Abstract
Reduced insulin/insulin-like growth factor (IGF) signaling may be a natural way for the reduction of dietary nutrients to extend lifespan. While evidence challenging this hypothesis is accumulating with Caenorhabditis elegans, for Drosophila melanogaster it is still thought that insulin/IGF and the mechanisms of dietary restriction (DR) might as yet function through overlapping mechanisms. Here, we aim to understand this potential overlap. We found that over-expression of dFOXO in head fat body extends lifespan and reduces steady-state mRNA abundance of insulin-like peptide-2 under conditions of high dietary yeast, but not when yeast is limiting. In contrast, conditions of DR that increase lifespan change only insulin-like peptide-5 (ilp5) mRNA abundance. Thus, reduction of ilp5 mRNA is associated with longevity extension by DR, while reduction of insulin-like peptide-2 is associated with the diet-dependent effects of FOXO over-expression upon lifespan. To assess whether reduction of ilp5 is required for DR to extend lifespan, we blocked its diet-dependent change with RNAi. Loss of the ilp5 dietary response did not diminish the capacity of DR to extend lifespan. Finally, we assessed the capacity of DR to extend lifespan in the absence of dFOXO, the insulin/IGF-responsive transcription factor. As with the knockdown of ilp5 diet responsiveness, DR was equally effective among genotypes with and without dFOXO. It is clear from many Drosophila studies that insulin/IGF mediates growth and metabolic responses to nutrition, but we now find no evidence that this endocrine system mediates the interaction between dietary yeast and longevity extension.
Keywords: aging, Drosophila, insulin/IGF-1 signaling, longevity regulation
Introduction
Dietary restriction (DR) extends the lifespan of many animals (Mobbs et al., 2007). Adult life expectancy of mice is increased up to 65% when calorie intake is reduced (Weindruch et al., 1986). Methods of DR with Caenorhabditis elegans include dilution of axenic liquid media, dilution of bacteria on agar plates and behavioral mutants that reduce feeding rate (Walker et al., 2005). These operations routinely increase lifespan by 30–80%. Similar lifespan extension occurs with Drosophila melanogaster by diluting dietary yeast in adult nutrient media, and to a lesser extent by diluting dietary sugar (Chippindale et al., 1993; Chapman & Partridge, 1996; Carvalho et al., 2005). From these and other examples, longevity extension by DR appears to be a nearly universal animal trait, and may therefore occur through conserved physiological and molecular mechanisms. Accordingly, it is widely thought that we may learn a great deal about the regulation and causes of aging through the analysis of DR in invertebrate model systems.
A powerful approach for this purpose is to measure the ability of restricted nutrients to extend lifespan in genotypes lacking specific molecular functions. When DR induces the same degree of longevity extension in a mutant and its corresponding wild-type, DR must work independent of the gene's product. Conversely, when longevity extension is reduced or eliminated by a mutation, the product of the tested gene may participate in the mechanism by which DR extends lifespan. Applications of this approach have been recently reviewed for C. elegans and D. melanogaster (Partridge et al., 2005; Walker et al., 2005; Houthoofd et al., 2007; Tatar, 2007). Notably, analyses have aimed to determine whether longevity extension by DR involves insulin/insulin-like growth factor (IGF) signaling (IIS) because mutations of this pathway extend lifespan, and this endocrine system affects the nutrient responses of metabolism, growth and reproduction.
In C. elegans, mutants at the locus eat have reduced pumping of the pharynx, and correspondingly they eat less and are long lived (Lakowski & Hekimi, 1998). This longevity extension appears to be insulin/IGF independent because lifespan is still extended when eat is combined with mutants of daf-16, the FOXO transcription factor that is inactivated by signal transduction through the insulin/IGF receptor encoded by daf-2 (Lakowski & Hekimi, 1998). Likewise, longevity extension in diluted axenic media is effective in wild-type and null genotypes of daf-16 (Houthoofd et al., 2003). Overall, as daf-16 is required for daf-2 mutation to extend lifespan under normal food conditions, these data suggest that the mechanisms of DR in C. elegans are somehow independent of IIS. Clues to as what might fulfill the signaling of diet restriction are emerging from analysis of forkhead transcription factors other than daf-16 (Panowski et al., 2007).
These conclusions with C. elegans differ from the prevailing view for Drosophila (Clancy et al., 2002; Partridge et al., 2005). Yeast restriction in the fly robustly extends lifespan of both male and female flies, as do mutations of the insulin receptor (InR) and of the InR substrate homolog encoded by chico (Clancy et al., 2001; Tatar et al., 2001; Tu et al., 2002). Over-expression of dFOXO in adult fat body extends lifespan and simultaneously reduces mRNA of insulin-like peptide-2 (ilp2) but not of ilp3 or ilp5 produced in the medial secretory neurons (MSNs) of the adult brain (Hwangbo et al., 2004). Likewise, reduction of Jun kinase (JNK) signaling in the MSN extends lifespan and represses ilp2 (Wang et al., 2005). Furthermore, ablation of the MSN is sufficient to increase survival, lipids and carbohydrates (Wessells et al., 2004; Broughton et al., 2005), suggesting that insulin-like peptides from the MSN are key regulators of both longevity and metabolism. Consistent with these responses, insulin-like peptides are secreted from the MSN to induce a cascade of FOXO-mediated transcriptional responses when sugar-fed adults are switched to sugar–yeast diet (Gershman et al., 2007). Finally, in the sole report to directly test for interaction of DR and IIS, longevity extension upon restricted media was analyzed in chico mutants (Clancy et al., 2002). When diets were diluted from the maximum concentration of 15% sugar and yeast, chico-null and wild-type genotypes robustly increased lifespan, but chico mutants were longest lived upon diet with 6.5% sugar and yeast, while wild-type were longest lived upon diets with 8% of these nutrients. Given these differences in the optimal diet for longevity extension and the intersection of their longevity functions in the range of more dilute diets, IIS and DR were argued to ‘act through overlapping mechanisms’ (Clancy et al., 2002).
Here, our aim was to further resolve whether and how IIS modulates the effect of DR upon Drosophila longevity. We initially approached this question by looking for diets that optimized longevity extension when dFOXO was over-expressed in fat body. We shall report that dFOXO over-expression in head fat body extended lifespan in female flies fed with a high-yeast diet, but not when fed with a restricted diet. Because ilp2 mRNA was repressed when dFOXO extended lifespan on high-yeast diet, as previously reported (Hwangbo et al., 2004), we asked whether DR might slow down aging because it modulates this specific insulin-like peptide. We measured ilp mRNA in wild-type flies maintained on restricted and rich diets; unexpectedly, ilp5 was repressed by DR but ilp2 was not. To determine whether nutrient regulation of ilp5 might be essential for DR to extend lifespan, we inhibited the diet-dependent change in its mRNA via RNAi and measured the capacity of DR to extend longevity. Dietary restriction worked equally well in cohorts with and without nutrient-responsive change in ilp5. Finally, we explored whether dFOXO was required for DR to extend lifespan. Dietary restriction was equally efficient in dfoxo null and wild-type. Overall, these data suggest that the mechanism by which DR slows down Drosophila aging is independent of insulin/IGF, consistent with data from C. elegans but contrary to prevailing perceptions for the fly.
Results
dFOXO over-expression and DR
At the onset, we determined the effect of dFOXO over-expression upon lifespan in female flies maintained on media that varied in dietary yeast. We expressed cDNA of wild-type dfoxo (UAS–dfoxo+) using the P{Switch}S132 driver, which is an RU486-inducible Gal4 that is specific to head fat body (Roman et al., 2001). We previously reported data with this driver when RU486 was fed in yeast paste (Hwangbo et al., 2004). To quantitatively vary the amount of available dietary yeast, in the current work we presented the RU486 in agar–cornmeal–sugar–yeast media where all ingredient concentrations were held constant except for dietary yeast, which was set at 2%, 4%, 8% and 12% (w/v). RU486 fed to P{Switch}S132 flies induces transgenes at both low- and high-yeast concentrations, based on assays with UAS–gfp as a reporter (Supplementary Fig. S1).
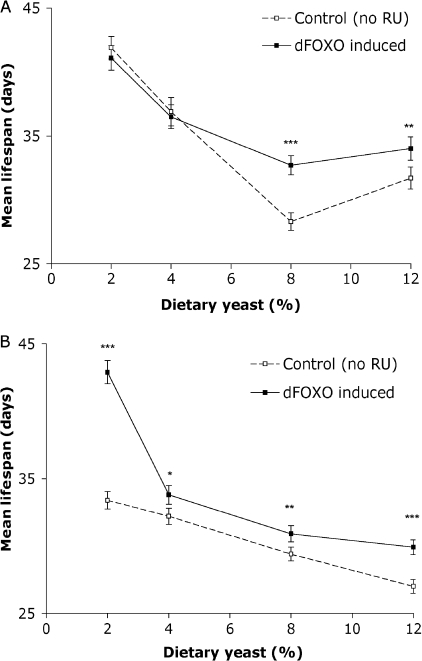
We measured survival for female flies maintained on a diet of each yeast concentration with or without RU486. As expected, survival of control (vehicle only) cohorts was enhanced in adults maintained upon yeast-restricted diets (Fig. 1A); mean lifespan upon 2% diet was 41.9 days while upon 12% diet it was 31.8 days (life table statistics are summarized in Table 1). Induction of UAS–dfoxo+ by RU486 increased lifespan by ∼32% in female flies maintained on relatively rich diets (8% and 12%). In contrast, among flies that were long lived by virtue of DR, expression of dFOXO in head fat body via P{Switch}S132 assured no additional longevity.
Fig. 1.
Dietary restriction in adult Drosophila when dFOXO is over-expressed in fat body by induction with RU486 relative to the same genotype without RU486 (vehicle only control). Mean lifespan with standard error. Asterisks indicate yeast concentrations where survival differed significantly by log-rank test (*P < 0.05; **P < 0.01; ***P < 0.0001). (A) Over-expression in the head fat body via P{Switch}S132. (B) Over-expression in visceral fat body via P{Switch}S1106.
Table 1.
Statistics of proportional hazard analysis for main and interaction effects of insulin peptide with diet and of FOXO with diet.
| Effect | DF | χ2 | P | Risk ratio | Lower CL | Upper CL |
|---|---|---|---|---|---|---|
| A. Insulin-like peptides | ||||||
| Diet | 1 | 1547.3 | < 0.000 | 1.155 | 1.151 | 1.167 |
| Genotype (vs. gal4/3RiA1) | 2 | 727.72 | < 0.000 | |||
| 3RiA1/+ | 1.912 | 1.823 | 2.006 | |||
| InsP3-gal4/+ | 0.868 | 0.830 | 0.909 | |||
| Diet*genotype | 2 | 57.29 | < 0.000 | |||
| diet*geno[3RiA1/+] | 1.016 | 1.007 | 1.025 | |||
| diet*geno[gal4/+] | 0.968 | 0.960 | 0.976 | |||
| B. dFOXO | ||||||
| Diet | 1 | 906.14 | < 0.000 | 1.226 | 1.210 | 1.242 |
| Genotype (vs. +/+) | 3 | 235.31 | < 0.000 | |||
| foxow24/foxo21 | 1.398 | 1.328 | 1.472 | |||
| foxo21/+ | 1.116 | 1.056 | 1.181 | |||
| foxow24/+ | 0.773 | 0.734 | 0.874 | |||
| Diet*genotype | 3 | 36.39 | < 0.000 | |||
| diet*geno[foxow24/foxo21] | 1.012 | 0.993 | 1.032 | |||
| diet*geno[foxo21/+] | 0.965 | 0.946 | 0.985 | |||
| diet*geno[foxow24/+] | 0.966 | 0.947 | 0.986 | |||
The P{Switch}S1106 driver is expressed in thoracic and abdominal fat bodies (Roman et al., 2001). We previously found that expression of UAS–dfoxo+ with this driver did not increase lifespan when RU486 was presented in yeast paste (Hwangbo et al., 2004), although a longevity benefit has been reported with RU486 delivered in sugar–yeast–agar diet (Giannakou et al., 2005). Here, we assess the effect of dFOXO regulated by P{Switch}S1106 when RU486 is presented in agar–sugar–cornmeal–yeast food media where yeast varied from 2% to 12% (Fig. 1B). At high concentrations of dietary yeast, over-expression of dFOXO increased adult survival a small amount. However, there was a 42% longevity extension with dFOXO expression in adults maintained on the most restricted diet. As a practical matter, these data show that sufficient RU486 can be consumed from restricted diets to induce FOXO-mediated longevity, providing a positive control to contrast with the absence of increased lifespan from P{Switch}S132 upon restricted diets. More generally, we see that longevity extension by dFOXO depends on interactions between where the gene is expressed and the concentration of the diet.
Messenger RNA of insulin-like peptides and DR
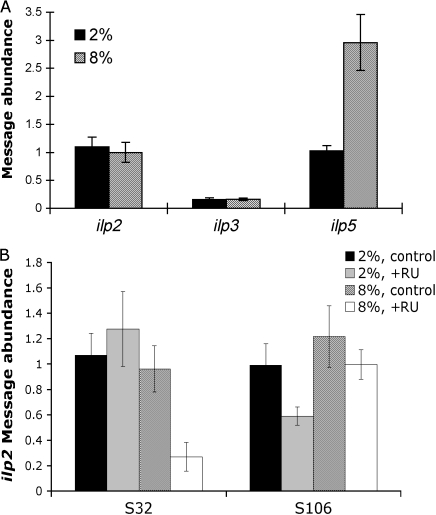
We previously found that mRNA of ilp2 was reduced when dFOXO was expressed in head fat body via P{Switch}S132 with RU486 delivered in yeast paste (Hwangbo et al., 2004). Here, we consider whether reduction in ilp2 might be responsible for the ability of DR to extend lifespan. As an initial analysis, we measured messenger RNA of insulin-like peptides from flies maintained on media with restricted (2%) and abundant (8%) dietary yeast, and with or without over-expression of dFOXO. The level of ilp2 and ilp3 mRNA remained constant across diets in wild-type flies, but the abundance of ilp5 was dramatically reduced upon a restricted diet (Fig. 2A). When UAS–dfoxo+ was expressed in head fat body (via P{Switch}S132), mRNA of ilp2 was repressed in flies fed with 8% yeast, consistent with our previous observations with yeast paste, but there was no change in ilp2 mRNA of flies upon a 2% yeast diet (Fig. 2B). When dFOXO was over-expressed from abdominal fat body (via P{Switch}S1106), ilp2 was reduced upon a 2% yeast diet – the condition where dFOXO over-expression most extends lifespan – but not upon an 8% yeast diet, where there was little longevity extension (Fig. 2B).
Fig. 2.
Abundance of transcripts for insulin-like peptides (ilp) isolated from adult heads. Within each replicate sample, ilp mRNA was normalized against mRNA of δ-tubulin. (A) mRNA of insulin-like peptide-2 (ilp2), ilp3 and ilp5 from flies maintained on 2% yeast and 8% yeast, abundance relative to ilp2 on 2% yeast (with standard error). (B) mRNA of ilp2 from flies on yeast concentrations of 2% and 8%, and with (+RU486) or without (control) induction of UAS–dFOXO in fat body by drivers P{Switch}S32 and P{Switch}S106.
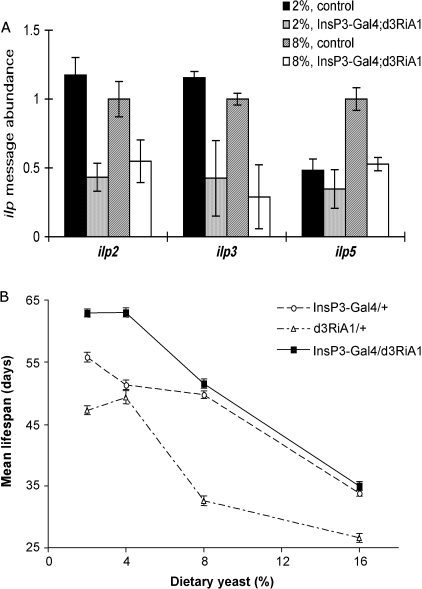
To directly assess whether regulation of ilp5 mRNA is required for DR to extend lifespan, we repressed ilp5 with RNAi and measured survival in a range of diet concentrations. An RNAi construct (UAS–d3RiA1) designed to suppress mRNA of ilp3 was found to also repress mRNA of ilp2 and ilp5 (Buch et al., 2007). We induced this construct in MSN from a driver with promoter sequence of ilp3 (InsP3-Gal4) that is expressed in late third instars and adults (Buch et al., 2007). Induction of the RNAi construct reduced ilp mRNA to a consistent low level across diets (Fig. 3A). Importantly, under these conditions, ilp5 mRNA no longer varied in response to reduction of dietary yeast, permitting us to determine whether loss of response at this specific ilp affected how DR extends lifespan. The response of lifespan to yeast concentration was strikingly similar among genotypes (Fig. 3B). We used survival regression with proportional hazard analysis (Parmer & Machin, 1995) to formally estimate the impact of genotype, diet and their interaction upon survival (Tatar, 2007). Diet restriction improved survival: on average, mortality increased by a factor of 1.16 per unit increase of yeast concentration (risk ratio, Table 1). Relative to the genotype with repressed ilp mRNA and analyzed across all diets, overall mortality was ∼2-fold higher in the 3RiA1/+control (risk ratio confidence interval: 1.82–2.01) but ∼13% less in the InsP3-Gal4/+control, despite the small advantage the ilp-RNAi provides in mean lifespan at some diets relative to this control (risk ratio confidence interval: 0.83–0.91) (Table 1). The differences in the effect of ilp-RNAi relative to the two control strains caution that background genetic effects may confound interpretations on how ilp reduction affects aging because overall survival should be sensitive to deleterious effects of mutation at potentially thousands of genes affecting any aspect of viability (Tatar, 1999). It is far less likely, however, for arbitrary second-site genes to affect the interaction between diet and lifespan because this is a specific rather than general phenotype. Central to the focus of our question and consistent with both controls, there was little interaction between the diet and genotype main effects; the mortality ratios for genotype-by-diet interaction did not meaningfully differ from what we would expect if loss of the ilp5 dietary response did not affect DR (mortality ratio = 1.0) (Table 1).
Fig. 3.
The impact of RNA interference against insulin-like peptide(ilp). (A) mRNA abundance of ilp2, ilp3 and ilp5 isolated from heads when adults were maintained on yeast concentrations of 2% and 8% (with standard error). The control genotypes were InsP3-Gal4/+ and d3RiA1/+0. The interference genotype was d3RiA1; InsP3-Gal4. Within each sample, ilp mRNA was normalized by the abundance of δ-tubulin, and abundance is expressed relative to the quantity in control flies upon 8% yeast. (B) Mean lifespan (standard error) as a function of dietary yeast concentration in control and interference genotypes.
FOXO mutation and DR
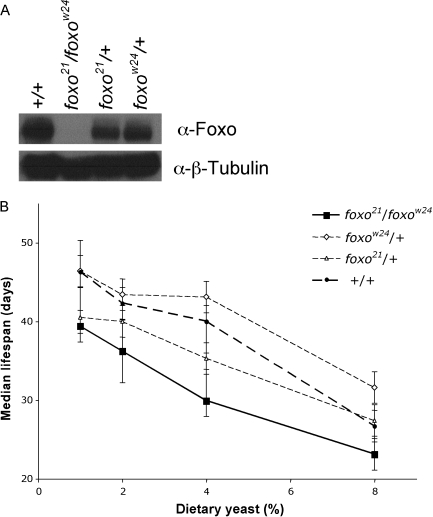
To assess whether dFOXO is required for extended lifespan upon DR, we measured survival in adults with dfoxo wild-type and mutant genotypes maintained on media that varied in yeast concentration. We compared a loss-of-function genotype with heterozyogote and wild-type controls. The null genotype foxo21/foxow24 produces no detectable dFOXO protein (Fig. 4A). Across four concentrations of dietary yeast, DR extends lifespan with nearly equal efficiency regardless of dfoxo genotype (Fig. 4B). Based on survival regression with proportional hazard analysis, DR extended lifespan with equal efficiency in flies with and without dFOXO (Table 1).
Fig. 4.
The impact of dfoxo mutation upon dietary restriction. (A) Western blot against dFOXO of wild-type (+/+), heterozygote (foxo21/+ and foxow24/+), and heteroallelic null (foxo21/foxow24) genotypes, with β-tubulin as loading control. (B) Mean lifespan (standard error) as a function of dietary yeast concentration for dfoxo genotypes.
Discussion
Dietary restriction and IIS affect Drosophila lifespan, and in many animals insulin signaling is the prominent endocrine response to nutrient status. From these relationships, we anticipate that mechanisms of Drosophila longevity extension by DR might overlap with those of IIS. Here, we evaluated this idea in several ways, but found no evidence to support the hypothesis.
Over-expression of dFOXO in adult fat bodies modulates lifespan and leads to reduction in mRNA of insulin-like ligand ilp2 (Hwangbo et al., 2004). Medial secretory neurons also produce ilp3 and ilp5, but neither of these change when dFOXO extends lifespan. A role for ilp2 in longevity control has also been suggested in the analysis of aging mediated by JNK, and this effect required dFOXO (Wang et al., 2005). If DR modulates aging through insulin-like signals, we might expect from these observations that ilp2 would be reduced in flies when DR extends longevity. However, we found this was not the case because ilp5 was the only insulin-like mRNA repressed by yeast-restricted diets.
Thus, while ilp2 appears to be associated with longevity control downstream of dFOXO and JNK, the otherwise static ilp5 is correlated with the effect of DR upon longevity. Accordingly, we investigated whether diet-induced reduction of ilp5 was required for DR to extend lifespan. Expression of RNAi in the MSN reduced mRNA of ilp2, ilp3 and ilp5 by approximately 50%. Importantly, this treatment eliminated the nutrient responsiveness of ilp5, permitting us to determine if this impaired the ability of DR to extend lifespan. We simultaneously analyzed the survival of more than 3800 adults distributed in three genotypes among four diets. Relative to fully fed adults, DR increased lifespan with equal efficiency with and without diet-responsive change in ilp5. We conclude that reduction of ilp5 is not sufficient to extend lifespan, although we cannot rule out that diet-responsive change in ilp5 may as yet be necessary for DR to modulate aging.
To investigate if a component of IIS distal to ligand synthesis contributes to DR, we evaluated whether dFOXO was required for restricted diet to extend lifespan. We compared longevity extension across four diets using 4100 flies representing confirmed, heterozygote and wild-type dfoxo genotypes. We found DR extends lifespan with equal efficiency with and without dFOXO.
Despite the apparent independence of insulin/IGF and DR in the control of Drosophila lifespan, we found that dFOXO over-expressed from fat body extends lifespan in a nutrient-dependent manner. dFOXO expressed from the head fat body only extends lifespan upon high-yeast diets, while dFOXO expressed from visceral fat body best extends lifespan upon a restricted diet. At face value, longevity extension induced by dFOXO over-expression in fully fed flies would be consistent with expectations if DR functions through IIS: dFOXO would be intrinsically induced on restricted diets, and over-expression on full diets copies this effect. This explanation, however, is not supported by our analysis with dFOXO loss-of-function genotypes. Some insight is provided by the association of reduced ilp2 and lifespan when dFOXO is expressed in different fat bodies. Reduction of ilp2 may increase lifespan independent of nutrition, but head and visceral fat bodies have unique conditions from which they repress brain synthesis of ilp2.
Our observations are consistent with current perspectives from C. elegans where DR efficiently extends lifespan in mutants of the insulin/IGF signaling pathway (Walker et al., 2005; Houthoofd et al., 2007; Panowski et al., 2007). Analysis of the interaction between DR and insulin/IGF was also studied in the context of the pituitary development mutations of mice. The Ames dwarf has deficient pituitary production of prolactin, growth hormone and thyroid-stimulating hormone, and these animals consequently have reduced circulating IGF and insulin. Because DR extends lifespan to the same extent in Ames and wild-type mice, it is thought that the mechanisms of Ames longevity assurance are independent of those induced by restricted diet (Bartke et al., 2001). However, mice that only lack the growth hormone response because of knockout of the growth hormone receptor locus appear to be refractory to the effect of DR upon longevity (Bonkowski et al., 2006). Our results are consistent with the observations from C. elegans and the initial conclusion from mice, but differ from the sole report of insulin/IGF and DR interactions with Drosophila (Clancy et al., 2002) where wild-type and homozygous mutants of chico were aged on a series of sugar–yeast diets. Lifespan of chico females was optimal on a diet of 8% sugar and yeast, while the longevity of wild-type flies was highest upon a diet of 6.5% sugar and yeast. In both genotypes, survival was reduced as nutrients were diluted below these optima. Importantly, the plots of longevity versus diet were parallel in the range of nutrients at concentrations greater than each genotype's optimum. These results were interpreted to represent overlap in the mechanism of insulin/IGF and DR (Clancy et al., 2002). However, DR occurs in the range of diets where nutrient dilution increases lifespan, not where lifespan is reduced by malnutrition. The parallelism in the range where restricted diet increases lifespan suggests that DR is equally efficient in these genotypes (Tatar, 2007), as we find for mutants of dfoxo and for inhibition of ilp5 dynamics. Overall, these data suggest that the mechanisms of DR function may be independent of insulin/IGF in Drosophila. This argument is also consistent with recent work on diet and olfaction where sensory modulation of Drosophila longevity was not associated with changes in ilp mRNA (Libert et al., 2007). We are still faced, therefore, with our basic problem: how does reduced nutrient intake modulate systems that extend fly longevity?
Experimental procedures
Food medium and rearing conditions
Adults were collected from larvae grown on diet of cornmeal (5.2%), sugar (11.0%), autolyzed yeast (2.5%; SAF brand) and agar (0.79%) (w/v in 100 mL water) with 0.2% Tegosep (methyl 4-hydroxybenzoate, Sigma, St Louis, MO, USA). Media for adults used this standard diet except with yeast at concentrations at 1%, 2%, 4%, 8%, 12% or 16% w/v as specified in each experiment. All flies were maintained at 25 °C, 40% relative humidity and 12 h light:12 h dark. RU486 (mifepristone, Sigma) was dissolved in ethanol and added to the media at a concentration of 200 µmol; ethanol alone was added to the media of the control treatments.
Drosophila stocks
Over-expression of dFOXO was studied with the genotypes w1118; UAS–dfoxo+/P{Switch}S132 and +/+; UAS–dfoxo+/P{Switch}S1106. These were generated from crosses among the stock w1118; P{w[+mW.hs] = Switch1}S132/CyO (Roman et al., 2001 after backcrossing to w1118; +/+; +/+, the stock+/+; P{w[+mW.hs] = Switch1}S1106/P{w[+mW.hs] = Switch1}S1106 after backcrossing to Dahomey wild-type (Giannakou et al., 2005), and +/+; UAS–dfoxo+/UAS–dfoxo+ after backcrossing to Dahomey wild-type (Giannakou et al., 2005). Inhibition of ilp mRNA was conducted with offspring from crosses of InsP3-Gal4 (w1118;; ilp3-Gal4/ilp3-Gal4), d3RiA1 and w1118; +/+; +/+ (Buch et al., 2007). The dilp3-promoter construct was made by cloning an 860 bp polymerase chain reaction (PCR) fragment (primers, GCTAACTGATGATGTTTGGCCC and GACACTTGGCCAACACACACAC) into a pCaSpeRAUG-Gal4 vector. The dilp3 RNAi construct was made by cloning a 370 bp fragment of dilp3 (using GCATCGAGATGAGGTGTC and CTCGGCTTGGCAGC) into pCRII-TOPO-Vector (Invitrogen, Carlsbad, CA, USA), then cut with EcoRI and ligated with Sym-pUAST vector (Buch et al., 2007). dFOXO nulls were derived from crosses of yw;; foxo21/TM6B Tb Hu e (Junger et al., 2003) and yw;; foxow24/TM6B Tb Hu e (Weber et al., 2005; prior to use, both stocks were backcrossed to yw for four generations. dFOXO heterozygote genotypes were derived from crosses of the mutant allele stocks to the yw stock used for the backcross.
Analysis of dilp mRNA and FOXO protein
Western blot
Thirty 4-day-old to 5-day-old female flies were homogenized in 400 µL of loading buffer (Sambrook & Russell, 2001). Samples were boiled for 5 min and spun for 10 min; 7 µL of the extract was loaded in a 7% Tris–acetate gel (Invitrogen, EA 03552), and transferred to polyvinylidene difluoride membranes following the manufacturer's instructions. Guinea pig α-dFOXO antibody was kindly provided by Dr H. Broihier (Case Western Reserve University, Cleveland, OH, USA), and used in 1 : 5000 dilution. α−β Tubulin (hybridoma center) was used at 1 : 5000 dilution.
PCR
Transcript levels of Drosophila insulin-like peptides were measured with quantitative PCR. Live flies were frozen in liquid nitrogen and stored at –80 °C. Heads are separated using a funnel with fine mesh. Because heads can rapidly thaw and then loose RNA, all sample preparations were conducted with iced reagents and containers prior to RNAase inactivation. Total RNA from 75 heads of 7-day-old female flies was prepared using Trizol reagent (Invitrogen). RNA purity and amount were measured spectrophotometrically (NanoDrop, Thermo Scientific, Wilmington, DE, USA). DNase-treated total RNA was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) according to the supplier's protocol. Real-time PCR was performed using iTaq SYBR Green Supermix with ROX (Bio-Rad) and ABI prism 7300 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). mRNA of each gene was normalized relative to GAPDH2 by the method of comparative CT (Livak & Schmittgen, 2001).
The primers were:
ilp2 F ‘TGAGTATGGTGTGCGAGG’, R ‘CTCTCCACGATTCCTTGC’;
ilp3 F ‘GAACTTTGGACCCCGTGAA’, R ‘TGAGCATCTGAACCGAACT’;
ilp5 F ‘CAAACGAGGCACCTTGGG’, R ‘AGCTATCCAAATCCGCCA’;
GAPDH2 F ‘GCGGTAGAATGGGGTGAGAC’, R ‘TGAAGAGCGAAAACAGTAGC’.
Demography and survival analysis
Adult female flies were aged in 1 L demography cages. Cages were made from clear food service containers each with a ventilated lid, a gasket-covered aperture and a 25-mm-diameter plastic tube affixed to an opening along the cage side near the floor. Food vials (25 × 95 mm) were attached via the tube and changed every 2 days, at which time dead flies were removed and recorded. Female flies were collected with light CO2 anesthesia over a 48-h period of emergence to permit maturation and mating. Female flies were introduced at a density of 100 per demography cage. For each level of diet, at least three replicate cages were initiated per treatment.
Survival analysis was conducted with JMP statistical software with data from replicate cages combined. Kaplan–Meier methods were used to estimate mean adult lifespan with standard error. Proportional hazard analysis was used to measure how a tested genotype modified the ability of DR to increase lifespan (Tatar, 2007). Within a trial, all recorded deaths were analyzed simultaneously in a survival regression model where main effects are genotype (G) as a nominal variable and diet (D) as a continuous variable, and where the interaction parameter G × D estimates how genotype modifies the impact of diet on mortality. Survival regression with proportional hazards fits a linear model for the additive effects of these parameters, and estimates coefficients (βi) and error for each parameter i (Parmer & Machin, 1995). The transformation exp(βi) gives the hazard ratio, the effect upon relative mortality of one unit increment in the parameter i. When the hazard ratio is close to unity, there is little effect of the variable upon survival.
Acknowledgments
For providing stocks, we thank E. Hafen (Zurich, Switzerland), K. Weber (Portland, ME, USA) and L. Partridge (London, England). H. Broihier (Case Western Reserve University) graciously provided the dFOXO antibody. Funding was provided by The Ellison Medical Foundation, and NIH AG024360 and AG021953.
Supplementary material
The following supplementary material is available for this article:
UASs-gfp transgene expression is driven by P{Switch}S132 with RU486 in diets of low- and high-yeast concentration. Sets of 1-day-old to 2-day-old female flies (P{Switch}S132 > UAS-gfp) were fed media containing 2% or 8% yeast, with or without 200 µM RU486 for 5 days. Per treatment, we prepared four biological samples each with 10 heads. Samples were homogenized in 120 µL of green fluorescent protein (GFP) assay buffer (10 mM Tris-HCl pH 8.4, 100 mM NaCl, 1 mM MgCl2, 10 mM dithiothreitol), centrifuged at 10 000 r.p.m. at 4 °C and the supernatant further diluted with an additional 120 µL of GFP assay buffer. In duplicate wells, each sample was measured on a SpectraMax M5 (Molecular Devices, Sunnyvale, CA, USA) in 96-well plates and corrected for blank. RU486 induces GFP expression upon both 2% and 8% yeast diets.
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1474-9726.2008.00373.x
(This link will take you to the article abstract).
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Bartke A, Wright C, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the life span of long-lived mice. Nature. 2001;414:412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc. Natl. Acad. Sci. USA. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton SJ, Piper MDW, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L. Longer life span, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl. Acad. Sci. USA. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch S, Melcher C, Bauer M, Katzenberger J, Pankratz MJ. Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Curr. Biol. 2008 doi: 10.1016/j.cmet.2008.02.012. in press. [DOI] [PubMed] [Google Scholar]
- Carvalho GB, Kapahi P, Benzer S. Direct quantification of food intake reveals compensatory ingestion upon dietary restriction in Drosophila. Nat. Methods. 2005;2:813–815. doi: 10.1038/nmeth798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proc. R. Soc. Lond. B. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- Chippindale AK, Leroi AM, Kim SB, Rose MR. Phenotypic plasticity and selection in Drosophila life-history evolution. I. Nutrition and the cost of reproduction. J. Evol. Biol. 1993;6:171–193. [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Hafen E, Leevers SJ, Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002;296:319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
- Gershman B, Puig O, Hang L, Peitzsch RM, Tatar M, Garofalo RS. High-resolution dynamics of the transcriptional response to nutrition in Drosophila: a key role for dFOXO. Physiol. Genomics. 2007;29:24–34. doi: 10.1152/physiolgenomics.00061.2006. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science. 2005;307:675. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- Houthoofd K, Braeckman BP, Johnson TE, Vanfleteren JR. Life extension via dietary restriction is independent of the Ins/IGF-1 signaling pathway in Caenorhabditis elegans. Exp. Gerontol. 2003;38:947–954. doi: 10.1016/s0531-5565(03)00161-x. [DOI] [PubMed] [Google Scholar]
- Houthoofd K, Gems D, Johnson TE, Vanfleteren JR. Dietary restriction in the nematode Caenorhabditis elegans. Interdiscip. Top. Gerontol. 2007;35:98–114. doi: 10.1159/000096558. [DOI] [PubMed] [Google Scholar]
- Hwangbo DS, Gershman B, Tu MP, Palmer MR, Tatar M. Drosophila dFOXO controls life span and regulates insulin signaling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Junger MA, Rintelen F, Stocker H, Wasserman DA, Vegh M, Radimerski T, Greenberg ME, Hafen E. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J. Biol. 2003;2(3):20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315:1133–1137. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mobbs CV, Yen K, Hof PR, editors. Mechanisms of Dietary Restriction in Aging and Disease. Vol. 35. Basel: Karger; 2007. [Google Scholar]
- Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–555. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- Parmer MKB, Machin D. Survival Analysis: A Practical Approach. Chichester: Wiley; 1995. [Google Scholar]
- Partridge L, Piper MDW, Mair W. Dietary restriction in Drosophila. Mech. Ageing Dev. 2005;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Roman G, Endo K, Zong L, Davis RL. P{Switch}, a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2001;98:12602–12607. doi: 10.1073/pnas.221303998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell D. Cold Spring Harbor Laboratory Press: New York; 2001. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- Tatar M. Transgenes in the analysis of life span and fitness. Am. Nat. 1999;154:S67–S81. doi: 10.1086/303284. [DOI] [PubMed] [Google Scholar]
- Tatar M. Diet restriction in Drosophila melanogaster: design and analysis. Interdiscip. Top. Gerontol. 2007;35:115–136. doi: 10.1159/000096559. [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu M-P, Yin C-M, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Tu M-P, Epstein D, Tatar M. The demography of slow aging in male and female Drosophila mutant for the insulin-receptor substrate homolog chico. Aging Cell. 2002;1:75–80. doi: 10.1046/j.1474-9728.2002.00010.x. [DOI] [PubMed] [Google Scholar]
- Walker G, Houthoofd K, Vanfleteren JR, Gems D. Dietary restriction in C. elegans: from rate-of-living effects to nutrient sensing pathways. Mech. Ageing Dev. 2005;26:929–937. doi: 10.1016/j.mad.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Weber K, Johnson N, Champlin D, Patty A. Many P-element insertions affect wing shape in Drosophila melanogaster. Genetics. 2005;169:1461–1475. doi: 10.1534/genetics.104.027748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J. Nutr. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging Drosophila. Nat. Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
UASs-gfp transgene expression is driven by P{Switch}S132 with RU486 in diets of low- and high-yeast concentration. Sets of 1-day-old to 2-day-old female flies (P{Switch}S132 > UAS-gfp) were fed media containing 2% or 8% yeast, with or without 200 µM RU486 for 5 days. Per treatment, we prepared four biological samples each with 10 heads. Samples were homogenized in 120 µL of green fluorescent protein (GFP) assay buffer (10 mM Tris-HCl pH 8.4, 100 mM NaCl, 1 mM MgCl2, 10 mM dithiothreitol), centrifuged at 10 000 r.p.m. at 4 °C and the supernatant further diluted with an additional 120 µL of GFP assay buffer. In duplicate wells, each sample was measured on a SpectraMax M5 (Molecular Devices, Sunnyvale, CA, USA) in 96-well plates and corrected for blank. RU486 induces GFP expression upon both 2% and 8% yeast diets.