Abstract
Structure–function studies of rhodopsin kinase (RK; EC 2.7.1.125) require a variety of mutants. Therefore, there is need for a suitable system for the expression of RK mutant genes. Here we report on a study of expression of the RK gene in baculovirus-infected Sf21 cells and characterization of the enzyme produced as purified to near homogeneity. Particular attention has been paid to the post-translational modifications, autophosphorylation and isoprenylation, found in the native bovine RK. The protein produced has been purified using, successively, heparin-Sepharose, Mono Q, and Mono S FPLC (fast protein liquid chromatography) and was obtained in amounts of about 2 mg from 1 liter of cell culture. The enzyme from the last step of purification was obtained in two main fractions that differ in the level of phosphorylation. The protein peak eluted first carries two phosphate groups per protein, whereas the second protein peak is monophosphorylated. Further, while both peaks are isoprenylated, the isoprenyl groups consist of mixtures of C5, C10, C15, and C20 isoprenyl moieties. From these results, we conclude that the above expression system is suitable for some but not all aspects of structure–function studies.
Keywords: G-protein-coupled receptor kinases, rod outer segment, autophosphorylation, isoprenylation, Spodoptera frugiperda cells
Rhodopsin kinase (RK; EC 2.7.1.125), specific to the mammalian retina, phosphorylates rhodopsin and thus initiates the desensitization cascade (2, 3). RK, a protein of about 62 kDa molecular mass, has been studied extensively, and methods are available for its purification from bovine rod cell outer segment (ROS) (4, 5). We are interested in structure–function studies of this enzyme that require preparation of a variety of mutants (6). These, in turn, require expression of the RK mutant genes in a suitable system and a method for purification in adequate amounts. In this paper, we report on expression of the RK gene in baculovirus-infected Sf21 insect cells and purification and characterization of the produced protein.
The baculovirus-infected insect cell system has been used for the production of a large variety of proteins. In particular, several G-protein-coupled receptor kinases have been produced by using this system (7–9). In the present work, particular attention has been paid to characterization of post-translational modifications of the protein as produced in the insect cells, specifically, isoprenylation and autophosphorylation that have been found in RK from bovine ROS (10, 11). We find that RK is produced in satisfactory amounts. On purification it distributes into two main fractions that differ from each other in regard to having either one or two phosphate groups per protein. Both fractions are isoprenylated; however, there is heterogeneity in the isoprenyl groups, these being mixtures of C5–C20 chain lengths. RK from the insect cells [RK(BV)] is indistinguishable from RK as prepared from bovine ROS [RK(ROS)] in regard to specific activity, temperature sensitivity, pH optimum, and mobility in SDS/PAGE. We conclude that the above expression system promises to be useful in some but not all structure–function studies of the enzyme.
MATERIALS AND METHODS
Materials
n-Dodecyl β-d-maltoside (DM) was from Anatrace (Maumee, OH). Bovine retinae were from J. A. Lawson Corp. (Lincoln, NE). Heparin-Sepharose, Mono Q, and Mono S were from Pharmacia. Insect medium and supplements were from GIBCO/BRL and Sigma. (R,S)-[5-3H]Mevalonolactone, inorganic [32P]phosphate, and [γ-32P]ATP were from DuPont/NEN.
Buffers used were as follows: buffer A, 1.5 mM KH2PO4/8 mM Na2HPO4, pH 7.4/137 mM NaCl/2.7 mM KCl; buffer B, 20 mM 1,3-bis[tris(hydroxymethyl)methylamino]propane (BTP), pH 7.5; buffer C, buffer B containing 1 mM EDTA, 250 mM KCl, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 20 μg/ml leupeptin, 1 mM benzamidine⋅hydrochloride, and 0.25% DM; buffer D, buffer C without KCl and DM; buffer E, 10 mM BTP (pH 7.5) and 0.05% DM. Buffers F, G, and H were buffer E containing 100 mM, 50 mM, and 30 mM KCl, respectively.
The plasmid and baculovirus construct have been described elsewhere (5, 12).
Methods
Urea-Stripped ROS.
ROS were prepared from frozen retinae as described (13). They were washed under dim red light with 5 M urea in buffer B to remove extrinsic proteins and RK (14).
RK(ROS).
This was purified by the method of Palczewski et al. (15). From 100 frozen retinae, the yield of RK was 150 μg with specific activity of 80 units/mg of protein. One unit is the amount of enzyme that catalyzes 1 nmol of phosphate transfer per min at 30°C. Palczewski and co-workers (15, 16) reported specific activities of 550–900 units/mg in their RK preparations, whereas Rim and Oprian (17) reported a specific activity of 165 units/mg.
Assay of RK Activity.
The reaction mixture (20 μl) in a microcentrifuge tube contained 2 mM MgCl2, 100 μM [γ-32P]ATP (150–1,500 cpm/pmol), and an enzyme fraction (1–4 μl, containing up to 0–0.03 unit) in buffer B. Urea-stripped ROS were added to 20 μM concentration in the dark and the reaction was initiated at 30°C by illumination. Parallel tubes incubated in the dark under identical conditions but without RK or ROS served as controls. The reactions were followed by one of the two following assays.
Assay 1: Acid precipitation.
Reactions in mixtures as above were terminated by the addition of 20 μl of a solution containing 200 mM EDTA, 5 mM adenosine, and 100 mM NaF. Trichloroacetic acid (TCA) (40 μl of 20%) was added in the cold and the resulting precipitate was collected by centrifugation and washed four times with 0.7 ml of 10% TCA, each washing being followed by centrifugation at 10,000 × g for 10 min. The pellet was directly used for Cerenkov counting.
Assay 2: Filter binding assay.
Reactions in mixtures as above were terminated by the addition of 180 μl of a solution containing 0.8 M KH2PO4, 20 mM ATP, and 20 mM EDTA. Aliquots (90 μl) were applied to nitrocellulose filters (Protran, NC; Schleicher & Schuell) presoaked in 1 M KH2PO4 containing 20 mM ATP, using the micro-dot blot system (Bio-Rad). The filters were washed once with 250 μl of 1.0 M KH2PO4 and then soaked in 50 ml of 1.0 M KH2PO4 four times for 5 min each time. The radioactivity was measured by Cerenkov counting. This assay was convenient for handling large numbers of samples. However, the retention of phosphorylated ROS on the nitrocellulose filters varied between 60% and 90% in different experiments.
Expression and Purification of RK from Sf21 Cells.
Expression. Insect culture media: Grace’s medium with supplements of 3.33 g/liter lactalbumin hydrolysate and 3.33 g/liter Yeastolate (GIBCO/BRL) and containing 10% fetal bovine serum, 50 μg/liter gentamycin, and 0.1% Pluronic F-68 (GIBCO/BRL). Spodoptera frugiperda Sf21 cells grown as described (18) were harvested from a 250-ml culture (2.0 × 106 cells per ml) by centrifugation at 1,000 × g for 3 min. The pellet, as a suspension in 40 ml of the insect medium, was treated with 10 ml of a baculovirus suspension (5 × 107 plaque-forming units/ml) at a multiplicity of infection of 1.0. The suspension was kept at 27°C for 1 hr without agitation, 200 ml of fresh medium was added, and the total mixture was incubated in a 2.0-liter flask with shaking at 27°C. Mevalonolactone was added to a final concentration of 6 mM after 36 hr and, after a total of 72 hr post infection, the cells were harvested by centrifugation at 1,000 × g for 3 min. The cell pellet was washed twice with 50 ml each of buffer A. The pellet was used directly for purification of RK or was stored at −70°C.
Purification of RK: Crude extract.
Cells (5 × 108) were suspended in 20 ml of buffer C, and the suspension was homogenized with an Ultra-Turrax T25 (IKA-Works, Cincinnati, OH) at 24,000 rpm for 30 sec three times with 1-min intervals in between. After removal of the cell debris by centrifugation (10,000 × g for 10 min), 30 ml of buffer D was slowly added with swirling to the solution. The precipitate formed at this stage was removed by centrifugation at 100,000 × g for 1 hr and the supernatant (50 ml) was used in step 1 below.
Step 1: Fast protein liquid chromatography (FPLC) on heparin-Sepharose column.
The above supernatant was applied to a HiTrap-heparin-Sepharose column (5 ml), preequilibrated with buffer F, at a flow rate of 1 ml/min. The column was washed with 80 ml of the same buffer and elution was carried out in the same buffer, using a KCl gradient of 100–500 mM in 80 ml. The flow rate was 1 ml/min, 2-ml fractions being collected. Absorbance at 280 nm was recorded continuously in a 0.5-cm light path flow cell, and RK activity was measured by assay 2.
Step 2: FPLC on Mono Q HR 5/5.
The combined fractions containing RK activity were diluted 4-fold with buffer E and applied to a Mono Q HR column (1 ml), previously equilibrated with buffer G, at a flow rate of 0.3 ml/min. The column was washed with buffer G at the same flow rate until A280 dropped below 0.01 (50 ml). RK was eluted using a linear gradient of 50–500 mM KCl in buffer E, 2-ml fractions being collected. Absorbance at 280 nm and RK activity were monitored as above.
Step 3: FPLC on Mono S HR.
Combined fractions from the preceding column containing RK activity (8 ml) were diluted with buffer E and the solution (50 ml) was applied to a Mono S HR 5/5 column, preequilibrated with buffer H. RK was eluted by using a linear gradient (30–300 mM) of KCl in buffer E at a flow rate of 0.3 ml/min. One-milliliter fractions were collected and protein elution and RK activities were measured as above.
3H-Labeling in vivo of isoprenyl groups in RK.
Sf21 cells (100 ml, 2.0 × 106 cells per ml) infected and grown as described above were treated 36 hr after infection with 0.5 ml of an aqueous solution of dl-[3H]mevalonolactone (500 μCi; 1 μCi = 37 kBq). At 72 hr after infection, the cells were collected by centrifugation at 1,000 × g for 3 min, the pellet being washed two times with 25 ml each of buffer A. The pellet was used for purification of RK as described above.
32P-Labeling of RK in vivo.
A culture (100 ml) of infected Sf21 cells (2 × 106 cells per ml) was treated at 67 hr after infection with 1 mCi (1 ml) of inorganic [32P]phosphate in Grace’s medium lacking phosphate, and at 72 hr after infection, the cells were harvested and washed as described above. The pellet was used for purification of RK.
Determination of Isoprenoid Groups.
RK was purified from cells (2 × 108) grown in the presence of [3H]mevalonolactone as described above. Fractions from a Mono S column containing RK activity were acidified with trichloroacetic acid (to a final concentration of 15%). The precipitate was collected and washed twice with 1 ml each of cold acetone after being kept at 4°C for 12 hr each time. The pellet was air-dried and suspended in 0.6 ml of 0.5% formic acid. Methyl iodide (100 μl) was added and the mixture was kept under agitation in the dark for 50 hr (19, 20). Sodium carbonate (30 μl of a 35% aqueous solution) was added and the mixture was kept in the dark at room temperature for 28 hr. The mixture was then extracted four times with 400 μl each of a chloroform/methanol (9:1, vol/vol) mixture. The combined extract was dried under nitrogen and to the dried residue was added 40 μl of a 40% (vol/vol) aqueous acetonitrile solution containing 0.025% each of geraniol, farnesol, nerolidol, and geranylgeraniol. The total mixture, including the residue, was extracted twice with 200 μl each of 40% acetonitrile containing 25 mM phosphoric acid. Two further extractions with 200 μl each of 100% acetonitrile were carried out. Portions of 250 μl of the extracts were injected into a Vydac C18 reverse-phase HPLC column (4.6 mm × 25 cm). Elution was carried out first using a linear gradient (40–80%) of acetonitrile containing 25 mM phosphoric acid (30 ml), then by 5 ml of 80–100% acetonitrile gradient, and, finally, by 5 ml of 100% acetonitrile at a flow rate of 1 ml/min. Absorbance at 210 nm was monitored with standards—geraniol, farnesol, nerolidol, and geranylgeraniol—being run separately. Fractions (0.5 ml) were counted for radioactivity. Control sample was analyzed under identical condition but without methyl iodide treatment.
In vitro Autophosphorylation of RK.
The reaction mixture (1.5 ml) contained 1 mM MgCl2, 10 μM [γ-32P]ATP (about 15,000 cpm/pmol), 300 μg of RK, 0.1 mg/ml BSA, and 0.05% DM in buffer B. After incubation at 20°C for 90 min, ATP was removed by filtration through Sephadex G-50 and the eluate was analyzed on a Mono S column as described above.
Miscellaneous Methods.
Protein concentrations were determined by the Bradford method (21) using BSA as the standard. SDS/PAGE was performed by the method of Laemmli (22).
RESULTS
Expression of RK in Sf21 Cells.
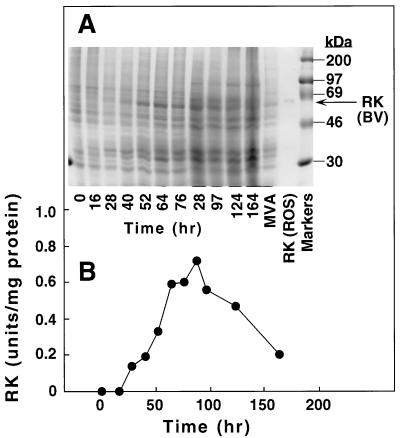
Initial studies attempted to optimize RK expression in baculovirus-infected cells by varying multiplicity of infection (moi) and following the time course of RK expression after infection. When an moi of 1.0 was used, a protein band corresponding to RK was visible on SDS/PAGE at 28 hr and its intensity increased subsequently (Fig. 1A). The RK activity profile (Fig. 1B) corresponded to the production of the RK band and reached a peak between 64 and 88 hr after infection.
Figure 1.
Expression of RK from baculovirus-RK-infected Sf21 cells as a function of time. (A) Aliquots (1 × 105 cells) of cell culture taken at the time points shown were processed for SDS/PAGE (10% gel) as described in the text. The proteins were visualized by Coomassie blue staining. (B) Aliquots (5 ml) of cell culture were processed for preparation of crude extracts for RK analysis. Values obtained in mock-infected cells (13.5% ± 3.3% of maximal activity) have been subtracted.
Purification of RK from Infected Cells.
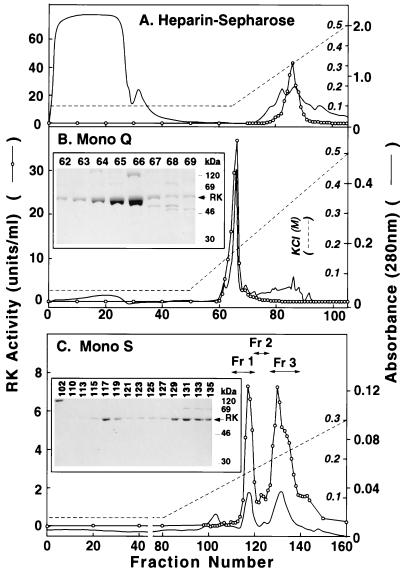
Step 1: Chromatography on heparin-Sepharose. Heparin-Sepharose has been used previously for purification of RK from ROS (4), and this served as the first step in the present procedure. Starting with 5 × 108 cells, applying the total crude extract (50 ml) to a column and elution as described in the text gave a single peak of RK activity (Fig. 2A). This appeared at a concentration of 0.28 M KCl when a buffer containing 0.05% DM was used and at 0.21 M KCl when the detergent Tween 80 (0.4%) replaced DM. The total pooled peak (12.0 ml) represented about 10-fold purification, and the recovery of activity was essentially quantitative (Table 1).
Figure 2.
Chromatographic Steps in Purification of RK. (A) Heparin-Sepharose. The crude extract (50 ml) prepared from 5 × 108 infected cells was applied to a HiTrap-Heparin column and elution was performed. A280 (solid line) (scale on the outside) and RK activity (○) (scale inside A) are shown. (B) FPLC on Mono Q. Fractions 83–88 from A above (12 ml) were diluted and applied on Mono Q and elution was performed with a linear salt gradient. (Inset) Fractions 62–69 as examined by SDS/PAGE. (C) Fractions 63–66 (8 ml) from B above were chromatographed by FPLC on Mono S. RK activity was assayed. The three fractions indicated were pooled. (Inset). SDS/PAGE analysis of fractions 102–135.
Table 1.
Purification of RK expressed from Sf21 cells
| Step | Vol, ml | Total protein, mg | Specific activity, nmol Pi/min/mg | Purification, fold | Yield, % |
|---|---|---|---|---|---|
| Crude | 50.0 | 128 | 1.53 | 1 | 100 |
| Heparin-Sepharose | 12.0 | 13.5 | 15.0 | 9.8 | 100 |
| Mono Q FPLC | 8.0 | 2.39 | 69.3 | 45 | 86 |
| Mono S FPLC | |||||
| Fraction 1 | 8.0 | 0.210 | 90.4 | 59 | 10 |
| Fraction 2 | 2.0 | 0.043 | 41.9 | 27 | 1 |
| Fraction 3 | 15.0 | 0.345 | 96.5 | 63 | 17 |
Starting material was 250 ml of cell culture, 5 × 108 cells. Details are in the text.
Step 2: FPLC chromatography on Mono Q HR.
The pooled fractions (12.0 ml) from step 1, after dilution (4-fold), were chromatographed on Mono Q HR (Materials and Methods). As seen in Fig. 2B, RK again eluted as a single peak. The total volume in combined fractions was 8.0 ml. It represented 86% recovery with a specific activity of 69 units/mg of protein (Table 1).
Step 3: FPLC on Mono S HR.
Subsequent chromatography of the pooled peak from step 2 on Mono S HR resolved the RK enzymatic activity into two major peaks (Fig. 2C) separated by one minor peak, the three fractions being eluted at 0.12 M (fraction 1), 0.14 M (fraction 2), and 0.17 M (fraction 3) KCl, respectively. The recovery at this step was 32%.
Finally, the procedure produced homogenous (purity >90%) RK with a final yield of 27% (Table 1). Fractions 1 and 3 both showed specific activity of 90–97 units/mg and, on SDS/PAGE, gave a band with the mobility of RK(ROS) (62 kDa) (Fig. 2C Inset). Specific activity of RK eluted in fraction 2 was lower (42 units/mg) and its mobility was lower, corresponding to 64 kDa.
Phosphorylation States of Purified RK Fractions.
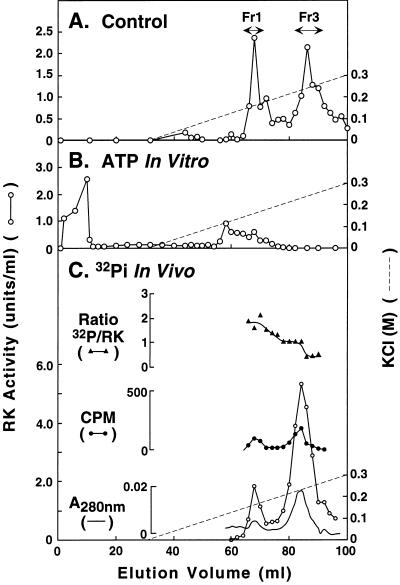
In vitro phosphorylation. RK(BV) from step 2 (Fig. 2) was treated with ATP as in Materials and Methods. The product when chromatographed on the Mono S HR column gave the pattern shown in Fig. 3B. For comparison, the elution pattern of the same RK without ATP treatment is shown in Fig. 3A. Thus, on ATP treatment, the activity peak corresponding to fraction 3 disappeared and the peak corresponding to fraction 1 was greatly reduced. Instead, most of the RK activity appeared in the flow-through volume. Even when applied to a column preequilibrated in the absence of salt, RK did not bind (not shown). This result shows that fractions 1 and 3 differ in their phosphorylation state and that incorporation of phosphate groups into RK decreases its interaction with the Mono S HR column.
Figure 3.
Characterization of phosphorylation states in RK(BV). (A) Elution pattern of RK from Mono S FPLC. ○, RK activity. (B) Elution pattern of RK from Mono S after in vitro phosphorylation with ATP. (C) Elution pattern of RK from Mono S FPLC after infected cells had been grown in medium containing inorganic [32P]phosphate.
In vivo phosphorylation of RK(BV).
RK was prepared from Sf21 cells that had been grown in the presence of [32P]phosphate. The purification procedure was taken to FPLC on Mono S HR. The chromographic pattern is shown in Fig. 3C. Although the radioactivity in the fractions was low, two 32P-containing peaks of RK were obtained; these peaks superimposed with the activity peaks and corresponded to fractions 1 and 3 of the control (Fig. 3A). The molar ratio of 32P to protein in the two fractions was 1.90 ± 0.37 (Fig. 3C). The amount of RK in fraction 1 relative to that in fraction 3 in this 32P-labeling experiment was low compared with the control (Fig. 3A). This is likely to be due to the depleted phosphate concentration in the growth medium used in the labeling experiment.
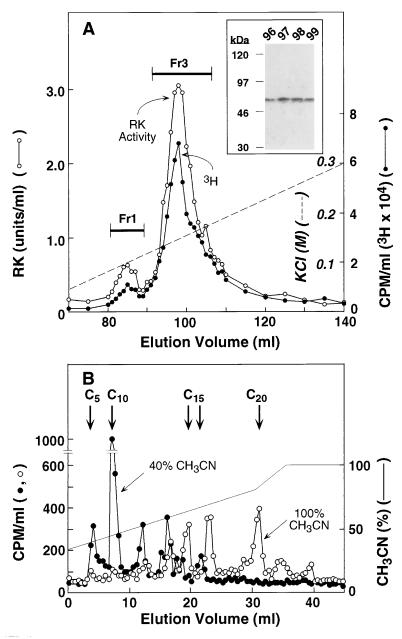
Isoprenylation of RK(BV).
RK was prepared from Sf21 cells grown in the presence of [3H]mevalonic acid and the purification procedure was taken to FPLC on Mono S HR (step 3). As seen in Fig. 4A, two 3H-containing peaks, corresponding to fractions 1 and 3, were obtained. The specific labeling of RK was further verified in selected fractions (96–99) by SDS/PAGE followed by fluorography (Fig. 4A Inset). It is concluded that RK in both fraction 1 and fraction 3 is isoprenylated. No radioactivity seems to be associated with fraction 2, although the small amounts of both protein and radioactive material make the reading difficult.
Figure 4.
(A) Elution pattern of RK, with 3H-labeled isoprenyl groups, from FPLC on Mono S. Sf21 cells were grown in the presence of [3H]mevalonolactone and RK was purified as described in the text. (Inset) SDS/PAGE (10%) analysis of fractions 96–99. 3H radioactivity was detected by fluorography. (B) Analysis of isoprenoid groups in [3H]RK(BV) by HPLC. HPLC was on a C18 Vydac column. Conditions for release of isoprenoid groups from [3H]RK as the corresponding alcohols and elution with a CH3CN gradient are described in the text. The arrows indicate the elution positions for C5 (mevalonic acid), C10 (geraniol), C15 (trans-farnesol and nerolidol), and C20 (geranylgeraniol).
Isoprenoid composition.
The isoprenoid composition of 3H-labeled RK in the above experiment was determined by sulfonium salt cleavage and acetonitrile extraction followed by HPLC (23). Each step in the procedure showed a recovery of at least 80%. Short-chain isoprenoids such as isopentenyl or dimethylallyl (C5) and geranyl (C10) were extracted using 40% aqueous acetonitrile, whereas extraction of farnesyl (C15) and geranylgeranyl (C20) groups required 100% acetonitrile (Fig. 4B). The distribution of the 3H radioactivity in the different chain lengths was: C5 plus C10, 43%; C15, 36%, and C20, 21%.
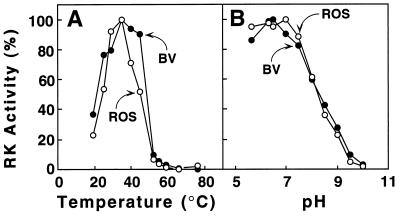
Optimal Temperature and pH Dependence of RK(BV).
These two properties were compared side by side for RK(ROS) and RK(BV). As seen in Fig. 5, both preparations exhibited the same temperature and pH optimum.
Figure 5.
Comparison of RK(ROS) and RK(BV) Properties. (A) Temperature optimum. (B) pH optimum. Reaction conditions and assays for RK activity are described in the text. RK activity was measured by using urea-washed ROS membranes as the substrate. To determine the pH optimum, 50 mM BTP buffer was used instead of 20 mM BTP (the pH values were 5.63, 6.3, 6.5, 7.0, 7.5, 8.0, 8.5, 9.0, 9.5, and 10.0).
DISCUSSION
We have described the expression of the bovine RK gene in baculovirus-infected insect cells and have purified the enzyme produced to near homogeneity. The purified RK is obtained in reasonable amounts, about 2 mg/liter of cell culture, and appears promising for some structure–function studies.
Wild-type RK gene and some of its mutants have already been expressed in the insect system by other groups (5, 24, 25), as have a number of related G-protein-coupled receptor kinases (7–9, 26). Because, in our proposed studies of RK, accurate post-translational modifications are an important consideration, we have paid special attention to this aspect of RK as produced in the insect system. Evidently, such modifications were not checked in the RK’s previously prepared using the insect system. Often in this system, post-translational modifications, especially glycosylation, have been found to be deficient (refs. 27 and 28 and unpublished work on the opsin gene expression by S. Kaushal in this laboratory).
Palczewski and co-workers (4) showed that RK from bovine ROS can be autophosphorylated at two major sites (Ser-448 and Thr-449) and one minor site (Ser-21). When purified from ROS, RK was obtained mainly as a mixture of mono- and diphosphorylated forms that did not separate on heparin-Sepharose (4). These workers also found that RK(ROS) purified in the mono- and diphosphorylated forms can undergo further autophosphorylation in vitro by ATP. Following this treatment, RK was found to elute from heparin-Sepharose earlier than RK without the treatment. Our results with RK from the insect cells parallel the above findings by Palczewski and colleagues. First, RK(BV) is also a mixture of phosphorylated species, which, like their ROS counterparts, do not separate on heparin-Sepharose (Fig. 2A). They do, however, separate on chromatography on a Mono S column (Fig. 2C). Further confirmation of the phosphate content in the RK main fractions seen in Fig. 2C came from in vivo labeling of RK using inorganic [32P]phosphate (Fig. 3B). Twice as many phosphate groups were incorporated per RK molecule in fraction 1 relative to fraction 3. We conclude that RK in fraction 1 carries two phosphate groups, while that in fraction 3 has one phosphate group. Second, RK(BV) can also be further phosphorylated in vitro, and this results in a shift in the position of its elution from the Mono S HR column (Fig. 3B) and from the heparin-Sepharose column (not shown) similar to the one seen by Palczewski et al. with RK(ROS). Thus, in these aspects of phosphorylation states, RK(BV) closely resembles RK(ROS).
The -Cys-Val-Leu-Ser carboxyl-terminal tetrapeptide sequence in RK falls under the consensus sequence for farnesylation and carboxymethylation (for review, see ref. 29). Indeed, Inglese et al. (11, 30) showed that bovine RK produced in COS-7 cells was carboxymethylated and contained a farnesyl group. Previously, the baculovirus expression system was found to be selective for the farnesylation of expressed H-Ras (31) but not selective for the isoprenylation of the expressed G protein γ1 subunit (32). Our analysis of this post-translational modification of RK(BV) shows that the bulk of RK (Fig. 2C, fractions 1 and 3, and Fig. 4A) produced is isoprenylated, whereas a small amount, about 7% (fraction 2 of Fig. 2C), possessing lower specific activity does not appear to be isoprenylated. However, isoprenylation is found to be strikingly heterogeneous. In this analysis, we took precautions to trap the short-chain carbon components (23). More than 40% of the protein contains C5 or C10 moieties, about 20% contains a C20 (geranylgeranyl) group, and about 40% contains a farnesyl group (C15). Therefore, our results show that, as with glycosylation, the insect expression system lags in completing the specific isoprenylation modification.
In summary, the insect system enables the preparation of RK in reasonable amounts. RK(BV) resembles RK from ROS in its specific activity, pH and temperature optima, and phosphorylation states. Thus, the system can be used to generate mutants of RK. A serious limitation of the system seems to be the incomplete and heterogeneous isoprenylation of the proteins.
Acknowledgments
We have benefitted greatly from discussions with Prof. U. L. RajBhandary (Massachusetts Institute of Technology, Biology Department) and with Drs. P. Reeves, R. Thurmond, and C. Creuzenet. We thank Dr. Sang Seok Koh (Whitehead Institute) for initial help with the baculovirus system. Ms. Judy Carlin’s patient assistance during the preparation of the manuscript is gratefully acknowledged. This work was supported by Grant GM28289 from the National Institutes of Health and by a Human Frontier Science Program Award LT449/96 (C.B.).
ABBREVIATIONS
- RK
rhodopsin kinase
- ROS
rod cell outer segment
- RK(BV)
RK from baculovirus-infected insect cells
- RK(ROS)
RK from bovine ROS
- DM
n-dodecyl β-d-maltoside
- BTP
1,3-bis[tris(hydroxymethyl)methylamino]propane
Footnotes
This is paper 25 in the series “Structure and function in rhodopsin.” Paper 24 is ref. 1.
References
- 1.Hwa J, Garriga P, Liu X, Khorana H G. Proc Natl Acad Sci USA. 1997;94:10577–10582. doi: 10.1073/pnas.94.20.10571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhn H. Biochemistry. 1978;17:4389–4395. doi: 10.1021/bi00614a006. [DOI] [PubMed] [Google Scholar]
- 3.Wilden U, Hall S W, Kuhn H. Proc Natl Acad Sci USA. 1986;83:1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buczylko J, Gutmann C, Palczewski K. Proc Natl Acad Sci USA. 1991;88:2568–2572. doi: 10.1073/pnas.88.6.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C, Inglese J, Lefkowitz R J, Hurley J B. J Biol Chem. 1995;270:18060–18066. doi: 10.1074/jbc.270.30.18060. [DOI] [PubMed] [Google Scholar]
- 6.Khorana H G. J Biol Chem. 1992;267:1–4. [PubMed] [Google Scholar]
- 7.Kim C M, Dion S B, Onorato J J, Benovic J L. Receptor. 1993;3:39–55. [PubMed] [Google Scholar]
- 8.Benovic J L, Gomez J. J Biol Chem. 1993;268:19521–19527. [PubMed] [Google Scholar]
- 9.Kunapuli P, Benovic J L. Proc Natl Acad Sci USA. 1993;90:5588–5592. doi: 10.1073/pnas.90.12.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palczewski K, Ohguro H, Premont R T, Inglese J. J Biol Chem. 1995;270:15294–15298. doi: 10.1074/jbc.270.25.15294. [DOI] [PubMed] [Google Scholar]
- 11.Inglese J, Glickman J F, Lorenz W, Caron M G, Lefkowitz R J. J Biol Chem. 1992;267:1422–1425. [PubMed] [Google Scholar]
- 12.Lorenz W, Inglese J, Palczewski K, Onorato J J, Caron M G, Lefkowitz R J. Proc Natl Acad Sci USA. 1991;88:8715–8719. doi: 10.1073/pnas.88.19.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papermaster D S. Methods Enzymol. 1982;81:240–246. doi: 10.1016/s0076-6879(82)81037-9. [DOI] [PubMed] [Google Scholar]
- 14.Shichi H, Somers R L. J Biol Chem. 1978;253:7040–7046. [PubMed] [Google Scholar]
- 15.Palczewski K. Methods Neurosci. 1993;15:217–225. [Google Scholar]
- 16.Palczewski K, McDowell J H, Hargrave P A. J Biol Chem. 1988;263:14067–14073. [PubMed] [Google Scholar]
- 17.Rim J, Oprian D D. Biochemistry. 1995;34:11938–11945. doi: 10.1021/bi00037a035. [DOI] [PubMed] [Google Scholar]
- 18.Summers M D, Smith G E. A Manual of Methods for Baculovirus Vectors and Insect Cell Culture Procedures. College Station, TX: Texas A&M University; 1987. [Google Scholar]
- 19.Kamiya Y, Sakurai A, Tamura S, Takahashi N. Agric Biol Chem. 1979;43:1049–1053. [Google Scholar]
- 20.Casey P J, Solski P A, Der C J, Buss J E. Proc Natl Acad Sci USA. 1989;86:8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Farnsworth C C, Casey P J, Howald W N, Glomset J A, Gelb M H. Methods. 1990;1:231–240. [Google Scholar]
- 24.Ohguro H, Rudnicka-Nawrot M, Buczylko J, Zhao X, Taylor K A, Walsh K A, Palczewski K. J Biol Chem. 1996;271:5215–5224. doi: 10.1074/jbc.271.9.5215. [DOI] [PubMed] [Google Scholar]
- 25.Green N M, Williams D S, Newton A C. J Biol Chem. 1997;272:10341–10344. doi: 10.1074/jbc.272.16.10341. [DOI] [PubMed] [Google Scholar]
- 26.Stoffel R M, Randall R R, Phemont R T, Lefkowitz R J, Inglese J. J Biol Chem. 1994;269:27791–27794. [PubMed] [Google Scholar]
- 27.Grunewald S, Haase W, Reilnder H, Michael H. Biochemistry. 1996;35:15149–15161. doi: 10.1021/bi9607564. [DOI] [PubMed] [Google Scholar]
- 28.DeCaluwe L L J, Van Oostrum J, Janssen J J M, DeGrip W J. Methods Neurosci. 1993;15:307–321. [Google Scholar]
- 29.Casey P J, Seabra M C. J Biol Chem. 1996;271:5289–5292. doi: 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- 30.Inglese J, Koch W J, Caron M G, Lefkowitz R J. Nature (London) 1992;359:147–150. doi: 10.1038/359147a0. [DOI] [PubMed] [Google Scholar]
- 31.Buss J E, Qilliam L A, Kato K, Casey P J, Wong S G, Clarke S, McCormick F, Bokoch G M, Der C J. Mol Cell Biol. 1991;11:1523–1530. doi: 10.1128/mcb.11.3.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalman V K, Erdman R A, Maltese W A, Robishaw J D. J Biol Chem. 1995;270:14835–14841. doi: 10.1074/jbc.270.24.14835. [DOI] [PubMed] [Google Scholar]