutations in these domains have been linked to corneal dystrophy. The fourth FAS1 domain was expressed in Escherichia coli B834 (DE3) (a methionine auxotroph) and purified by DEAE anion-exchange and gel-filtration chromatography. The FAS1 domain was crystallized using the vapour-diffusion method. A SAD diffraction data set was collected to a resolution of 2.5 Å at 100 K. The crystal belonged to space group P61 or P65 and had two molecules per asymmetric unit, with unit-cell parameters a = b = 62.93, c = 143.27 Å, α = β = 90.0, γ = 120.0°.
1. Introduction
The protein BigH3 is an RGD-containing collagen-associated protein in the extracellular matrix which is induced by transforming growth factor-β (TGF-β) in various cells (Skonier et al., 1992 ▶, 1994 ▶; Bae et al., 2002 ▶). It is involved in many cell processes such as cell growth, differentiation, wound healing, angiogenesis and apoptosis (Kim et al., 2003 ▶; Bae et al., 2002 ▶). It is also related to corneal, vascular and renal diseases (O’Brien et al., 1996 ▶; Munier et al., 1997 ▶; Park et al., 2004 ▶).
The protein BigH3 has a molecular weight of about 77 kDa, but undergoes partial cleavage in the C-terminal region to generate 68–70 kDa isoforms (Skonier et al., 1994 ▶). It has been detected in the extracellular matrix (ECM) of various tissues. Most cells express BigH3 at low levels, except for fibroblasts, in which expression is higher. It binds to various extracellular matrix components, such as fibronectin, laminin and some collagens (Billings et al., 2002 ▶; Kim, Park et al., 2002 ▶). Recently, it has been reported that bovine collagen VI is colocalized with BigH3 and that a disulfide bond may be formed between the two (Hanssen et al., 2003 ▶). Moreover, it interacts with integrins (α3β1, α1β1 and αVβ5) in various cell types (Kim, Park et al., 2002 ▶; Bae et al., 2002 ▶; Ohno et al., 1999 ▶; Kim, Jeong et al., 2002 ▶) through the RGD motif at the C-terminus of BigH3. These results imply that BigH3 plays an important role in cell–cell interactions.
The protein BigH3 is composed of four homologous domains known as FAS1 domains, which were originally found in Drosophila melanogaster fasciclin 1, an insect neuronal cell-adhesion molecule. FAS1 domains exist in many secreted and membrane-anchor proteins from various organisms, including humans, bacteria, insects, plants and yeast (Kawamoto et al., 1998 ▶). Generally, proteins containing FAS1 domains have multiple tandem FAS1 domains along with other types of domains. In humans, BigH3 is a secreted protein that has four FAS1 domains.
Point mutations of BigH3 bring about several phenotypically different corneal dystrophies: R555W is found in granular Groenouw type I, R124C in Lattice type I, R124H in Avellino and R555Q in Thiel-Behnke (Munier et al., 2002 ▶). In addition, the type of corneal dystrophy determines the protein deposit type. For example, the BigH3 mutant R555Q forms a protein deposit of curly fibres, whereas the R555W mutant gives rise to fuchsinophilic granular deposits (Klintworth, 2003 ▶; Klintworth et al., 2004 ▶). This linkage means that a specific mutation of BigH3 induces a specific type of corneal dystrophy disease. Corneal dystrophy results in a reduction of visual power and in eventual blindness owing to the accumulation of protein deposits in the cornea. Development of a clinical therapy for corneal dystrophy has been slow because it is not known how the protein deposit accumulates progressively in the cornea (Klintworth et al., 1998 ▶; Streeten et al., 1999 ▶). The structure of the FAS1 domains from D. melanogaster have been determined (Clout et al., 2003 ▶), but this study did not reliably explain the effect of the corneal dystrophy mutations. In addition, an NMR structure of the fourth FAS1 domain of human BigH3 (PDB code 1x3b) has recently been determined, although no analysis of the structure has been reported to date. Thus, it is not clear why mutations induce the accumulation of pathological amyloid deposits and result in corneal dystrophies. To answer this question, we tried to determine the structure of the fourth FAS1 domain of human BigH3. This study reports the crystallization and preliminary analysis of the fourth FAS1 domain of human BigH3. We expressed the SeMet-substituted protein and obtained a crystal that diffracted to 2.5 Å. The structure of the fourth FAS1 domain of human BigH3 may provide new information about the physical and biochemical mechanisms of BigH3 protein aggregation caused by the R555Q and R555W mutations.
2. Materials and methods
2.1. Protein expression
DNA fragments corresponding to the fourth FAS1 domain (amino acids 502–636) were inserted into the pRSET(A) vector (Invitrogen). In order to obtain SeMet-substituted protein, the cloned DNA was transformed into Escherichia coli B834 (DE3), which is a methionine auxotroph. The transformed cells were cultured in M9 medium containing an amino-acid mixture [0.1 g lysine, threonine and phenylalanine, 0.05 g leucine, isoleucine, valine and l-(+)-selenomethionine] at 310 K until the OD600 reached 0.5. The protein was expressed by adding 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at 310 K for 4 h. The cells were harvested by centrifugation at 3500 rev min−1 for 10 min at 277 K.
2.2. Protein purification
Harvested cells were suspended in lysis buffer (20 mM Tris–HCl pH 8.0) and disrupted by sonication. The lysate was centrifuged at 12 000 rev min−1 for 15 min at 277 K. The supernatant obtained was loaded onto a DEAE anion-exchange column equilibrated with lysis buffer. The resin was washed with the same buffer and the FAS1 domain was eluted with a salt gradient from 0 to 0.3 M NaCl. The presence of the protein was confirmed by SDS–PAGE of each fraction. For further purification, collected fractions were loaded onto a gel-filtration column (Superdex200, Amersham Bioscience) with buffer A (20 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM β-mercaptoethanol). The purified protein was concentrated to 20 mg ml−1 using a Centricon device with a10 kDa pore size (Amicon Ultra, Millipore).
2.3. Crystallization and X-ray diffraction
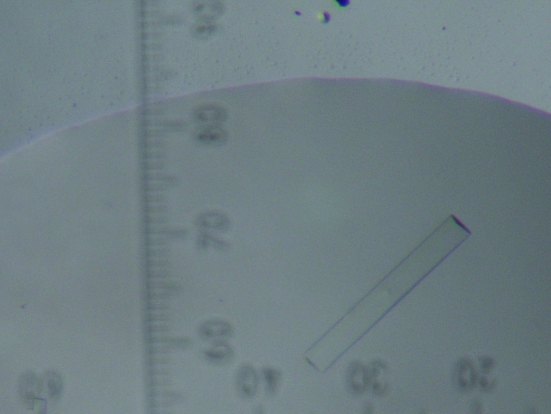
The initial crystallization conditions were determined by screening solutions using the microbatch method (Index HT kit, Hampton Research). The optimal crystallization conditions were obtained using the vapour-diffusion method at 290 K. A crystal (Fig. 1 ▶) was grown in a mixture containing equal volumes of the protein and reservoir buffer (4.0 M sodium formate pH 7.0) over 3–4 weeks.
Figure 1.
Crystal of the SeMet-substituted fourth FAS1 domain of human BigH3. The dimensions of the crystal are approximately 0.1 × 0.4 × 0.02 mm.
Crystals were transferred to a cryoprotectant composed of reservoir solution with 20%(v/v) ethylene glycol prior to data collection. Data were collected on the 4A MXW beamline at Pohang Light Source (PAL), South Korea. SAD data were collected from an SeMet-incorporated protein crystal at 100 K. A total of 270 images (0–270°) were obtained using a 1° oscillation width at the selenium peak wavelength (0.97939 Å) without an inverse-beam experiment. All collected data were indexed, integrated and scaled with DENZO and SCALEPACK from the HKL-2000 program package (Otwinowski & Minor, 1997 ▶).
3. Results and discussion
From the diffraction data, the SeMet-substituted fourth FAS1 domain crystal belongs to space group P61 or P65 and has unit-cell parameters a = b = 63.090, c = 143.374 Å, α = β = 90.0, γ = 120.0°. The diffraction data set had a resolution range of 50–2.5 Å with 100% completeness and an R sym of 10.7% (Table 1 ▶). There were two molecules per asymmetric unit and the Matthews coefficient V M was calculated to be 2.93 Å3 Da−1, which corresponds to a solvent content of 58%.
Table 1. Diffraction statistics.
Values in parentheses are for the highest resolution shell.
| Wavelength (Å) | 0.97939 |
| Resolution (Å) | 50–2.5 |
| Space group | P61 or P65 |
| Unit-cell parameters | a = 62.93, c = 143.29 |
| Total/unique reflections | 190248/11191 |
| Completeness (%) | 100 (100) |
| Rsym† (%) | 10.7 (60.7) |
| Average I/σ(I) | 35.38 (5.04) |
| Resolution range for phasing | 50–3.5 |
| Redundancy | 16.9 (15.5) |
| Anomalous signal-to-noise | |
| P61 | 1.99 (3.54) |
| P65 | 1.99 (3.54) |
| Figure of merit | |
| P61 | 0.34 (0.33) |
| P65 | 0.33 (0.32) |
R
sym = 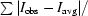
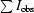 , where I
obs is the observed intensity of an individual reflection and I
avg is the average over symmetry equivalents.
, where I
obs is the observed intensity of an individual reflection and I
avg is the average over symmetry equivalents.
The FAS1-domain structure from D. melanogaster and the NMR structure of the fourth domain of human BigH3 were used as models to solve this structure by molecular replacement. However, initial molecular-replacement attempts were not successful. Therefore, we attempted to use the SAD method to solve the phase problem. Because the fourth FAS1 domain of BigH3 contains four methionine residues, we expected to find eight selenium sites using the programs SOLVE (Terwilliger & Berendzen, 1999 ▶) and SHELX (Sheldrick & Schneider, 1997 ▶), but only four selenium sites were found, giving partial phase information that was not sufficient for manual tracing. Table 1 ▶ shows phase information including the anomalous signal-to-noise and figure of merit. Structure determination of this protein is currently in progress using the molecular-replacement method with the partially phased map.
Acknowledgments
Experiments at PLS were supported in part by MOST and POSTECH. We thank Sun-Sin Cha and Ghyung-Hwa Kim for assistance at beamlines 6B and 4A of PLS. This work was supported by a Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean government (MOST; No. R112000078010010) and by Korea Research Foundation Grants (KRF-2004-005-C00112 and R08-2004-000-10403-02-004) funded by the Korean Government. KSK and YJL acknowledge support from KOSEF through CBMH.
References
- Bae, J. S., Lee, S. H., Kim, J. E., Choi, J. Y., Park, R. W., Park, J. Y., Park, H. S., Sohn, Y. S., Lee, D. S., Lee, E. B. & Kim, I. S. (2002). Biochem. Biophys. Res. Commun.294, 940–948. [DOI] [PubMed]
- Billings, P. C., Whitbeck, J. C., Adams, C. S., Abrams, W. R., Cohen, A. J., Engelsberg, B. N., Howard, P. S. & Rosenbloom, J. (2002). J. Biol. Chem.277, 28003–28009. [DOI] [PubMed]
- Clout, N. J., Tisi, D. & Hohenester, E. (2003). Structure, 11, 197–203. [DOI] [PubMed]
- Hanssen, E., Reinboth, B. & Gibson, M. A. (2003). J. Biol. Chem.278, 24334–24341. [DOI] [PubMed]
- Kawamoto, T., Noshiro, M., Shen, M., Nakamasu, K., Hashimoto, K., Kawashima-Ohya, Y., Gotoh, O. & Kato, Y. (1998). Biochim. Biophys. Acta, 1395, 288–292. [DOI] [PubMed]
- Kim, J. E., Jeong, H. W., Nam, J. O., Lee, B. H., Choi, J. Y., Park, R. W., Park, J. Y. & Kim, I. S. (2002). J. Biol. Chem.277, 46159–46165. [DOI] [PubMed]
- Kim, J. E., Kim, S. J., Jeong, H. W., Lee, B. H., Choi, J. Y., Park, R. W., Park, J. Y. & Kim, I. S. (2003). Oncogene, 22, 2045–2053. [DOI] [PubMed]
- Kim, J. E., Park, R. W., Choi, J. Y., Bae, Y. C., Kim, K. S., Joo, C. K. & Kim, I. S. (2002). Invest. Ophthalmol. Vis. Sci.43, 656–661. [PubMed]
- Klintworth, G. K. (2003). Front. Biosci.8, d687–d713. [DOI] [PubMed]
- Klintworth, G. K., Bao, W. & Afshari, N. A. (2004). Invest. Ophthalmol. Vis. Sci.45, 1382–1388. [DOI] [PubMed]
- Klintworth, G. K., Valnickova, Z. & Enghild, J. J. (1998). Am. J. Pathol.152, 743–748. [PMC free article] [PubMed]
- Munier, F. L. et al. (2002). Invest. Ophthalmol. Vis. Sci.43, 949–954. [PubMed]
- Munier, F. L., Korvatska, E., Djemai, A., Le Paslier, D., Zografos, L., Pescia, G. & Schorderet, D. F. (1997). Nature Genet.15, 247–251. [DOI] [PubMed]
- O’Brien, E. R., Bennett, K. L., Garvin, M. R., Zderic, T. W., Hinohara, T., Simpson, J. B., Kimura, T., Nobuyoshi, M., Mizgala, H., Purchio, A. & Schwartz, S. M. (1996). Arterioscler. Thromb. Vasc. Biol.16, 576–584. [DOI] [PubMed]
- Ohno, S., Noshiro, M., Makihira, S., Kawamoto, T., Shen, M., Yan, W., Kawashima-Ohya, Y., Fujimoto, K., Tanne, K. & Kato, Y. (1999). Biochim. Biophys. Acta, 1451, 196–205. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Park, S. W., Bae, J. S., Kim, K. S., Park, S. H., Lee, B. H., Choi, J. Y., Park, J. Y., Ha, S. W., Kim, Y. L., Kwon, T. H., Kim, I. S. & Park, R. W. (2004). Exp. Mol. Med.36, 211–219. [DOI] [PubMed]
- Sheldrick, G. M. & Schneider, T. R. (1997). Methods Enzymol.277, 319–343. [PubMed]
- Skonier, J., Bennett, K., Rothwell, V., Kosowski, S., Plowman, G., Wallace, P., Edelhoff, S., Disteche, C., Neubauer, M., Marquardt, H., Rodgers, J. & Purchio, A. F. (1994). DNA Cell Biol.13, 571–584. [DOI] [PubMed]
- Skonier, J., Neubauer, M., Madisen, L., Bennett, K., Plowman, G. D. & Purchio, A. F. (1992). DNA Cell Biol.11, 511–522. [DOI] [PubMed]
- Streeten, B. W., Qi, Y., Klintworth, G. K., Eagle, R. C. Jr, Strauss, J. A. & Bennett, K. (1999). Arch. Ophthalmol.117, 67–75. [DOI] [PubMed]
- Terwilliger, T. C. & Berendzen, J. (1999). Acta Cryst. D55, 849–861. [DOI] [PMC free article] [PubMed]