Abstract
The cellular targets for estramustine, an antitumor drug used in the treatment of hormone-refractory prostate cancer, are believed to be the spindle microtubules responsible for chromosome separation at mitosis. Estramustine only weakly inhibits polymerization of purified tubulin into microtubules by binding to tubulin (Kd, ≈30 μM) at a site distinct from the colchicine or the vinblastine binding sites. However, by video microscopy, we find that estramustine strongly stabilizes growing and shortening dynamics at plus ends of bovine brain microtubules devoid of microtubule-associated proteins at concentrations substantially below those required to inhibit polymerization of the microtubules. Estramustine strongly reduced the rate and extent both of shortening and growing, increased the percentage of time the microtubules spent in an attenuated state, neither growing nor shortening detectably, and reduced the overall dynamicity of the microtubules. Significantly, the combined suppressive effects of vinblastine and estramustine on the rate and extent of shortening and dynamicity were additive. Thus, like the antimitotic mechanisms of action of the antitumor drugs vinblastine and taxol, the antimitotic mechanism of action of estramustine may be due to kinetic stabilization of spindle microtubule dynamics. The results may explain the mechanistic basis for the benefit derived from combined use of estramustine with vinblastine or taxol, two other drugs that target microtubules, in the treatment of hormone-refractory prostate cancer.
Estramustine (EM) is a chemotherapeutic drug used for the treatment of advanced prostate carcinoma (1–5). EM inhibits growth and induces mitotic arrest in many cultured cells, including the human prostate carcinoma cell lines DU 145 and PC3 (6–8). EM disrupts interphase and mitotic microtubule networks in cells (5–10) and an EM-resistant prostate carcinoma cell line has been shown to have a higher level of expressed βIII and βIV tubulin isotypes than EM-sensitive cells (11, 12). Whereas EM is known to bind to certain other proteins, including a secretory protein that is thought to facilitate EM accumulation in tumor cells called the estramustine-binding protein (13, 14) and the the P-glycoprotein efflux pump (15), results of most studies indicate that microtubules are the drug’s principal cellular target.
However, the mechanism by which EM affects microtubule organization and function in cells has remained unclear. The clinically used form of EM is the prodrug, EM phosphate (EMP), which is rapidly dephosphorylated to produce EM in the body (16). EMP is not active in cells because it cannot penetrate the plasma membrane. However, EMP is active in vitro and weakly inhibits polymerization and induces depolymerization of microtubule-associated protein (MAP)-containing microtubules (17–23). Fifty percent inhibition of MAP-containing microtubule polymerization in vitro occurs at an EMP concentration of ≈100 μM (21). EMP also has been shown to bind weakly to brain MAPs (between 2 and 20 sites per MAP molecule) with a binding constant of ≈15–20 μM (21, 23). EM has also been shown to bind weakly to MAPs and to inhibit microtubule polymerization in vitro (3, 24). Based upon these and other related findings, the antimitotic mechanism of action of EM was thought to be due to a unique action on MAPs (19, 21–24).
However, results of other studies have not been consistent with the idea that EM acts on MAPs. Recent studies have shown that EM binds to tubulin (kd ≈23 μM; refs. 11 and 25) and that it has different affinities for tubulins composed of specific β-tubulin isotypes (11). In addition, it weakly inhibits polymerization and induces depolymerization of MAP-free microtubules in vitro (9). In the same work, EM did not remove MAPs from taxol-stabilized microtubules and in other studies it had no effect on the binding of MAPs to microtubules (21) or to microfilaments (26). These studies indicate that EM may act on microtubules through an interaction with tubulin.
Microtubules are intrinsically dynamic polymers and considerable evidence now indicates that the dynamics of mitotic spindle microtubules, not just their presence, are critically required for proper spindle function (27–29). Both in vitro and in cells microtubule ends switch between growing and shortening states (called dynamic instability), due to a stochastic gain and loss at the microtubule ends of a stabilizing GTP or GDP⋅Pi cap (30–34). Also, at steady state, microtubules can grow predominantly at one end and shorten at the opposite end, a dynamic behavior termed treadmilling (35, 36). Extremely rapid microtubule dynamics during mitosis appear to be essential for building the mitotic spindle and for the complex movements of the chromosomes (29, 37, 38).
Microtubules are the targets for a large number of antimitotic agents including the antitumor drugs taxol and vinblastine. Most such agents either inhibit or promote microtubule polymerization and, at appropriately high concentrations, they either increase or decrease the spindle microtubule mass. For example, vinblastine and colchicine depolymerize spindle microtubules, whereas taxol increases spindle microtubule mass (38–40). However, we have recently found that while these and a number of other antimitotic drugs affect the microtubule polymer mass at relatively high drug concentrations, they all strongly suppress the dynamics of microtubules in the absence of significant effects on polymer mass at relatively low concentrations (40–46).
In this study we show that high concentrations of EM inhibit the polymerization of MAP-free tubulin into microtubules in vitro, in confirmation of the work of Dahllof et al. (9). However, at steady-state in vitro at EM concentrations that did not appreciably affect the microtubule polymer mass, the drug strongly stabilized the dynamics of the microtubules. The results indicate that, like taxol and vinblastine, EM may exert its antiproliferative effects by stabilizing spindle microtubule dynamics through a novel interaction with tubulin.
MATERIALS AND METHODS
Purification of Tubulin.
Microtubule protein (tubulin plus MAPs) was isolated from bovine brain by three cycles of polymerization and depolymerization (45). Tubulin devoid of detectable MAPs by Coomassie staining on SDS gels was obtained by phosphocellulose chromatography. The tubulin solution was quickly frozen as drops in liquid nitrogen and stored at −70°C until used. Protein concentration was determined by the method of Bradford (47) using BSA as the standard.
Determination of Steady-State Microtubule Polymer Mass.
In all experiments, tubulin pellets were thawed and centrifuged at 4°C to remove any aggregated or denatured tubulin. Tubulin (13 μM) was mixed with Strongylocentratus purpuratus flagellar axonemal seeds in 87 mM 1–4 piperazinediethanesulfonic acid (Pipes), 36 mM 2-morpholinoethanesulfonic acid (Mes), 1.8 mM MgCl2, 1 mM EGTA (pH 6.8) (PMME buffer) containing 1.5 mM GTP and incubated at 37°C for 35–45 min to attain steady state (48). The microtubules were sedimented by centrifugation (150,000 × g for 1 hr) and microtubule pellets were solubilized in PMME buffer at 0°C for protein determination.
Determination of Microtubule Dynamics by Video Microscopy.
Tubulin (13 μM) was polymerized to steady state onto flagellar seeds in the absence or presence of EM (a gift from Beryl Hartley-Asp, Pharmacia, Lund, Sweden). The seed concentration was adjusted to achieve 3–6 seeds per microscope field. After 35 min of incubation, samples of microtubule suspensions (4 μl) were prepared for video microscopy and the dynamics of individual microtubules were recorded at 37°C and analyzed as described (48). The microtubules were observed for a maximum of 45 min after they had reached steady state. We considered a microtubule to be in a growth phase if it increased in length by >0.2 μm at a rate >0.15 μm/min, and in a shortening phase if it shortened in length by >0.2 μm at a rate >0.3 μm/min. Microtubules undergoing length changes equal to or less than 0.2 μm over the duration of six data points were considered to be in an attenuated state. The same tubulin preparation was used for all experiments; 20–30 microtubules were analyzed for each experimental condition.
The catastrophe frequency [a catastrophe is a transition from the growing or attenuated state to shortening (32)] was determined by dividing the number of catastrophes by the sum of the total time spent in the growing plus attenuated states for all microtubules for a particular condition. The rescue frequency [a rescue is a transition from shortening to growing or attenuation, excluding new growth from a seed (32)] was calculated by dividing the total number of rescue events by the total time spent shortening for all microtubules for a particular condition. Dynamicity is the sum of all growing and shortening events divided by the total time measured, including time spent in the attenuated state.
Vinblastine and Colchicine Binding.
Binding of vinblastine to tubulin was measured by a column centrifugation method. Tubulin (5 μM) and vinblastine (6 μM) containing trace amounts of [3H]vinblastine (Amersham; specific activity 11 Ci/mmol; 1 Ci = 37 GBq) were incubated with or without EM (0–50 μM) in PEM buffer (50 mM Pipes/0.18 mM MgCl2/1 mM EGTA, pH 6.8) at 34°C for 15 min, and 90 μl was centrifuged through 1-ml columns of Biogel P6 (Bio-Rad) equilibrated in the same buffer. Radioactivity and protein in the eluates were then determined. Background radioactivity, measured in the absence of tubulin, was ≤1% of the experimental values. The stoichiometry of vinblastine binding to tubulin controls was 0.65 ± 0.03 mol vinblastine per mol tubulin. Data are the average of two experiments and each experiment was carried out in duplicate. For colchicine binding, tubulin (3.6 μM) and EM (0–50 μM) were incubated for 20 min at 34°C, after which 5 μM colchicine containing trace amounts of [3H]colchicine (New England Nuclear; specific activity 1.03 Ci/mol) was added and incubation continued for an additional hour. Colchicine binding was determined by a DE-81 filter paper assay (49). The stoichiometry of colchicine binding to tubulin controls was 0.80 ± 0.04 mol colchicine per mol tubulin. Experiments were performed three times.
Fluorescence Measurements.
Fluorescence measurements were performed using a Perkin–Elmer LS50B spectrofluorometer. Spectra were taken by multiple scans and buffer blanks were subtracted from all measurements. The inner filter effects were corrected as described by Sackett (50) and empirically by measuring the change of fluorescence intensity of a tryptophan solution equivalent to the tubulin concentration in the presence of EM (51). EM did not quench the fluorescence of tryptophan in solution after inner filter effect correction. The excitation and emission wavelengths were 295 nm and 336 nm, respectively. The fraction of binding sites (β) occupied by EM was determined using the following relationship: β = (Fo − F)/(Fo − Fm), where Fo is the fluorescence intensity of tubulin in the absence of EM, F is the corrected fluorescence intensity when the tubulin and EM are in equilibrium, and Fm is the calculated fluorescence intensity of the fully liganded tubulin. Fm was determined by plotting 1/(Fo − F) versus 1/L (L = total ligand concentration) and extrapolating 1/L = 0. The association constant, Ka, was determined using the relationship: Ka = (β/1 − β) × 1/Lf, where Lf = L − β [C] and [C] is the molar concentration of ligand binding sites assuming a single binding site per tubulin dimer.
RESULTS
Inhibition of Microtubule Polymerization by EM.
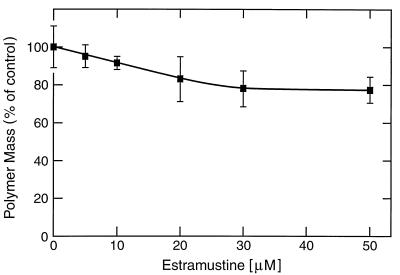
We first analyzed the ability of EM to inhibit polymerization of phosphocellulose purified (MAP-free) tubulin into microtubules in vitro. Polymerization was initiated by adding the tubulin to axoneme seeds, and at steady state the polymer was sedimented and quantitated (see Materials and Methods). EM inhibited microtubule polymerization in a concentration-dependent manner but relatively weakly; only 18% inhibition occurred at 20 μM EM (Fig. 1). The results confirm that EM inhibits the polymerization of MAP-free tubulin into microtubules with low potency (9).
Figure 1.
Effects of EM on steady-state microtubule polymer mass. Tubulin (13 μM) was polymerized to steady state onto the ends of flagellar seeds by incubation at 37°C for 35 min in the absence or presence of different EM concentrations. The microtubule polymer mass was determined as described in Materials and Methods. Data are from five independent experiments. (Error bars = SEM.)
Stabilization of Microtubule Dynamics by EM.
To determine whether concentrations of EM that minimally inhibit microtubule polymerization could stabilize microtubule dynamics, we initially determined the number of microtubules on axonemes by video microscopy before and 15 min after 3-fold dilution in PMME buffer, in both the presence and absence of 20 μM EM. Prior to dilution, 100% of the axonemes contained at least one relatively long microtubule (5–12 μm in length). After dilution, only 8% of control axonemes (a total of 177 axoneme constructs) contained a short microtubule (≤2 μm in length), whereas 20% of the axonemes (a total of 123 axoneme constructs) incubated with EM contained a short microtubule. Thus, EM appeared to stabilize microtubules against dilution-induced disassembly.
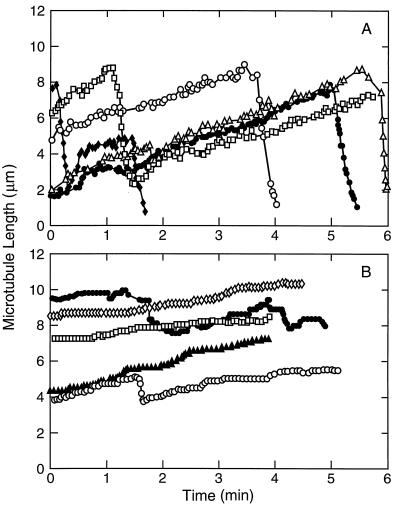
Based on the aforementioned results, we then analyzed the effects of EM on the dynamics of individual microtubules at steady state by video microscopy. Under the experimental conditions used, microtubule growth occurred predominantly at the plus ends of the seeds as determined by the growth rates, the number of microtubules that grew, and the relative lengths of the microtubules at the opposite ends of the seeds (32, 45, 46, 52–54). Several life history traces of length changes at the plus ends of individual microtubules with time in the absence of EM are shown in Fig. 2A. As previously documented, the microtubules predominantly alternated between phases of growing and shortening, but also spent a small fraction of time in an attenuated state, neither growing nor shortening detectably (43–46, 52–55). Addition of EM suppressed dynamics; 20 μM of EM visibly reduced the growing and shortening rates and increased the percentage of time that the microtubules spent in the attenuated state (Fig. 2B).
Figure 2.
Growing and shortening length changes of microtubules at plus ends at steady state in the absence (A) and presence of 20 μM EM (B). Lengths of individual microtubules were measured as described in Materials and Methods.
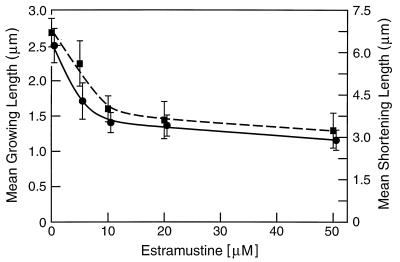
The actions of EM on the individual dynamic instability parameters were determined quantitatively (Table 1). EM strongly suppressed the rate and extent of shortening and growing in a concentration-dependent manner. EM (20 μM) reduced the shortening rate by 51% and the mean length shortened per shortening event by 46% (Table 1; Fig. 3). EM (5 μM) reduced the growing rate by 27% and at the highest concentration studied (50 μM) EM reduced the mean growing rate by 45%. The average length that the microtubules grew per growing event was also strongly decreased by EM (Fig. 3). For example, the mean length grown was reduced by 45% at 20 μM EM.
Table 1.
Effects of EM alone and EM combined with vinblastine on dynamics
| No vinblastine
|
0.05 μM vinblastine
|
||||||
|---|---|---|---|---|---|---|---|
| 0 μM EM | 5 μM EM | 10 μM EM | 20 μM EM | 50 μM EM | 0 μM EM | 10 μM EM | |
| Rate (μm/min) | |||||||
| Growing | 1.25 ± 0.5 | 0.91 ± 0.7 | 0.86 ± 0.5 | 0.81 ± 0.4 | 0.69 ± 0.4 | 0.69 ± 0.6 | 0.55 ± 0.4 |
| Shortening | 16.7 ± 5.5 | 16.8 ± 6.8 | 12.6 ± 8.1 | 8.2 ± 5.8 | 6.8 ± 5.6 | 7.3 ± 5.6 | 3.4 ± 3.0 |
| Percent time in phase | |||||||
| Growing | 83.8 | 75.2 | 68.6 | 60.0 | 59.0 | 61.0 | 48.0 |
| Shortening | 12.2 | 10.1 | 11.5 | 9.2 | 11.8 | 14.5 | 11.7 |
| Attenuation | 4.0 | 14.7 | 19.9 | 30.8 | 29.2 | 24.5 | 40.3 |
| Transition frequencies (min−1) | |||||||
| Catastrophe | 0.34 ± 0.06 | 0.28 ± 0.07 | 0.37 ± 0.06 | 0.20 ± 0.05 | 0.26 ± 0.05 | 0.28 ± 0.06 | 0.28 ± 0.06 |
| Rescue | 0.85 ± 0.24 | 1.34 ± 0.44 | 2.06 ± 0.40 | 1.61 ± 0.36 | 1.47 ± 0.29 | 1.6 ± 0.40 | 2.1 ± 0.50 |
| Dynamicity (dimer/sec) | 87 | 67 | 57 | 35 | 34 | 41 | 18 |
Valves are ± SD.
Figure 3.
Microtubule length changes per growing (circles) or shortening (squares) event as a function of EM concentration. The average length microtubules grew during growing events was determined by dividing the summed growing lengths for all microtubules for a particular condition by the total number of growing events measured for that condition. The mean shortening length per shortening event was determined similarly. (Error bars = SEM.)
The transition frequencies among the growing, shortening, and attenuated states, which may reflect the gain and loss of the stabilizing tubulin–GTP or tubulin–GDP–Pi cap at microtubule ends, are considered to be important in the regulation of microtubule dynamics in cells (55, 56). EM had no significant effect on the catastrophe frequency and only increased the rescue frequency 1.7-fold at the highest EM concentration examined (Table 1). Thus, EM may not affect the mechanism governing the gain and loss of the stabilizing cap.
At or near steady state, both in vitro and in cells, microtubules spend a considerable fraction of time in an attenuated or pause phase (43–46, 52–55). EM strongly decreased the fraction of time the microtubules spent growing and increased the percentage of time that the microtubules spent in the attenuated state (Table 1). For example, 20 μM EM increased the percentage of time spent in the attenuated phase 7.5-fold (Table 1). Dynamicity is a measure of the overall visually detectable growth and shortening at a microtubule end (44–46). EM suppressed dynamicity in a concentration dependent manner (Table 1); 5 μM EM suppressed dynamicity by 23% and 20 μM EM reduced the dynamicity by 61%.
Interaction of EM with Tubulin.
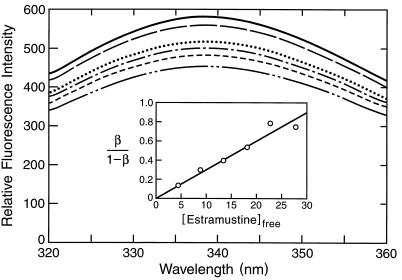
We found that EM quenched the intrinsic fluorescence of tubulin in a concentration-dependent manner (Fig. 4). The binding constant determined from the data was 30 ± 9 μM (Fig. 4 Inset), a value consistent with previous data (11, 25). These data also indicate that the binding of EM to tubulin induces a conformational change in tubulin. Additional evidence indicating that EM binding to tubulin induces a conformational change in tubulin is that EM increased the fluorescence of bis (8-anilinonaphthalene 1-sulfonate) when bound to tubulin (data not shown).
Figure 4.
Effects of EM on tubulin fluorescence. Tubulin (5 μM) was incubated in the absence (solid line) or presence of 5 μM (— —), 10 μM (⋅⋅⋅⋅), 15 μM (— - —), 20 μM (- - - -), and 25 μM (— - - —) EM at 25°C for 20 min and the fluorescence determined as described in Materials and Methods. Data in the Inset are representative of four replicate experiments.
The colchicine and vinblastine binding domains are two important drug binding regions in soluble tubulin (39). In this study we found that EM does not appear to bind to the colchicine domain. When added prior to the addition of colchicine, 30 μM and 50 μM EM inhibited colchicine binding by only 7% and 9%, respectively, and when colchicine was added prior to or simultaneously with EM, EM has no detectable effect on the binding of colchicine to tubulin. Tubulin (5 μM) was also incubated with 6 μM [3H]vinblastine in the absence or presence of EM (5–50 μM) and the amount of vinblastine bound to tubulin was determined (see Materials and Methods). EM had no detectable effect on the binding of vinblastine to tubulin at the concentrations examined and, thus, EM also does not appear to bind to the vinca alkaloid binding domain. Laing et al. (11) recently obtained similar evidence indicating that EM does not bind either to the colchicine or vinblastine binding domains. Thus, vinblastine, colchicine, and EM stabilize microtubule dynamics by binding to tubulin at distinct sites.
The Combined Suppressive Effects of EM and Vinblastine on Dynamics.
EM is used clinically in combination with vinblastine for the treatment of hormone-refractory prostate cancer (1, 4). Whereas the potency of vinblastine is considerably greater than that of EM, it is interesting to note that vinblastine stabilizes microtubule dynamics in a manner somewhat, but not entirely, similar to that of EM (Table 1, Fig. 3) (44, 45, 52). Because vinblastine and EM stabilize dynamics by binding to different sites in tubulin, we wanted to determine whether the stabilizing effects of the two drugs when present simultaneously might be additive or possibly synergistic. We used 10 μM EM, which by itself decreased dynamicity ≈1.5-fold, and 50 nM vinblastine, which by itself decreased dynamicity ≈2.1-fold (Table 1, see also ref. 52). The two drugs together reduced dynamicity 4.8-fold, indicating that the combined suppressive effect of vinblastine and EM on dynamicity is greater than additive (Table 2). Similarly, EM and vinblastine together produced greater than additive suppression of the shortening rate and not quite additive but significantly increased suppression of the growing rate (Table 1).
Table 2.
Additive stabilizing effects of 10 μM EM and 0.05 μM vinblastine on microtubule dynamics
| Fold decrease
|
||||
|---|---|---|---|---|
| EM only | Vinblastine only | Expected if additive | Actual | |
| Dynamicity | 1.55 | 2.12 | 3.67 | 4.8 |
| Shortening rate | 1.32 | 2.28 | 3.6 | 4.9 |
| Growing rate | 1.45 | 1.81 | 3.26 | 2.27 |
DISCUSSION
In this study we found that EM stabilized microtubule dynamics. EM exerted its strongest suppressive effects on the rate and extent of shortening and growing and the percentage of total time that the microtubules spent growing (Table 1). Interestingly, the ability of EM to inhibit microtubule polymerization was relatively weak (Fig. 1; ref. 9) compared with its ability to stabilize microtubule dynamics (Fig. 1, Table 1). Similar results, but not as striking as those reported here with EM, have also been observed with vinblastine (52) and colchicine (53). We also found that EM bound to tubulin at a site distinct from those of colchicine and vinblastine and that EM and vinblastine exerted additive suppressive effects on dynamics.
It is not known whether EM acts at microtubule ends like vinblastine or whether it binds to the microtubule surface like taxol. Because EM reduces the growing rate, EM could be acting at microtubule ends. If so, its mechanism might involve steric hindrance or alteration of the microtubule lattice at the end in a way that makes tubulin incorporation energetically unfavorable. Because EM induces a conformational change in tubulin (Fig. 4), binding of EM to tubulin at the end of the microtubule could reduce the rate of shortening by inducing a conformational change in the microtubule lattice that increases tubulin–tubulin interactions in a manner proposed for vinblastine.
Interestingly, EM did not significantly reduce the catastrophe frequency, an action one might expect for a drug that acts at microtubule ends (Table 1). Because catastrophes may involve loss of the stabilizing tubulin–GTP-Pi or tubulin–GTP cap at the microtubule end, it is possible that EM does not act at the extreme end of the microtubule, but rather that it binds to the microtubule surface. Similar to the mechanism of action of taxol (54) the presence of few EM molecules bound at the surface of a microtubule near an end could alter the tubulin lattice in a way that stabilizes dynamics. However, a mechanism involving the binding of EM to the microtubule surface cannot easily account for the fact that EM inhibits microtubule growth. A possibility consistent with the low potency of EM is that it binds to the end of the microtubule, but weakly or transiently in a way that does not affect the frequency of cap loss but does reduce the growing rate.
EM appears to bind to a novel binding site in tubulin. However, despite the differences in the molecular sites of action, EM, colchicine, vinblastine, and taxol all stabilize microtubule dynamics at low concentrations without significantly altering the microtubule polymer mass (43–45, 53, 54). These findings support the hypothesis that the action of these agents responsible for the most potent antimitotic and antiproliferative activities and for the killing of tumor cells may be the kinetic stabilization of spindle microtubule dynamics (38).
EM in combination with vinblastine exerts synergistic cytotoxicity (14, 57) indicating that antimitotic agents that act on microtubules can exert additive or synergistic effects. Recent clinical trials have indicated that combinations of EM and vinblastine and EM and paclitaxel are significantly more effective in patients with advanced prostate cancer than EM alone (1, 4, 58). Vinblastine and EM have very different dose-limiting toxicities and the original basis for combining EM with vinblastine was the idea that increased antitumor activity might be obtained in conjunction with decreased toxicity because vinblastine acts on tubulin and EM was thought to act on MAPs (1, 4). However, EM appears to act by binding to tubulin and stabilizing microtubule dynamics. The finding described here that EM in combination with vinblastine exerted suppressive effects on microtubule dynamics that were stronger than their individual additive effects may provide a mechanistic explanation for the improved responses reported for combination therapy as compared with monotherapy. The combined use of drugs that act by stabilizing microtubule dynamics by different mechanisms could result in increased antitumor activity without some of the specific harmful side-effects of the individual drugs by minimizing the dose of the individual drugs. It would be highly desirable to explore the clinical use of combinations of such antimitotic drugs in other tumor types sensitive to microtubule-stabilizing drugs.
Acknowledgments
We thank Dr. Mary Ann Jordan for critically reading the manuscript. This work was supported by U.S. Public Health Services Grant NS13560 from the National Institute of Neurological Disorders and Stroke.
ABBREVIATIONS
- EM
estramustine
- EMP
EM phosphate
- MAP
microtubule-associated protein
References
- 1.Hudes G R, Greenberg R, Krigel R L, Fox S, Scher R, Litwin S, Watts P, Speicher L, Tew K, Comis R. J Clin Oncol. 1992;10:1754–1761. doi: 10.1200/JCO.1992.10.11.1754. [DOI] [PubMed] [Google Scholar]
- 2.Amato R J, Logothetis C J, Dexeus F H, Stella A, Kilbourn R G, Fritz K. Proc Am Assoc Cancer Res. 1992;32:186. (abstr). [Google Scholar]
- 3.Tew K D, Glusker J P, Hartley-Asp B, Hudes G, Speicher L A. Pharmacol Ther. 1992;56:323–339. doi: 10.1016/0163-7258(92)90023-s. [DOI] [PubMed] [Google Scholar]
- 4.Seidman A, Scher H I, Petrylak D, Dershaw D D, Curley T. J Urol. 1992;147:931–934. doi: 10.1016/s0022-5347(17)37426-8. [DOI] [PubMed] [Google Scholar]
- 5.Hudes G R, Nathan F E, Khater C, Greenberg R, Gomella L, Stern C, McAleer C. Semin Oncol. 1995;22:41–45. [PubMed] [Google Scholar]
- 6.Stearns M E, Tew K D. Cancer Res. 1985;45:3891–3897. [PubMed] [Google Scholar]
- 7.Hartley-Asp B. Prostate. 1984;5:93–100. doi: 10.1002/pros.2990050109. [DOI] [PubMed] [Google Scholar]
- 8.Eklov S, Nilsson S, Larson A, Bjork P, Hartley-Asp B. Prostate. 1992;20:43–50. doi: 10.1002/pros.2990200106. [DOI] [PubMed] [Google Scholar]
- 9.Dahllöf B, Billstrom A, Cabral F, Hartley-Asp B. Cancer Res. 1993;53:4573–4581. [PubMed] [Google Scholar]
- 10.Sheridan V R, Speicher L A, Tew K D. Eur J Cell Biol. 1991;54:268–276. [PubMed] [Google Scholar]
- 11.Laing N, Dahllof B, Hartley-Asp B, Ranganathan S, Tew K D. Biochemistry. 1997;36:871–878. doi: 10.1021/bi961445w. [DOI] [PubMed] [Google Scholar]
- 12.Ranganathan S, Dexter D W, Benetatos C A, Chapman A E, Tew K D, Hudes G R. Cancer Res. 1996;56:2584–2589. [PubMed] [Google Scholar]
- 13.Eklov S, Mahdy E, Webster K, Bjork P, Malmstrom P-U, Busch C, Nilsson S. Anticancer Res. 1996;16:1819–1822. [PubMed] [Google Scholar]
- 14.Batra S, Karlsson R, Witt L. Int J Cancer. 1996;68:644–649. doi: 10.1002/(SICI)1097-0215(19961127)68:5<644::AID-IJC15>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Yang C P, Shen H J, Horwitz S B. J Natl Cancer Inst. 1994;86:723–725. doi: 10.1093/jnci/86.9.723. [DOI] [PubMed] [Google Scholar]
- 16.Gunnarsson P O, Andersson S-B, Johansson S A, Nilsson T, Plym-Forshell G. Eur J Clin Pharmacol. 1984;26:113–119. doi: 10.1007/BF00546718. [DOI] [PubMed] [Google Scholar]
- 17.Friden B, Wallin M, Deinum J, Prasad V, Luduena R F. Arch Biochem Biophys. 1987;257:123–130. doi: 10.1016/0003-9861(87)90550-9. [DOI] [PubMed] [Google Scholar]
- 18.Tew K D, Stearns M E. Pharmacol Ther. 1989;43:299–319. doi: 10.1016/0163-7258(89)90012-0. [DOI] [PubMed] [Google Scholar]
- 19.Kanje M, Deinum J, Wallin M, Ekstrom P, Edstrom A, Hartley-Asp B. Cancer Res. 1985;45:2234–2239. [PubMed] [Google Scholar]
- 20.Wallin M, Deinum J, Friden B. FEBS Lett. 1985;179:289–293. doi: 10.1016/0014-5793(85)80536-6. [DOI] [PubMed] [Google Scholar]
- 21.Friden B, Rutberg M, Deinum J, Wallin M. Biochem Pharmacol. 1991;42:997–1006. doi: 10.1016/0006-2952(91)90281-9. [DOI] [PubMed] [Google Scholar]
- 22.Moraga D, Rivas-Berrios A, Farias G, Wallin M, Maccioni R B. Biochim Biophys Acta. 1992;1121:97–103. doi: 10.1016/0167-4838(92)90342-b. [DOI] [PubMed] [Google Scholar]
- 23.Burns R G. Cell Motil Cytoskel. 1990;17:167–173. doi: 10.1002/cm.970170304. [DOI] [PubMed] [Google Scholar]
- 24.Stearns M E, Tew K D. J Cell Sci. 1988;89:331–342. doi: 10.1242/jcs.89.3.331. [DOI] [PubMed] [Google Scholar]
- 25.Speicher L A, Laing N, Barone L R, Robbins J D, Seamon K B, Tew K D. Mol Pharmacol. 1994;46:866–872. [PubMed] [Google Scholar]
- 26.Pedrotti B, Islam K. FEBS Lett. 1997;403:123–126. doi: 10.1016/s0014-5793(96)01524-4. [DOI] [PubMed] [Google Scholar]
- 27.Haydeon J J, Bowser S S, Rieder C. J Cell Biol. 1990;111:1039–1045. doi: 10.1083/jcb.111.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skibbens R V, Skeen V P, Salmon E D. J Cell Biol. 1993;122:859–875. doi: 10.1083/jcb.122.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wordeman L, Mitchison T J. In: Microtubules. Hyams J, Lloyd C, editors. New York: Wiley–Liss; 1994. pp. 287–301. [Google Scholar]
- 30.Mitchison T, Kirschner M W. Nature (London) 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 31.Horio T, Hotani H. Nature (London) 1986;321:605–607. doi: 10.1038/321605a0. [DOI] [PubMed] [Google Scholar]
- 32.Walker R A, O’Brien E T, Pryer N K, Soboeiro M F, Voter W A, Erickson H P, Salmon E D. J Cell Biol. 1988;107:1437–1448. doi: 10.1083/jcb.107.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlier M-F. Int Rev Cytol. 1989;115:139–170. doi: 10.1016/s0074-7696(08)60629-4. [DOI] [PubMed] [Google Scholar]
- 34.Erickson H P, O’Brien E T. Annu Rev Biophys Biomol Struct. 1992;21:145–166. doi: 10.1146/annurev.bb.21.060192.001045. [DOI] [PubMed] [Google Scholar]
- 35.Margolis R, Wilson L. Nature (London) 1981;293:705–771. doi: 10.1038/293705a0. [DOI] [PubMed] [Google Scholar]
- 36.Rodionov V I, Borisy G G. Science. 1997;275:215–218. doi: 10.1126/science.275.5297.215. [DOI] [PubMed] [Google Scholar]
- 37.McIntosh J R, Hering G E. Annu Rev Cell Biol. 1991;7:403–426. doi: 10.1146/annurev.cb.07.110191.002155. [DOI] [PubMed] [Google Scholar]
- 38.Wilson L, Jordan M A. Chem Biol. 1995;2:569–573. doi: 10.1016/1074-5521(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 39.Hamel E. In: Microtubule Proteins. Avila J, editor. Boca Raton, FL: CRC; 1990. pp. 89–191. [Google Scholar]
- 40.Wilson L, Jordan M A. In: Microtubules. Hyams J S, Lloyd C W, editors. New York: Wiley–Liss; 1994. pp. 59–83. [Google Scholar]
- 41.Jordan M A, Thrower D, Wilson L. Cancer Res. 1991;51:2212–2222. [PubMed] [Google Scholar]
- 42.Jordan M A, Thrower D, Wilson L. J Cell Sci. 1992;102:401–416. doi: 10.1242/jcs.102.3.401. [DOI] [PubMed] [Google Scholar]
- 43.Jordan M A, Toso R J, Thrower D, Wilson L. Proc Natl Acad Sci USA. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhamodharan R I, Jordan M A, Thrower D, Wilson L, Wadsworth P. Mol Biol Cell. 1995;6:1215–1229. doi: 10.1091/mbc.6.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toso R J, Jordan M A, Farrell K W, Matsumoto B, Wilson L. Biochemistry. 1993;32:1285–1293. doi: 10.1021/bi00056a013. [DOI] [PubMed] [Google Scholar]
- 46.Panda D P, Singh J P, Wilson L. J Biol Chem. 1997;272:7681–7687. doi: 10.1074/jbc.272.12.7681. [DOI] [PubMed] [Google Scholar]
- 47.Bradford M M. Anal Biochem. 1976;72:248–354. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 48.Panda D, Goode B L, Feinstein S C, Wilson L. Biochemistry. 1995;34:11117–11127. doi: 10.1021/bi00035a017. [DOI] [PubMed] [Google Scholar]
- 49.Wilson L, Creswell K M, Chin D. Biochemistry. 1975;14:5586–5592. doi: 10.1021/bi00697a008. [DOI] [PubMed] [Google Scholar]
- 50.Sackett D L. Biochemistry. 1995;34:7010–7019. doi: 10.1021/bi00021a012. [DOI] [PubMed] [Google Scholar]
- 51.Lee J C, Harrison D, Timasheff S N. J Biol Chem. 1975;250:9276–9282. [PubMed] [Google Scholar]
- 52.Panda D, Jordan M A, Chu K C, Wilson L. J Biol Chem. 1996;271:29807–29812. doi: 10.1074/jbc.271.47.29807. [DOI] [PubMed] [Google Scholar]
- 53.Panda D, Daijo J E, Jordan M A, Wilson L. Biochemistry. 1995;34:9921–9929. doi: 10.1021/bi00031a014. [DOI] [PubMed] [Google Scholar]
- 54.Derry W B, Wilson L, Jordan M A. Biochemistry. 1995;34:2203–2211. doi: 10.1021/bi00007a014. [DOI] [PubMed] [Google Scholar]
- 55.Shelden E, Wadsworth P. J Cell Biol. 1993;120:935–945. doi: 10.1083/jcb.120.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belmont L D, Hyman A A, Sawin K E, Mitchison T J. Cell. 1990;62:579–589. doi: 10.1016/0092-8674(90)90022-7. [DOI] [PubMed] [Google Scholar]
- 57.Mareel M M, Strome G A, Dragonetti C H, Debruyne G K, Hartley-Asp B, Segers J L, Rabaey M L. Cancer Res. 1988;48:1842–1849. [PubMed] [Google Scholar]
- 58.Hudes, G. R., Nathan, F., Khather, C., Hass, N., Cornfield, M., Greenberg, R., Gomella, L., Litwin, S., Ross, E., Roethke, S. & McAleer, C. (1997) J. Clin. Oncol., in press. [DOI] [PubMed]