Abstract
LXRα is an orphan member of the nuclear hormone receptor superfamily that displays constitutive transcriptional activity. We reasoned that this activity may result from the production of an endogenous activator that is a component of intermediary metabolism. The use of metabolic inhibitors revealed that mevalonic acid biosynthesis is required for LXRα activity. Mevalonic acid is a common metabolite used by virtually all eukaryotic cells. It serves as a precursor to a large number of important molecules including farnesyl pyrophosphate, geranylgeranyl pyrophosphate, cholesterol, and oxysterols. Inhibition of LXRα could be reversed by addition of mevalonic acid and certain oxysterols but not by other products of mevalonic acid metabolism. Surprisingly, the constitutive activity of LXRα was inhibited by geranylgeraniol, a metabolite of mevalonic acid. These findings suggest that LXRα may represent a central component of a signaling pathway that is both positively and negatively regulated by multiple products of mevalonate metabolism.
Nuclear hormone receptors compose a superfamily of ligand-modulated transcription factors (1, 2). Included in this superfamily are the receptors for steroid hormones, retinoids, vitamin D, and thyroid hormone. Over the past decade, additional members of this family have been identified that lack known ligands. The implicit hope is that orphan receptors can be used to uncover signaling molecules that regulate novel physiologic networks. Indeed, orphan receptors have led to the identification of fatty acids as nutritional ligands for peroxisome proliferato activated receptor (PPAR)α/δ (3, 4), 15-deoxy-Δ12,14-prostaglandin J2 as an adipogenic PPARγ ligand (5, 6), and farnesol metabolites as activators of farnesoid X receptor (FXR) (7).
In the presence of their specific ligands, nuclear hormone receptors alter the transcriptional rate of specific genes. These target genes are determined by selective interactions between the conserved DNA binding domain (Fig. 1A) and their cognate response elements. However, ligand-dependent regulation of these genes is mediated by a complex C-terminal region, the ligand binding domain (LBD) (Fig. 1A). In addition to binding ligand, this region contains several embedded functional activities including a dimerization interface, a co-repressor interaction domain, and a transcriptional activation function (1). These activities are tightly integrated. For example, dimerization can lead to allosteric changes that promote high affinity ligand binding (8). Once ligand is bound, an additional conformation change occurs that both releases the corepressor and promotes interaction with coactivators. These transcriptional coactivators can modify chromatin structure as well as the basal transcriptional machinery. Thus, ligand binding switches the transcriptional status of the LBD from a repressed to an active state.
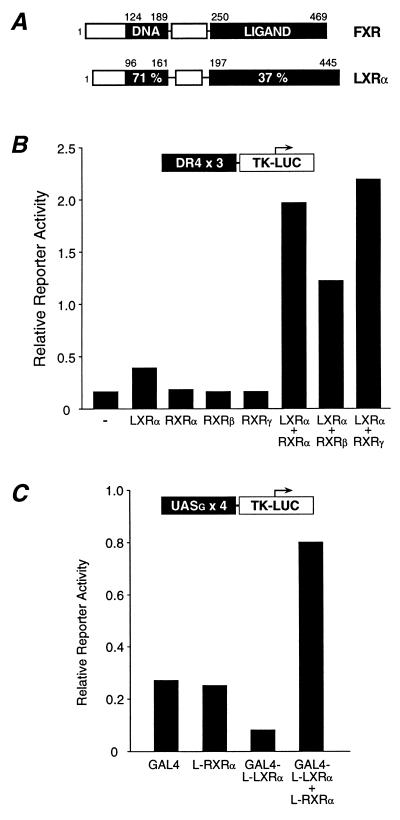
Figure 1.
The LXRα–RXR heterodimer displays constitutive activity. (A) Schematic diagram illustrating the amino acid sequence identity between FXR and LXRα. (B) The LXRα–RXR heterodimer is constitutively active in a cell-based transient transfection assay. CV-1 cells were transfected with the DR4x3 TK-Luc reporter and the indicated expression vectors. Luciferase and β-galactosidase activity were assayed 40 h posttransfection. (C) Constitutive activity is mediated by the LBDs of LXRα and RXR. Cells were transfected with the GAL4 reporter and vectors expressing the GAL4 DBD, the RXRα LBD, and the LBD of LXRα linked to the GAL4-DBD.
Unlike the classical nuclear receptors, several orphan nuclear receptors have been described that are active in the apparent absence of ligand. Included among these orphan receptors are rat RLD-1 (9) and its human ortholog, LXRα (10). Previous studies have shown that both LXRα and RLD-1 form heterodimers with the 9-cis retinoic acid receptor (RXR) that binds response elements composed of a direct repeat separated by four nucleotides (DR4) (9, 10). When bound to this response element, the LXRα–RXR heterodimer displays substantial constitutive activity (9). Similar results have been reported for LXRβ (11–13).
It remains unclear how LXRα reaches its transcriptionally active state. One possibility is that LXRα is in fact a ligand-activated receptor with the putative ligand produced endogenously within cells. Ligands/activators for several ex-orphan receptors including PPAR and FXR are products of intermediary metabolism, so we decided to examine whether the constitutive activity of LXRα required endogenous lipid metabolism. We found that inhibitors of mevalonic acid (MVA) biosynthesis repressed LXRα activity and that this repression was relieved by addition of MVA and oxysterols including 20(S)-hydroxycholesterol (OH-Chol) and 22(R)-OH-Chol but not by other products of MVA metabolism. In contrast, geranylgeraniol (GG-OH), another metabolite of MVA, was itself an inhibitor of LXRα. These findings suggest that LXRα may represent a central component of a signaling pathway that is differentially regulated by multiple products of MVA metabolism.
MATERIALS AND METHODS
Cell Culture and Transfection.
CV-1 cells were grown and transfected as described (14) in media containing DMEM supplemented with 10% lipid-stripped fetal bovine serum. Reporter constructs (300 ng/105 cells), cytomegalovirus- driven receptor (50–100 ng/105 cells), and β-galactosidase expression vectors (500 ng/105 cells) were added as indicated. Expression vectors contained the cytomegalovirus IE promoter/enhancer (pCMX) upstream of wild-type human LXRα, human RXRα, rat RXRβ, or mouse RXRγ. GAL4 fusions contained the human LXRα LBD (amino acids 166–447) fused to the C-terminal end of the yeast GAL4 DNA binding domain (amino acids 1–147). The human RXRα LBD expression construct contained the human RXRα LBD (amino acids 203–462) (14). The LXRα reporter construct, DR4x3 TK-LUC, contained three copies of the mouse mammary tumor virus-derived response element linked upstream of the herpes virus thymidine kinase promoter. Other expression vectors and reporter constructs have been described (14). Cells were exposed to the compounds for 40 h then harvested and assayed for luciferase and β-galactosidase activities. Each experimental point was performed in triplicate and varied by less than 10%. Luciferase activity was normalized to the β-galactosidase control and plotted as relative reporter activity or as fold-activation relative to untreated cells. Each experiment was repeated three or more times with similar results.
Electrophoretic Mobility Shift Assays.
Proteins were translated in a rabbit reticulocyte lysate system (TNT, Promega) and incubated for 30 min at room temperature with 100,000 cpm of Klenow-labeled probes in 10 mM Tris (pH 8), 100 mM KCl, 6% glycerol, 0.05% Nonidet P-40, 1 mM DTT, and 100 ng/μl poly(dI·dC) and then electrophoresed through a 5% nondenaturing polyacrylamide gel in 0.5× TBE (45 mM Tris-base/45 mM boric acid/1 mM EDTA). The LXRα (10) and Nurr1 (15) response elements were as described.
Reagents and Chemical Synthesis.
(20R, 22R)-di-OH-Chol and (20S, 22S)-di-OH-Chol were synthesized as follows. [20(22)E]-3β-acetoxycholesta-5,20(22)-diene was prepared from pregnenolone (Sigma) by modifications of published methods (16, 17): mp 124–125°C [literature 124.5 ± 0.5°C (16)]; single component on GC and Ag+-HPLC (18), characterized by 1H and 13C NMR and GC—MS. Osmium-catalyzed asymmetric dihydroxylation (100 mg) using AD-mix-β (Aldrich) under conditions described (19) gave a 55:45 mixture (67% yield) of the 3β-acetates of (20R, 22R)-cholest-5-ene-3β,20,22-triol [(20R, 22R)-di-OH-Chol, I] and (20S, 22S)-cholest-5-ene-3β,20,22-triol [(20S, 22S)-di-OH-Chol, II]. The acetates of the two triols were resolved by semi-preparative Ag+-HPLC (18) using 7.5% acetone in hexane as the solvent. The (20R, 22R)-isomer, mp 196–197°C [literature 192–194°C (20)] and the (20S, 22S)-isomer (obtained as a glassy material) were characterized by MS and by 1H NMR: acetate of I, 0.895 (H-18), 0.897 (H-26), 0.910 (H-27), 1.027 (H-19), 1.217 (H-21), 3.387 (H-22S), 4.605 (H-3), and 5.377 (H-6); acetate of II, 0.881 (H-18), 0.904 (H-26), 0.909 (H-27), 1.022 (H-19), 1.064 (H-21), 3.728 (H-22R), 4.604 (H-3), and 5.375 (H-6). Saponification of their 3β-acetate esters gave I (84% yield), mp 176–178°C [literature 178–180°C (21), 179–180°C (22)] and II (82% yield), mp 168–170°C [literature 169–171°C (22)]. The triols I and II, single components on thin-layer chromatography and HPLC, were further characterized by MS, GC—MS (trimethylsilyl ether), and 1H-NMR: I, 0.896 (H-18), 0.897 (H-26), 0.910 (H-27), 1.017 (H-19), 1.218 (H-21), 3.388 (H-22S), 3.530 (H-3), and 5.355 (H-6); and II, 0.883 (H-18), 0.902 (H-26), 0.909 (H-27), 1.011 (H-19), 1.064 (H-21), 3.727 (H-22R), 3.530 (H-3), and 5.352 (H-6).
All other MVA products and mevastatin were purchased from Cayman Chemicals (Ann Arbor, MI), Research Plus (Bayonne, NJ), Steraloids (Wilton, NH), or Sigma. LG268 and lovastatin were obtained from Ligand Pharmaceuticals (La Jolla, CA) and Merck, respectively.
RESULTS
Constitutive Activation by LXRα Is Mediated by the LBD.
Previous studies have shown that LXRα-RXR heterodimers exhibit constitutive activity when assayed on a DR4 response element (9). Consistent with these results, we find that LXRα displays minimal activity when expressed in CV-1 cells containing endogenous RXR. However, coexpression of LXRα with exogenous RXR-α, -β, or -γ results in a significant enhancement of constitutive activity (Fig. 1B). In principle, this constitutive activity could be mediated by a ligand-independent transactivation domain that has been identified in the N terminus of other nuclear receptors (23, 24). Alternatively, an endogenous ligand may activate the LXRα–RXR heterodimer. To distinguish among these possibilities, we asked whether the observed constitutive activity could be localized to the LBD. Thus, a chimeric protein containing the LBD of LXRα was fused to the DNA binding domain of the yeast transcription factor GAL4. Unexpectedly, GAL4-LXRα acted as a transcriptional suppressor (Fig. 1C). However, coexpression of GAL4-LXRα with the LBD of RXRα resulted in apparent constitutive activity (Fig. 1C). This indicates that the constitutive activity of the LXRα–RXR complex requires the LBDs of both receptors and is consistent with the possibility that the LXRα–RXR heterodimer is activated by an endogenous ligand.
Constitutive Activation by LXRα Requires MVA Biosynthesis.
If an endogenous ligand is activating LXRα, it is presumably recognizing the heterodimeric complex. A requirement of heterodimer formation for ligand responsiveness has been demonstrated for the insect ecdysone receptor and for mammalian FXR (7). Of interest, sequence comparison between the LBD of LXRα and other nuclear receptors indicates that this receptor is most closely related to mammalian FXR (Fig. 1A and data not shown). We have shown previously that the FXR–RXR heterodimer is responsive to farnesol, a product of MVA metabolism (Fig. 3A), so we wondered whether other endogenous products of MVA metabolism could be responsible for constitutive activation of the LXRα.
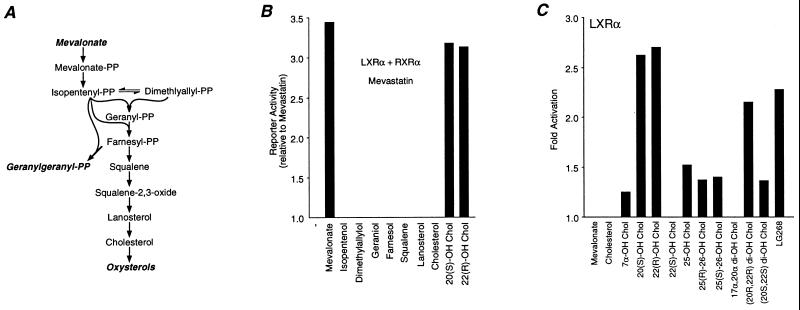
Figure 3.
Activation of LXRα by-products of MVA metabolism. (A) Schematic illustration of the MVA biosynthetic pathway. (B) LXRα is activated by MVA and oxysterols. Cells were transfected as in Fig. 2 and then treated with 7.5 μM mevastatin in the presence of 200 μM MVA, isopentenol, or dimethylallylol; 50 μM geraniol, farnesol, or squalene; 25 μM lanosterol and Chol; 10 μM 20(S)-OH-Chol or 22(R)-OH-Chol. (C) Activation of LXRα by oxysterols. Cells were transfected with the DR4x3 reporter and an LXR expression vector and then treated with 10 μM of the indicated oxysterol or 100 nM LG268.
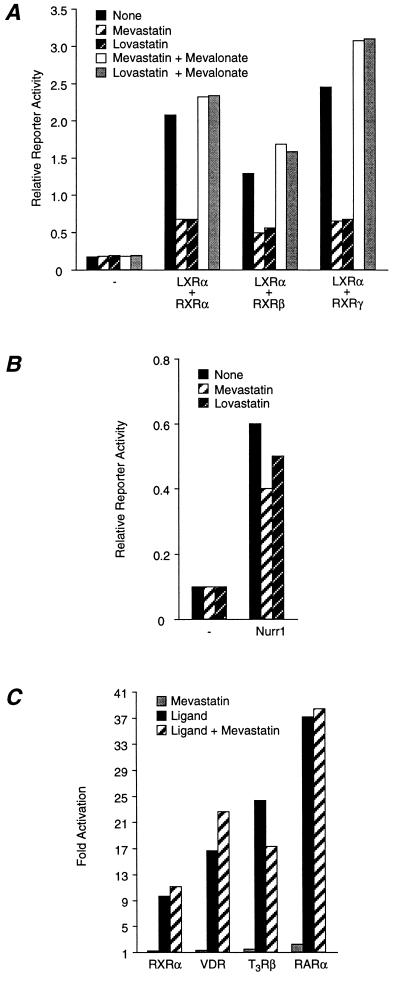
To address this possibility, we sought to block endogenous MVA production with inhibitors of 3-hydroxy-3-methylglutaryl-CoA (HMG–CoA) reductase, the rate-limiting enzyme in the biosynthesis of MVA (25). As seen in Fig. 2A, the constitutive activity of the LXRα–RXR heterodimer is dramatically reduced when HMG–CoA reductase is inhibited with mevastatin (7.5 μM) or lovastatin (7.5 μM). Inhibition was due to decreased MVA production because transcriptional activity could be restored by the addition of exogenous MVA (200 μM) (Fig. 2A). Inhibition was specific; mevastatin and lovastatin had little or no effect on the basal activity of the reporter or on the constitutive activity of another orphan receptor, Nurr1 (Fig. 2B). Similarly, inhibition of HMG–CoA reductase had little effect on ligand-dependent activation of classical nuclear receptors including RXR, vitamin D receptor, thyroid hormone receptor (T3R), or the all-trans retinoic acid receptor (RAR) (Fig. 2C). Taken together, these data suggest that the constitutive activity of LXRα is mediated by MVA or a MVA-derived metabolite.
Figure 2.
The constitutive activity of LXRα–RXR requires MVA biosynthesis. (A) Inhibition of constitutive activity by HMG–CoA reductase inhibitors. CV-1 cells were transfected as in Fig. 1B and treated with 7.5 μM mevastatin, 7.5 μM lovastatin, or 200 μM MVA. (B) MVA is not required for the constitutive activity of Nurr1. Cells were transfected as indicated and then incubated with 7.5 μM mevastatin or 7.5 μM lovastatin. (C) Mevastatin does not inhibit ligand-dependent activation by nuclear hormone receptors. Cells were transfected with TK-Luc-based reporter constructs and receptor expression vectors and then treated with appropriate ligands (100 nM) as follows: CRBPII/hRXRα/LG268; SPP1x3/hVDR/1,25-di-OH-VD3; MLVx2/hTRβ/l-triiodothyronine and DR5x2/hRARα/Am580.
MVA and Oxysterols Activate LXRα.
Fig. 3A illustrates the major pathways of MVA metabolism (25). MVA undergoes two rounds of phosphorylation and is then converted to the 5-carbon isoprenoid isopentenyl-PP and its isomer dimethylallyl-PP. An isopentenyl group is then sequentially added to dimethylallyl-PP to produce the 10-, 15- and 20-carbon isoprenoids geranyl-PP, farnesyl-PP, and GG-PP, respectively. In an attempt to identify a MVA metabolite responsible for activation of LXRα, we inhibited MVA production with mevastatin and then treated cells with products of MVA metabolism. Because isoprenyl-PPs may not readily enter cells, we used their corresponding isoprenyl alcohols (50 μM) because they can enter cells and are presumably converted to their PP derivatives (26–28). Although MVA was able to restore the transcriptional activity of LXRα, other isoprenpoids in the pathway were inactive (Fig. 3B). This implies that the endogenous activator of LXRα lies between MVA and isopentenyl-PP, suggesting that MVA itself, MVA-P, MVA-PP, or perhaps a yet to be described MVA metabolite is an endogenous activator of LXRα.
In addition to the isoprenyl-PPs, the MVA pathway gives rise to a large number of other compounds, including oxysterols (25) (Fig. 3A). Surprisingly, we found that specific oxysterols, including 20(S)-OH-Chol (20α-OH-CHOL, 10 μM) and 22(R)-OH-Chol (10 μM) were also able to activate LXRα in the presence of mevastatin (Fig. 3B), suggesting that they also modulate LXRα.
We next tested whether exogenous MVA or oxysterols activated LXRα under conditions in which HMG–CoA reductase remains active. However, when both LXRα and RXR were coexpressed, the resulting constitutive activity (Fig. 1B) could potentially mask further activation by the test compounds. To minimize this possibility, assays were performed in RXR-expressing cells transfected with LXRα alone, conditions that result in minimal constitutive activity (Fig. 1B). Under these conditions (Fig. 3C), MVA did not further activate LXRα. In contrast, 20(S)-OH-Chol (10 μM) and 22(R)-OH-Chol (10 μM) remained active, and the extent of activation by these oxysterols was as high as or greater than that observed with the RXR-selective ligand LG268 (Fig. 3C). Activation by 20(S)-OH-Chol and 22(R)-OH-Chol was specific because these compounds had no effect on nuclear receptors with known ligands (data not shown). Moreover, other oxysterols, including 7α-OH-Chol, 25-OH-Chol, 26-OH-Chol, and 17α,20α-di-OH-Chol, were inactive or weakly activated LXRα. Similar activation by oxysterols was reported recently (29, 30). This hierarchy of response is distinct from that observed with the sterol-response element binding protein (31, 32), suggesting that LXRα defines a unique oxysterol response pathway.
The data in Fig. 3C suggest that hydroxylation of Chol at the 20 and 22 positions results in the most active oxysterols. In addition, the stereochemistry around the 22 position appeared crucial as 22(R)-OH-Chol was active while the 22(S) isomer was inactive. This raised the possibility that (20R, 22R)-di-OH-Chol may be a more effective activator of LXRα. To test this possibility, we synthesized (20R, 22R)-di-OH-Chol as well as its (20S, 22S)-isomer. (20S, 22S)-di-OH-Chol (10 μM) was a weak activator, and (20R, 22R)-di-OH-Chol (10 μM) retained activity although it was not more effective than either 20(S)- or 22(R)-OH-Chol (Fig. 3C). It should be noted that the absolute configuration of the 20-OH function in 20(S)-OH-Chol was the same as in (20R, 22R)-di-OH-Chol. Thus, the most effective sterol activators that we tested were 20(S)- and 22(R)-OH Chol.
Geranylgeraniol Is a Negative Regulator of LXRα.
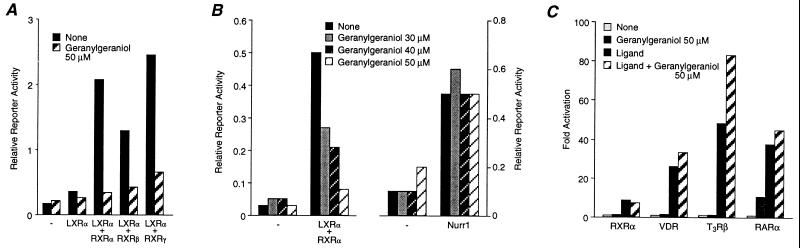
Farnesyl-PP represents a key branch point in the MVA pathway because it can be metabolized along multiple pathways (Fig. 3A). As shown above, one of these pathways leads to the production of oxysterols that activate LXRα. Another major product of farnesyl-PP is GG-PP. Thus, we decided to examine whether GG-OH could modulate LXRα. Unexpectedly, 50 μM GG-OH inhibited the constitutive activity of LXRα–RXR heterodimers (Fig. 4A). Inhibition was specific; GG-OH did not inhibit the constitutive activity of Nurr1 (Fig. 4B) or the ligand-dependent activity of classical nuclear receptors (Fig. 4C). These data suggest that GG-OH, or a metabolite, may serve as an endogenous antagonist of LXRα. This is in contrast to MVA and 20(S)- or 22(R)-OH-Chol, which are natural activators of this receptor.
Figure 4.
Geranylgeraniol inhibits the constitutive activity of LXRα-RXR. (A) Inhibition of LXRα–RXR by geranylgeraniol. (B) Geranylgeraniol does not inhibit the constitutive activity of Nurr1 or (C) ligand-dependent activation by nuclear receptors. Transient transfections were performed as above followed by exposure of cells to 30–50 μM geranylgeraniol.
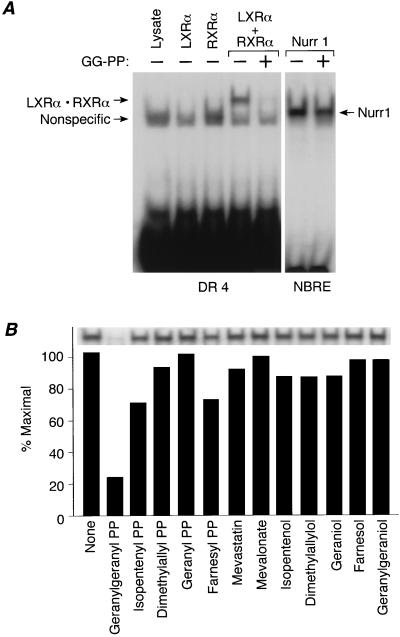
It is unclear how GG-OH (or its metabolite) inhibits LXRα activity. Two protein GG-transferases have been identified that transfer GG groups to specific sequences at the carboxy termini of proteins (type I: Cys-Xaa-Xaa-Leu/Phe; type II: Cys-Xaa-Cys, Cys-Cys, Cys-Cys-Xaa-Xaa) (33–37). It is unlikely that the LXRα–RXR heterodimer can be modified in this fashion because both receptors lack a consensus sequence for protein prenyltransferases (LXRα: Trp-Asp-Val-His-Glu-COOH; RXR: Pro-His-Gln-Met-Thr-COOH). An alternative possibility is that GG-OH, or its metabolite GG-PP, binds directly to the LXRα–RXR heterodimer and causes the complex to dissociate. This would provide a mechanism to account for the antagonistic activity of GG-OH. This hypothesis was tested directly using an electrophoretic mobility shift assay. As shown in Fig. 5A, neither LXRα nor RXR bound to DNA itself. As expected, when the two subunits were mixed in vitro, a heterodimer was formed that bound DNA. In contrast, the heterodimeric complex no longer bound to DNA in the presence of GG-PP (Fig. 5A). Inhibition of DNA binding was specific; other receptors such as Nurr1 were not effected. Moreover, inhibition was specific for GG-PP; GG-OH and other isoprenoids had little effect on the DNA binding activity of LXRα–RXR (Fig. 5B). These data raise the surprising possibility that GG-PP may be a direct antagonist of LXRα.
Figure 5.
Geranylgeranyl-PP inhibits the DNA binding activity of LXRα–RXR. (A) GG-PP inhibits the DNA binding activity of LXRα-RXR complexes. Mobility shift assays were performed as described with 1 μl of LXRα, 0.4 μl of hRXRa, or 1.4 μl of Nurr1 and 17.5 μM GG-PP. (B) Inhibition of DNA binding is specific to GG-PP. Mobility shift assays were performed as in Fig. 5A with the following compounds: 25 μM of each prenyl-PP, 10 μM mevastatin, 200 μM MVA, or 50 μM of each prenyl alcohol.
DISCUSSION
LXRα Is an Isoprenoid Sensor.
Our data suggest that LXRα is an orphan receptor that is both positively and negatively regulated by distinct products of the MVA biosynthetic pathway. By inhibiting endogenous MVA biosynthesis, we were able to show that the constitutive activity of this receptor requires the availability of MVA. Thus, when MVA production is inhibited, constitutive transcriptional activity can be restored by addition of exogenous MVA at appropriate concentrations (200 μM). In contrast, isopentenyl alcohol and other downstream isoprenyl alcohols were incapable of restoring activity. However, for isopentenyl alcohol to enter the MVA pathway, it must first be converted to isopentenyl-PP. Although it is unknown whether this conversion can occur, exogenous farnesol and GG-OH may be converted to their corresponding diphosphate derivatives (26–28). If isopentenyl alcohol is phosphorylated in CV-1 cells, then our data would suggest that the endogenous activator of LXRα is a metabolite that lies upstream of isopentenyl-PP (Fig. 3A). This implicates MVA, MVA phosphate, or a yet-to-be described derivative as an endogenous activator of LXRα. If isopentenyl alcohol is not effectively converted to isopentenyl-PP, we can conclude that the endogenous regulator of LXRα must lie upstream of farnesyl-PP because farnesol could not restore activity to LXRα despite being converted to farnesyl-PP. It is of interest to note that MVA or an immediate derivative has been implicated in the control of cell proliferation (38). Our data suggest a possible connection between this regulatory process and LXRα and/or LXRβ (11–13).
Although it remains unclear which MVA product activates LXRα, our data raise the possibility that an isoprenyl-PP may activate this receptor. This is of special interest because these compounds are produced intracellularly and may be trapped within cells. These compounds could represent an example of a nuclear receptor whose ligand is made and retained within cells. In this case, the concentrations of the isoprenyl-PP signal would be uniquely determined by each cell, thus providing a means for individual cells to maintain a signaling status that is distinct from its neighbor. This is in contrast to classical nuclear receptors where the hormone is produced at a distant site and is made available to each cell by transport and diffusion.
In addition to MVA, we also show that 20(S)-OH-Chol, 22(R)-OH-Chol, and (20R, 22R)-di-OH-Chol can activate LXRα. Activation by these and other oxysterols has been demonstrated recently (29, 30). The doses required for activation (10 μM) by these compounds are similar to the presumed concentrations of these compounds in the adrenal gland (3–7 μM) (39). Of interest, at similar concentrations, 22(R)-OH-Chol and (20R, 22R)-di-OH-Chol act as intermediary substrates in the cytochrome P450scc-mediated conversion of Chol to pregnenolone (40). The production of pregnenolone by this enzyme is the rate-limiting step in steroid hormone biosynthesis. This raises the possibility that LXRα responds to changing concentrations of these key intermediates and may play a role in the regulation of steroid hormone biosynthesis. However, it is unclear whether 20(S)-OH-Chol, 22(R)-OH-Chol, or (20R, 22R)-di-OH-Chol serves as the in vivo ligands for LXRα. Rather, they may be metabolized to a more active ligand. A more detailed understanding of the physiological role of LXRα will require definitive identification of the actual ligand for this receptor.
(20R, 22R)-di-OH-Chol is not commercially available, so it was necessary to develop an efficient synthesis. A previous biosynthetic approach resulted in 9.6% yield by incubation of (22R)-22-OH-Chol with placental mitochondria (41). Previous chemical syntheses of (20R, 22R)-di-OH-Chol were either very lengthy or gave the desired isomer in low yields (20–22, 42, 43). In the present study, osmium-catalyzed asymmetric dihydroxylation (19) of (20(22)E)-3β-acetoxycholesta-5,20(22)-diene gave a 55:45 mixture of the 3β-acetates of (20R, 22R)-di-OH-Chol and its (20S, 22S)-isomer, which could be resolved by semi-preparative Ag+-HPLC. It should be noted that simple dihydroxylation of (20(22)E)-3β-acetoxycholesta-5,20(22)-diene with osmium tetroxide (43) or reduction (under a variety of conditions) of 3β,20α-dihydroxycholest-5-en-22-one (21) gives mixtures in which formation of the (20S, 22S)-isomer is highly favored over the (20R, 22R)-isomer. Thus, the current synthesis represents an efficient route for the preparation of the (20R, 22R)-isomer of di-OH-Chol.
Our most interesting findings are the demonstration that GG-OH or a metabolic derivative is an antagonist of LXRα. This is the first demonstration of a nuclear receptor that possesses both naturally occurring agonists and antagonists. This indicates that distinct products of MVA metabolism can either activate or inhibit signaling through this receptor. Thus, LXRα may function as a key sensor of flux through the MVA pathway. The mechanism by which GG-PP inhibits LXRα remains unclear. One possibility raised by our data is that GG-PP binds to the receptor and inhibits heterodimer formation and/or DNA binding. Further testing of this hypothesis will require a more detailed examination of the potential ligand binding properties of GG-PP. In any event, our findings imply that, in addition to protein prenylation, GG may contribute to additional signaling pathways.
Finally, our data indicate that, upon interaction with RXR, LXRα is converted from a transcriptional repressor to an activator. Transcriptional repression by other receptors results from an interaction between the unliganded LBD and the transcriptional corepressors SMRT/N-COR. In the presence of ligand, the corepressor is released. One interpretation of our findings is that the LXRα LBD interacts with a corepressor in cells. Upon dimerization with RXR, LXRα acquires the potential to bind an endogenous ligand that results in corepressor release and subsequent activation. A requirement of heterodimer formation for ligand binding has been demonstrated for the closely related insect ecdysone receptor, whose ligand is also a 22-OH steroid. Thus, future experiments aimed at identification of LXRα ligands should account for the potential contribution of RXR in establishing the ligand binding complex.
Acknowledgments
We thank David Mangelsdorf (CMX-human LXRα, LXREx3 TK-Luc) and Richard Heyman (LG268) for the contribution of reagents and Debu Chakravarti and Peter Tontonoz for critical reading of the manuscript. This work was supported by the Howard Hughes Medical Institute (R.M.E.), the March of Dimes (R.M.E.), the Tobacco-Related Disease Research Program (B.M.F.), the National Institutes of Health (HL-49122; G.J.S.), and the Robert A. Welch Foundation (C-583; G.J.S.). R.M.E. is an Investigator of the Howard Hughes Medical Institute at the Salk Institute for Biological Studies.
ABBREVIATIONS
- LBD
ligand binding domain
- RXR
9-cis retinoic acid receptor
- DR4
direct repeat with a 4-nt gap
- FXR
farnesoid X receptor
- HMG–CoA
3-hydroxy-3-methylglutaryl–CoA
- Chol
cholesterol
- GG
geranylgeranyl
- OH
hydroxy
- MVA
mevalonic acid
References
- 1.Mangelsdorf D J, Evans R M. Cell. 1995;83:841–50. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 2.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans R M. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forman B M, Chen J, Evans R M. Proc Natl Acad Sci USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kliewer S A, Sundseth S S, Jones S A, Brown P J, Wisely G B, Koble C S, Devchand P, Wahli W, Willson T M, Lenhard J M, Lehmann J M. Proc Natl Acad Sci USA. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forman B M, Tontonoz P, Chen J, Brun R P, Spiegelman B M, Evans R M. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 6.Kliewer S A, Lenhard J M, Willson T M, Patel I, Morris D C, Lehmann J M. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 7.Forman B M, Goode E, Chen J, Oro A E, Bradley D J, Perlmann T, Noonan D J, Burka L T, McMorris T, Lamph W W, Evans R M, Weinberger C. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 8.Yao T P, Forman B M, Jiang Z, Cherbas L, Chen J D, McKeown M, Cherbas P, Evans R M. Nature (London) 1993;366:476–479. doi: 10.1038/366476a0. [DOI] [PubMed] [Google Scholar]
- 9.Apfel P W, Benbrook D, Lernhardt O, M A, Gilles S, Pfahl M. Mol Cell Biol. 1994;14:7025–7035. doi: 10.1128/mcb.14.10.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willy P J, Umesono K, Ong E S, Evans R M, Heyman R A, Mangelsdorf D J. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 11.Teboul M, Enmark E, Li Q, Wikstrom A C, Pelto-Huikko M, Gustafsson J-A. Proc Natl Acad Sci USA. 1995;92:2096–2100. doi: 10.1073/pnas.92.6.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song C, Kokontis J M, Hiipakka R A, Liao S. Proc Natl Acad Sci USA. 1994;91:10809–10813. doi: 10.1073/pnas.91.23.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinar D M, Endo N, Rutledge S J, Vogel R, Rodan G A, Schmidt A, Andres D A. Gene. 1994;147:273–276. doi: 10.1016/0378-1119(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 14.Forman B M, Umesono K, Chen J, Evans R M. Cell. 1995;81:541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 15.Wilson T E, Fahrner T J, Johnston M, Milbrandt J. Science. 1991;252:1296–1300. doi: 10.1126/science.1925541. [DOI] [PubMed] [Google Scholar]
- 16.Schmit J P, Piraux M, Pilette J F. J Org Chem. 1975;40:1586–1588. doi: 10.1021/jo00899a014. [DOI] [PubMed] [Google Scholar]
- 17.Schow S R, McMorris T C. J Org Chem. 1979;44:3760–3765. [Google Scholar]
- 18.Ruan B, Shey J, Gerst N, Wilson W K, Schroepfer G J., Jr Proc Natl Acad Sci USA. 1996;93:11603–11608. doi: 10.1073/pnas.93.21.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharpless K B, Amberg W, Bennani Y L, Crispino G A, Hartung J, Jeong K-S, Kwong H-L, Morikawa K, Wang Z-M, Xu D, Zhang X-L. J Org Chem. 1992;57:2768–2771. [Google Scholar]
- 20.Byon C Y, Gut M. J Org Chem. 1976;41:3716–3722. doi: 10.1021/jo00885a014. [DOI] [PubMed] [Google Scholar]
- 21.Chaudhuri N K, Nickolson R, Kimball H, Gut M. Steroids. 1970;15:525–539. doi: 10.1016/s0039-128x(70)80081-2. [DOI] [PubMed] [Google Scholar]
- 22.Morisaki M, Sato S, Ikekawa N. Chem Pharm Bull. 1977;25:2576–2583. [Google Scholar]
- 23.Hollenberg S M, Evans R M. Cell. 1988;55:899–906. doi: 10.1016/0092-8674(88)90145-6. [DOI] [PubMed] [Google Scholar]
- 24.Nagpal S, Friant S, Nakshatri H, Chambon P. EMBO J. 1993;12:2349–2360. doi: 10.1002/j.1460-2075.1993.tb05889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein J L, Brown M S. Nature (London) 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 26.Crick D C, Andres D A, Waechter C J. Biochem Biophys Res Commun. 1995;211:590–599. doi: 10.1006/bbrc.1995.1854. [DOI] [PubMed] [Google Scholar]
- 27.Crick D C, Waechter C J, Andres D A. Biochem Biophys Res Commun. 1994;205:955–961. doi: 10.1006/bbrc.1994.2758. [DOI] [PubMed] [Google Scholar]
- 28.Westfall D, Aboushadi N, Shackelford J E, Krisans S K, Andres D A. Biochem Biophys Res Commun. 1997;230:562–568. doi: 10.1006/bbrc.1996.6014. [DOI] [PubMed] [Google Scholar]
- 29.Lehmann J M, Kliewer S A, Moore L B, Smith-Oliver T A, Oliver B B, Su J-L, Sundseth S S, Winegar D A, Blanchard D E, Spencer T A, Willson T M. J Biol Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 30.Janowski B A, Willy P J, Devi T R, Falck J R, Mangelsdorf D J. Nature (London) 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 31.Taylor F R, Andres D A. Biochem Biophys Res Commun. 1992;186:182–189. doi: 10.1016/s0006-291x(05)80791-0. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Sato R, Brown M S, Hua X, Goldstein J L. Cell. 1994;77:53–62. doi: 10.1016/0092-8674(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 33.Reiss Y, Goldstein J L, Seabra M C, Casey P J, Brown M S. Cell. 1990;62:81–88. doi: 10.1016/0092-8674(90)90242-7. [DOI] [PubMed] [Google Scholar]
- 34.Gomez R, Goodman L E, Tripathy S K, O’Rourke E, Manne V, Tamanoi F. Biochem J. 1993;289:25–31. doi: 10.1042/bj2890025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khosravi-Far R, Clark G J, Abe K, Cox A D, McLain T, Lutz R J, Sinensky M, Der C J. J Biol Chem. 1992;267:24363–24368. [PubMed] [Google Scholar]
- 36.Moores S L, Schaber M D, Mosser S D, Rands E, O’Hara M B, Garsky V M, Marshall M S, Pompliano D L, Gibbs J B. J Biol Chem. 1991;266:14603–14610. [PubMed] [Google Scholar]
- 37.Omer C A, Gibbs J B. Mol Microbiol. 1994;11:219–225. doi: 10.1111/j.1365-2958.1994.tb00302.x. [DOI] [PubMed] [Google Scholar]
- 38.Cuthbert J A, Lipsky P E, Andres D A. Cancer Res. 1995;55:1732–1740. [PubMed] [Google Scholar]
- 39.Dixon R, Furutachi T, Lieberman S, Andres D A. Biochem Biophys Res Commun. 1970;40:161–165. doi: 10.1016/0006-291x(70)91060-0. [DOI] [PubMed] [Google Scholar]
- 40.Tuckey R C, Andres D A. J Steroid Biochem Mol Biol. 1992;42:883–890. doi: 10.1016/0960-0760(92)90097-3. [DOI] [PubMed] [Google Scholar]
- 41.Tuckey R C, Cameron K J. Steroids. 1993;58:230–238. doi: 10.1016/0039-128x(93)90024-h. [DOI] [PubMed] [Google Scholar]
- 42.Koreeda, M., Koizumi, N. & Teicher, B. A. (1976) Tetrahedron Lett. 4565–4568.
- 43.Bannai K, Morisaki M, Ikekawa N J. Chem Soc Perkin. 1976;1:2116–2120. [PubMed] [Google Scholar]