Abstract
We report evidence for proton-driven subunit rotation in membrane-bound FoF1–ATP synthase during oxidative phosphorylation. A βD380C/γC87 crosslinked hybrid F1 having epitope-tagged βD380C subunits (βflag) exclusively in the two noncrosslinked positions was bound to Fo in F1-depleted membranes. After reduction of the β–γ crosslink, a brief exposure to conditions for ATP synthesis followed by reoxidation resulted in a significant amount of βflag appearing in the β–γ crosslinked product. Such a reorientation of γC87 relative to the three β subunits can only occur through subunit rotation. Rotation was inhibited when proton transport through Fo was blocked or when ADP and Pi were omitted. These results establish FoF1 as the second example in nature where proton transport is coupled to subunit rotation.
FoF1–ATP synthases are found embedded in the membranes of mitochondria, chloroplasts, and bacteria, and are structurally and functionally conserved among species (1–5). During oxidative- and photo-phosphorylation, the synthases couple the movement of protons down an electrochemical gradient to the synthesis of ATP. The Fo sector is composed of membrane-spanning subunits (ab2c9–12 in Escherichia coli) that conduct protons across the membrane, whereas the F1 sector (α3β3γδɛ) is an extrinsic complex that contains the catalytic sites for ATP synthesis. F1 can be removed from the membrane in a soluble form that functions as an ATPase, and rebinding F1 to Fo in membranes restores the capacity to catalyze net ATP synthesis. A high-resolution structure of bovine F1 shows a hexamer of alternating α and β subunits surrounding a single γ subunit. The three catalytic sites of F1 are located on the three β subunits at α/β subunit interfaces (6).
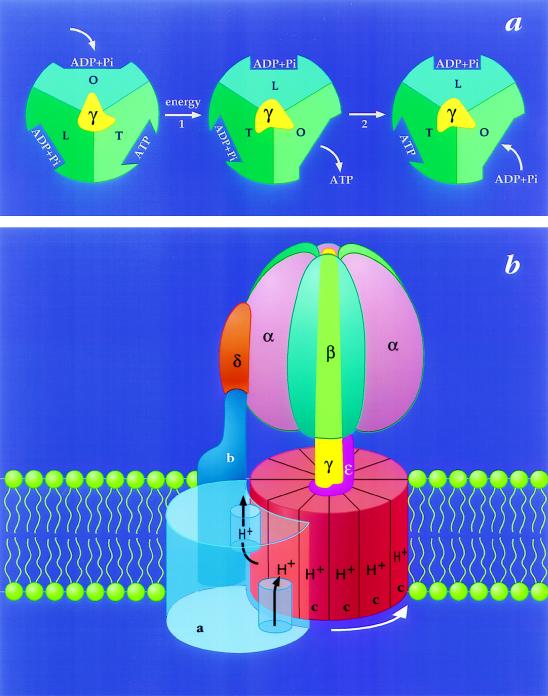
The model for energy coupling by FoF1–ATP synthases that has gained the most general support is called the binding change mechanism (7). According to this proposal, the major energy requiring step (Fig. 1a, step 1) is not the synthesis of ATP at catalytic sites, but rather the simultaneous and highly cooperative binding of substrates to, and release of products from, these sites (14, 15). Furthermore, it is proposed that these affinity changes are coupled to proton transport by the rotation of a complex of subunits that extends through FoF1. Rotation of the γ subunit in the center of F1 (Fig. 1a) is thought to deform the surrounding catalytic subunits to give the required binding changes (16), whereas rotation of the c-subunits relative to the single a-subunit in Fo (Fig. 1b) is believed to be required for completion of the proton pathway (8, 17, 18). The latter is analogous to the proton-driven subunit rotation that occurs within the bacterial flagellar motor (19).
Figure 1.
The binding change mechanism for FoF1 ATP synthases. This figure was adapted from ref. 8 and modified. (a) Looking up at F1 from the membrane. In step 1, the asymmetric γ subunit rotates 120° clockwise driving conformational changes in the three catalytic sites that alter their affinities for substrates and product. In this illustration, the catalytic sites remain stationary. In step 2, ATP forms spontaneously from tightly bound ADP and Pi. For additional details and alternative views see refs. 7, 9, and 10. (b) View from the side of FoF1. The a-subunit contains two partial channels, each in contact with a different side of the membrane. In order for a H+ to traverse the membrane it moves through one channel to the center of the membrane, binds to one of the c-subunits (at Asp-61), and then is carried to the other partial channel by rotation of the c-subunit complex. The c-subunits are anchored to γ (11), whereas the a-subunit is anchored through subunits b and δ to the periphery of the α3β3 hexamer (12, 13). Hence the rotation of c-subunits relative to the a-subunit in Fo will drive the rotation of γ relative to the α3β3 hexamer in F1.
Based on supportive evidence from several laboratories (6, 16, 20, 21), the rotary aspect of the binding change mechanism has remained a popular idea. However, a critical test for rotation only became possible recently. The crystal stucture of bovine mitochondrial F1 shows a specific interaction between a small α-helix of the γ subunit, which contains a Cys (E. coli γC87), and the “DELSEED” loop of one of the three β subunits (6). We substituted Cys for several different residues in this region of E. coli β (380DELSEED386) and found that the presence of an oxidant induced rapid and specific formation of a βD380C-γC87 disulfide bond in βD380C-F1 (8, 22). Using a dissociation/reassociation approach with the β-γ crosslinked βD380C-F1, we incorporated radiolabeled β subunits into the two noncrosslinked β subunit positions of F1. Following reduction of the crosslink and a short burst of ATP cleavage, radiolabeled and unlabeled β subunits in the hybrid F1 showed a similar capacity to form a disulfide bond with the γ subunit (8), indicating that γ rotates relative to β subunits during ATP hydrolysis. We then showed that hybrid F1 containing a βD380C-γC87 crosslink can be recoupled to Fo in F1-depleted membranes and we provided evidence that rotation of γ relative to β subunits also occurs during hydrolysis of ATP by FoF1 (23). Subsequently, additional evidence for subunit rotation during ATP hydrolysis was provided by using immobilized chloroplast F1 with a spectroscopic probe attached near the C terminus of the γ subunit. Recovery of polarized absorption after photobleaching was used to monitor rotational motion of γ during ATP hydrolysis by the tethered F1 (24). In a more recent study using immobilized bacterial F1, a fluorescent actin filament was attached to one end of the γ subunit and fluorescence microscopy was used to monitor its rotation during catalytic turnover (25). It was shown that MgATP could induce net unidirectional rotation of γ through many complete revolutions. However, studies thus far have not examined whether rotation of F1 subunits occurs in coupled FoF1 during the physiologically important reaction of ATP synthesis. We now extend our hybrid F1/crosslinking approach to provide the first clear indication that subunit rotation in FoF1 is an integral part of energy coupling during oxidative phosphorylation.
MATERIALS AND METHODS
Materials.
NADH, N,N′-dicyclohexylcarbodiimide (DCCD), carbonylcyanide p-trifluoro-methoxyphenylhydrazone (FCCP), N-ethylmaleimide (NEM), ATP, ADP, selenocystamine, and hexokinase were supplied by Sigma, 5,5′-dithiobis(2-nitrobenzoate) (DTNB) by Aldrich, DTT by American Bioanalytical (Natick, MA), lauryldimethylamine oxide (LDAO) by Calbiochem, [γ-32P]ATP by ICN, anti-Flag M2 antibody by Eastman Kodak, and anti-mouse IgG/alkaline phosphatase conjugate by Promega. Alkaline phosphatase color development reagents were from Bio-Rad, pyruvate kinase and lactate dehydrogenase from Boehringer Mannheim, and polyvinylidene difluoride (PVDF) membranes from NOVEX (San Diego). Other reagents and chemicals were the highest grade available.
Plasmids and E. coli Strains.
Plasmids p3U and pAU1, and mutants βD380C and βflagD380C/γC87S have been reported (22, 23). Mutant βD380C-F1 was expressed in strain JP17, which has a chromosomal deletion of most of the uncD gene coding for the β subunit (26). Mutant βflagD380C/γC87S-F1 was expressed in strain AN887, which has a Mu insertion that blocks expression of all unc genes from the chromosome (27).
Preparation of E. coli Membranes and Soluble F1.
Membranes were isolated and washed (28, 29) and soluble F1 was purified (8) as described. Membranes prepared from strain JP17 harboring pAU1 were depleted of F1 (28) with two additional washes with 10 mM Tris·acetate/1 mM EDTA, pH 7.5.
Preparation of Hybrid F1 and Reconstitution with F1-Depleted Membranes.
βD380C-F1 was treated with DTNB to induce disulfide bond formation between γC87 and the βD380C of one β subunit (8). The crosslinked enzyme and βflagD380C/γC87S-F1 were then treated under conditions that cause disassembly of subunits, mixed in a 1:1 ratio, and allowed to reassemble as hybrid F1 complexes as described previously (8). F1 hybrids that contain βD380C-γC87 can contain βflagD380C subunits only in the two noncrosslinked β positions. F1 hybrids containing the γC87S subunit can contain βflagD380C in any of the three β positions, but these hybrids will be incapable of forming a β–γ disulfide bond due to the γC87S mutation. However, oxidation of F1 containing βD380C and γC87S can yield low levels of a 101-kDa crosslinked product previously identified as a β–β dimer (ref. 8; see Fig. 2). Hybrid F1 (0.5 mg/ml) was recoupled to Fo in F1-depleted membranes (2 mg protein per ml) by incubation in TMg buffer (50 mM Tris-acetate/10 mM MgS04, pH 7.5) at 30°C for 15 min. Unbound F1 was removed by centrifuging at 100,000 × g in a Beckman Airfuge for 1 min. The membrane pellet was resuspended and washed twice with TSGMg buffer (50 mM Tris-acetate/250 mM sucrose/50 mM glucose/5 mM MgSO4, pH 7.4) and finally resuspended in the same buffer at 4 mg protein per ml.
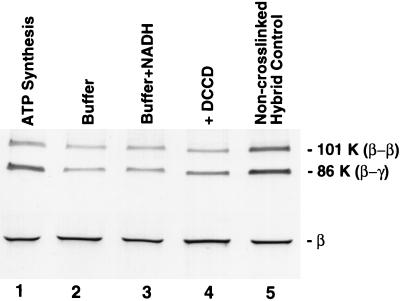
Figure 2.
Rotation of subunits in E. coli FoF1 under ATP synthesis conditions. Hybrid F1 was prepared so that complexes containing a βD380C/γC87 crosslink contained βflagD380C subunits only in the two noncrosslinked β positions. After rebinding hybrid F1 to F1-depleted membranes, aliquots (1 mg total protein per ml) were exposed to different conditions (described below) for 30 sec at 23°C, 20 mM DTT and 2 mM selenocystamine were added to rapidly reduce any disulfide bonds, and the membranes were incubated for an additional 30 sec before passage through a Sephadex G50-F centrifuge column (30), equilibrated with TSGMg buffer. Disulfide bond formation was induced as each sample eluted from the column into a tube containing DTNB (0.2 mM final concentration). An aliquot of each oxidized sample (equivalent to 0.4 μg of βflag-F1) was denatured under nonreducing conditions and used for SDS/PAGE and immunoblotting. The blot above shows bands containing the βflagD380C subunit. As shown in lanes 1–4, membranes were exposed to the following conditions: lane 1, conditions for ATP synthesis (TSGMg buffer containing 4 mM ADP/20 mM Pi/2 mM NADH/165 units hexokinase/ml); lane 2, same as for lane 1 except that ADP, Pi, and NADH were omitted; lane 3, same as for lane 1 except that ADP and Pi were omitted; lane 4, same as for lane 1 except that F1-depleted membranes were pretreated with DCCD prior to reconstitution with hybrid F1 (see Materials and Methods). For the “noncrosslinked hybrid” control in lane 5, hybrid F1 was prepared from dissociated subunits without prior crosslinking of γC87 to a βD380C subunit. Thus, epitope-tagged β subunit could assemble randomly in the three β positions around the γC87 subunit. After rebinding to membranes and exposure to ATP synthesis conditions (as for lane 1), reoxidation of this sample provided a measure of the amount of βflagD380C trapped in the β–γ crosslinked product when the orientation of γC87 is random relative to the three β positions.
DCCD Modification of Fo.
F1-depleted membranes (2 mg protein per ml) were incubated with 100 μM DCCD in TMg buffer at 0°C for 20 hr with slow stirring. Membranes were sedimented by centrifuging at 100,000 × g in an Airfuge for 1 min, washed twice with TMg buffer, and resuspended in the same buffer at 4 mg protein per ml.
ATP Synthesis Assay.
The ATP synthesis activity of reconstituted membranes was determined as the rate of glucose-6-32P formation. Each 250-μl aliquot contained 25 μg of membrane protein in TSGMg buffer with 4 mM ADP, 20 mM 32Pi, and 40 units of hexokinase. After preincubation at 23°C for 5 min, ATP synthesis was initiated by adding NADH (2 mM final concentration). Each timed sample was quenched by adding 25 μl of 5.5 M perchloric acid. Pi was precipitated (31) and glucose-6-32P in the supernatant was determined by Cerenkov counting. No significant ATP synthesis was detected in the presence of uncoupler (55 μM FCCP), and pretreatment of membranes with DCCD before reconstitution with F1 inhibited ATP synthesis by 92%. In a control experiment for the hexokinase trap, no detectable 32Pi was produced when 1 μM [γ-32P]ATP was incubated with 50 units of hexokinase plus 100 μg of reduced, reconstituted membranes in 1 ml of TSGMg buffer.
Electrophoresis and Immunological Detection of Proteins Containing the Flag Epitope.
SDS/PAGE (32) was performed on 4–15% acrylamide gradient gels (Ready gels, Bio-Rad). For nonreducing conditions, samples were denatured in the presence of 0.5 mM NEM instead of 2-mercaptoethanol. Proteins were transferred from the gel to a PVDF membrane at 250 mA for 90 min in 25 mM Tris/192 mM glycine/10% methanol/0.005% SDS (33). The blotted membrane was blocked with 5% nonfat, dried milk in TBST (10 mM Tris⋅HCl/150 mM NaCl/0.05% Tween-20, pH 8.0) and incubated with anti-Flag M2 antibody (0.4 μg/ml in TBST), then rinsed three times with TBST + 0.1 M NaCl. Bands containing the Flag epitope were then visualized colorimetrically using a secondary-antibody/alkaline phosphatase conjugate and quantitated using a Hewlett–Packard scanner (model C2501) and densitometry software from Biosoft (Milltown, NJ). Known amounts of βflag-F1 were run on a separate gel in the presence of 2-mercaptoethanol, blotted, and the Flag epitope in the β-subunit band of each sample was quantitated as described above. The results showed a range for which densitometry had a linear dependence on the amount of protein added. This provided a standard curve for determining the total βflag in aliquots of each experimental sample run on a preliminary reducing gel, so that aliquots containing identical amounts of βflag could be added to each lane of a nonreducing gel.
Protein Assay.
Protein concentrations were determined by a modified Lowry assay (34).
RESULTS AND DISCUSSION
F1-depleted membranes were reconstituted with hybrid F1 that contained a βD380C-γC87 disulfide crosslink with epitope-tagged βflagD380C subunits only at the two noncrosslinked β positions. Following reduction of the intersubunit disulfide, these reconstituted membranes were capable of catalyzing electron transport-driven ATP synthesis at a rate of 87 nmol⋅min−1·mg−1 membrane protein. To test for subunit rotation during ATP synthesis, the reconstituted membranes were reduced, incubated briefly under conditions for ATP synthesis, and then reoxidized. To preclude any contribution of ATP hydrolysis to subunit rotation under these conditions, hexokinase and glucose were present to trap ATP synthesized by FoF1 (see Materials and Methods). In the absence of subunit rotation, γC87 would be expected to reform a disulfide link to the original βD380C and thus the Flag epitope would not be detected in the β–γ crosslinked product (an 86-kDa band) on an immunoblot. However, if subunit rotation occurred during ATP synthesis, as predicted by the binding change mechanism (Fig. 1), then βflagD380C would be properly aligned to crosslink to γC87 in two-thirds of the FoF1 hybrid molecules containing γC87. Fig. 2 shows that exposure to ATP synthesis conditions resulted in a significant amount of Flag epitope in the β–γ band (lane 1), demonstrating that subunit rotation had occurred. In contrast, when ADP, Pi, and NADH were omitted, much less βflag was detected in the β–γ band (lane 2).
The binding change mechanism stipulates that ADP and Pi must bind at a catalytic site on F1 before protons can be transported through Fo down an electrochemical gradient. If these two events were not sequentially linked and proton transport could drive subunit rotation when catalytic sites were empty, energy would be wasted. The existence of this obligatory coupling is clearly demonstrated in Fig. 2 where, in the absence of ADP and Pi, the presence of NADH resulted in little βflag in the β–γ band (lane 3). This indicates that an electrochemical gradient alone is not sufficient to promote subunit rotation in FoF1; ADP and Pi must also be present.
Transport of protons through Fo can be blocked by covalent modification of one or more c-subunits with DCCD, and this also blocks ATP synthesis or hydrolysis by FoF1 (35). When F1-depleted membranes were treated with DCCD prior to reconstitution with hybrid F1, exposure of reconstituted membranes to ATP synthesis conditions, as for lane 1, showed considerably reduced amounts of βflag in the β–γ band (Fig. 2, lane 4), indicating that subunit rotation in F1 is blocked by modifying Fo with DCCD.
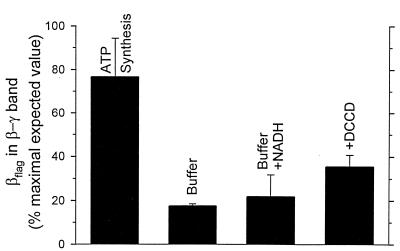
The amount of Flag epitope observed in the β–γ crosslinked product was quantitated and compared with that expected if the orientation of γ was randomized during turnover relative to the three surrounding β subunits and if 100% of the FoF1–ATP synthase complexes present in the membranes were catalytically active during the brief episode of ATP synthesis (Fig. 3). Conditions for ATP synthesis yielded 76% of this expected value, a reasonable correlation considering the probability that a fraction of FoF1 is bound to leaky or uncoupled membranes and would thus remain inactive and immobile during the experiment. In contrast, controls lacking NADH and/or ADP and Pi had only 17–21% of the expected value (Fig. 3, Buffer and Buffer+NADH). Furthermore, when F1-depleted membranes were treated with DCCD prior to binding hybrid F1, exposure to ATP synthesis condition yielded only 35% of the expected βflag in the 86-kDa band (Fig. 3, +DCCD). This emphasizes the tight functional linkage of Fo to subunit rotation in F1, and supports the plausibility of subunit rotation in Fo.
Figure 3.
Quantitation of the Flag epitope appearing in the β–γ crosslinked product. For immunoblots as in Fig. 2, the amount of βflag in the 86-kDa band of each sample was determined by scanning densitometry. The value obtained for a “noncrosslinked hybrid” control (see Fig. 2, lane 5) was multiplied by 0.8 to correct for its greater βflag content compared with the crosslinked hybrid F1 (Fig. 2, lanes 1–4). This value was set to 100%, representing the amount of βflag expected in the β–γ crosslinked product if each β subunit has an equal opportunity to crosslink to γ following reduction and exposure to conditions for ATP synthesis. Bars are labeled to indicate conditions as described for Fig. 2. Data from three separate experiments were averaged and the error bars represent standard deviations.
Previously, the bacterial flagellar motor was the only macromolecular complex known to use an electrochemical proton gradient to drive subunit rotation (19). The results presented in Figs. 2 and 3 provide strong support for the conclusion that the FoF1–ATP synthase is a second example. The recent visual observation of net unidirectional rotation during ATP hydrolysis by F1 (25) suggests the sequential participation of all three catalytic sites and that the direction of rotation will depend on whether it is driven by ATP hydrolysis or proton transport. In view of the close evolutionary relationship between FoF1 synthases and the V0V1 ATPases (36), it seems likely that the acidification of vacuoles also requires subunit rotation. In addition, it is becoming apparent that RecA (37, 38) and DNA and RNA helicases (38–40) may operate by a rotary-type mechanism. In analogy to the rotation of γ within the α3β3 hexamer of F1, a single strand of DNA or RNA is thought to rotate within a hexamer of subunits which show clear structural homologies with the F1-β subunit (6, 41–43). Whereas RecA and the helicases use ATP hydrolysis to drive rotation and the flagellar motor uses an electrochemical gradient, FoF1-ATP synthases appear to be unique in that they can use either. Thus, further analysis of rotational coupling in FoF1 may provide useful insights for these diverse systems.
Acknowledgments
We wish to thank Marcus L. Hutcheon for excellent technical assistance. This work was supported by Research Grant GM23152 from the National Institutes of Health, U.S. Public Health Service.
ABBREVIATION
- DCCD
N,N′-dicyclohexylcarbodiimide
References
- 1.Capaldi R A, Aggeler R, Wilkens S, Gruber G. J Bioenerg Biomembr. 1996;28:397–401. doi: 10.1007/BF02113980. [DOI] [PubMed] [Google Scholar]
- 2.Cross R L, Duncan T M. J Bioenerg Biomembr. 1996;28:403–408. doi: 10.1007/BF02113981. [DOI] [PubMed] [Google Scholar]
- 3.Deckers-Hebestreit G, Altendorf K. Annu Rev Microbiol. 1996;50:791–824. doi: 10.1146/annurev.micro.50.1.791. [DOI] [PubMed] [Google Scholar]
- 4.Howitt S M, Rodgers J W, Hatch L P, Gibson F, Cox G B. J Bioenerg Biomembr. 1996;28:415–420. doi: 10.1007/BF02113983. [DOI] [PubMed] [Google Scholar]
- 5.Nakamoto R K. J Membr Biol. 1996;151:101–111. doi: 10.1007/s002329900061. [DOI] [PubMed] [Google Scholar]
- 6.Abrahams J P, Leslie A G, Lutter R, Walker J E. Nature (London) 1994;370:621–628. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- 7.Boyer P D. Biochim Biophys Acta. 1993;1140:215–250. doi: 10.1016/0005-2728(93)90063-l. [DOI] [PubMed] [Google Scholar]
- 8.Duncan T M, Bulygin V V, Zhou Y, Hutcheon M L, Cross R L. Proc Natl Acad Sci USA. 1995;92:10964–10968. doi: 10.1073/pnas.92.24.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou J-M, Boyer P D. J Biol Chem. 1993;268:1531–1538. [PubMed] [Google Scholar]
- 10.Weber J, Wilke-Mounts S, Lee R S-F, Grell E, Senior A E. J Biol Chem. 1993;268:20126–20133. [PubMed] [Google Scholar]
- 11.Watts S D, Zhang Y, Fillingame R H, Capaldi R A. FEBS Lett. 1995;368:235–238. doi: 10.1016/0014-5793(95)00658-v. [DOI] [PubMed] [Google Scholar]
- 12.Wilkens S, Dunn S D, Capaldi R A. FEBS Lett. 1994;354:37–40. doi: 10.1016/0014-5793(94)01059-5. [DOI] [PubMed] [Google Scholar]
- 13.Lill H, Hensel F, Junge W, Engelbrecht S. J Biol Chem. 1996;271:32737–32742. doi: 10.1074/jbc.271.51.32737. [DOI] [PubMed] [Google Scholar]
- 14.Boyer P D, Cross R L, Momsen W. Proc Natl Acad Sci USA. 1973;70:2837–2839. doi: 10.1073/pnas.70.10.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kayalar C, Rosing J, Boyer P D. J Biol Chem. 1977;252:2486–2491. [PubMed] [Google Scholar]
- 16.Boyer P D, Kohlbrenner W E. In: Energy Coupling in Photosythesis. Selman B, Selman-Reiner S, editors. New York: Elsevier/North Holland; 1981. pp. 231–240. [Google Scholar]
- 17.Vik S B, Antonio B J. J Biol Chem. 1994;269:30364–30369. [PubMed] [Google Scholar]
- 18.Hatch L P, Cox G B, Howitt S M. J Biol Chem. 1995;270:29407–29412. doi: 10.1074/jbc.270.49.29407. [DOI] [PubMed] [Google Scholar]
- 19.Macnab R M. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. 2nd Ed. Neidhardt F C, editor. Vol. 1. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 123–145. [Google Scholar]
- 20.Kandpal R P, Boyer P D. Biochim Biophys Acta. 1987;890:97–105. doi: 10.1016/0005-2728(87)90073-9. [DOI] [PubMed] [Google Scholar]
- 21.Gogol E P, Johnston E, Aggeler R, Capaldi R A. Proc Natl Acad Sci USA. 1990;87:9585–9589. doi: 10.1073/pnas.87.24.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duncan T M, Zhou Y, Bulygin V, Hutcheon M L, Cross R L. Biochem Soc Trans. 1995;23:736–741. doi: 10.1042/bst0230736. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Duncan T M, Bulygin V V, Hutcheon M L, Cross R L. Biochim Biophys Acta. 1996;1275:96–100. doi: 10.1016/0005-2728(96)00056-4. [DOI] [PubMed] [Google Scholar]
- 24.Sabert D, Engelbrecht S, Junge W. Nature (London) 1996;381:623–625. doi: 10.1038/381623a0. [DOI] [PubMed] [Google Scholar]
- 25.Noji H, Yasuda R, Yoshida M, Kinosita K., Jr Nature (London) 1997;386:299–302. doi: 10.1038/386299a0. [DOI] [PubMed] [Google Scholar]
- 26.Lee R S, Pagan J, Wilke-Mounts S, Senior A E. Biochemistry. 1991;30:6842–6847. doi: 10.1021/bi00242a006. [DOI] [PubMed] [Google Scholar]
- 27.Gibson F, Downie J A, Cox G B, Radik J. J Bacteriol. 1978;134:728–736. doi: 10.1128/jb.134.3.728-736.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senior A E, Fayle D R H, Downie J A, Gibson F, Cox G B. Biochem J. 1979;180:111–118. doi: 10.1042/bj1800111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wise J G. J Biol Chem. 1990;265:10403–10409. [PubMed] [Google Scholar]
- 30.Penefsky H S. J Biol Chem. 1977;252:2891–2899. [PubMed] [Google Scholar]
- 31.Sugino Y, Miyoshi Y. J Biol Chem. 1964;239:2360–2364. [PubMed] [Google Scholar]
- 32.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson G L. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 35.Hermolin J, Fillingame R H. J Biol Chem. 1989;264:3896–3903. [PubMed] [Google Scholar]
- 36.Nelson N, Taiz L. Trends Biochem Sci. 1989;14:113–116. doi: 10.1016/0968-0004(89)90134-5. [DOI] [PubMed] [Google Scholar]
- 37.Bedale W, Cox M M. J Biol Chem. 1996;271:5725–5732. doi: 10.1074/jbc.271.10.5725. [DOI] [PubMed] [Google Scholar]
- 38.Yu X, Egelman E H. Nat Struct Biol. 1997;4:101–104. doi: 10.1038/nsb0297-101. [DOI] [PubMed] [Google Scholar]
- 39.Doering C, Ermentrout B, Oster G. Biophys J. 1995;69:2256–2267. doi: 10.1016/S0006-3495(95)80096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hingorani M M, Washington M T, Moore K C, Patel S S. Proc Natl Acad Sci USA. 1997;94:5012–5017. doi: 10.1073/pnas.94.10.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dombrowski A J, LaDine J R, Cross R L, Platt T. J Biol Chem. 1988;263:18810–18815. [PubMed] [Google Scholar]
- 42.Story R M, Weber I T, Steitz T A. Nature (London) 1992;355:318–325. doi: 10.1038/355318a0. [DOI] [PubMed] [Google Scholar]
- 43.Subramanya H S, Bird L E, Branningan J A, Wigley D B. Nature (London) 1996;384:379–383. doi: 10.1038/384379a0. [DOI] [PubMed] [Google Scholar]